
Benefits of adjuvant chemotherapy in elderly patients with stage IB–IIIB non-small cell lung cancer: a propensity-matched analysis
Highlight box
Key findings
• Adjuvant chemotherapy (ACT) may confer survival benefits to elderly patients with stage IB–IIIB non-small cell lung cancer (NSCLC).
• Subgroup analysis revealed that ACT was particularly beneficial for individuals aged 70–79 years, male, and with N1 stage NSCLC.
What is known and what is new?
• ACT is a standard treatment option for surgically resected NSCLC, but its efficacy in elderly patients has been debated.
• This study demonstrates that ACT may offer survival advantages in elderly patients with NSCLC, especially in certain subgroups such as those aged 70–79 years and with N1 stage disease.
What is the implication, and what should change now?
• Clinicians should consider ACT as a potential treatment option for elderly patients with stage IB–IIIB NSCLC, particularly those meeting the criteria identified in this study.
• Further research and clinical trials are warranted to validate these findings and refine patient selection criteria for ACT in the elderly NSCLC population.
IntroductionOther Section
With an estimated two million new cases and 1.76 million deaths per year, lung cancer is one of the most commonly diagnosed cancers and the leading cause of cancer-related deaths worldwide (1). Non-small cell lung cancer (NSCLC) accounts for approximately 85% of all lung cancers (2). Although surgery is the most effective curative treatment option for patients with NSCLC, some patients experience recurrence beyond the surgical margin even after receiving curative surgery (3).
In this day and age of targeted therapy and immunotherapy, cytotoxic chemotherapy is still used to treat operable NSCLC (4). Adjuvant chemotherapy (ACT) is used to prevent recurrence in patients who have had their NSCLC completely removed (5). According to national and local guidelines, ACT should be considered for stage IB disease and is strongly recommended for stages II and IIIA disease (6). The Lung Adjuvant Cisplatin Evaluation (LACE)-meta-analysis, which included mainly randomized clinical trials comparing ACT vs. observation in 4,584 patients undergoing surgical resection for early-stage disease, revealed a significant but modest 5.4% improvement in the chemotherapy arm’s 5-year survival rate, implying that we must “treat many to save few” in our daily practices (7,8). Improving patient selection is an important goal of ongoing chemotherapy trials because not all patients require ACT, but selecting those who will benefit remains difficult (9).
In actual practice, oncologists are more likely to believe that older patients cannot endure chemotherapy or that the survival advantage of ACT is not worth the hazards to these patients. According to SEER-Medicare database research, oncologists are increasingly giving ACT to patients aged 70–79 years in response to current guideline recommendations. However, physicians are still hesitant to use ACT on patients aged 80 years and older. The study demonstrated that between 2004 and 2011, the 5-year overall survival (OS) for patients in the 70–79 years age group improved by 7.6% with an increase in the use of ACT. The 5-year OS for patients over 80 years improved by just 1.0% (10). It is yet to be shown whether the rise in 5-year survival is the result of more ACT administered. As a result, further research to guide ACT choices in older NSCLC patients is required. We present this article in accordance with the STROBE reporting checklist (available at https://tcr.amegroups.com/article/view/10.21037/tcr-24-2/rc).
MethodsOther Section
Patient selection and data source
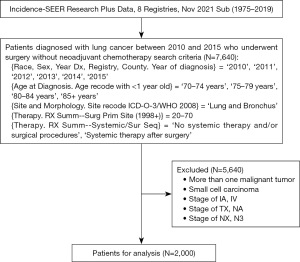
Data from the Surveillance, Epidemiology, and End Results (SEER) database were collected using SEER*Stat software from patients aged 70 years or older, with surgical resection, no preoperative chemotherapy, and pathologically confirmed stage IB, II, or III NSCLC as per the 7th edition American Joint Committee on Cancer staging system (AJCC 7th edition) (version 8.4.0.1; National Cancer Institute, USA). The following were the inclusion criteria: (I) diagnosed with lung cancer by histology between 2010 and 2015; (II) 70 years of age or older; (III) undergoing surgical resection; and (IV) without preoperative chemotherapy. The following were the exclusion criteria: (I) with more than one malignant tumor; (II) small cell carcinoma; (III) tumor-node-metastasis (TNM) stage of IA or IV; (IV) T stage of TX or NA; (V) N stage of NX or N3. After screening, 2,000 eligible patients were eventually included into the analysis. The data processing procedure is depicted in Figure 1. Because SEER is a publicly accessible database, the Institutional Review Board of Zhongshan Hospital Qingpu Branch assessed this study and determined that it was exempt from ethical review. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013).
Variables to investigate
Age, gender, race, laterality, primary tumor site, pathological type, histological grade, T stage, N stage, surgery, scope of regional lymph node removed (SRLNR), radiotherapy, chemotherapy, OS, and survival months were collected. Based on their treatment, patients were separated into ACT and non-ACT groups. This study’s primary outcome was OS. It was described as the period of time between the date of discovery and the date of death, whatever the cause.
Propensity score matching (PSM)
Treatment-related selection bias in retrospective cohort studies is unavoidable due to an imbalance in baseline characteristics. PSM can eliminate selection bias, compensate for differences in clinical characteristics across groups, and strengthen the findings of retrospective cohort research (11). To balance the baseline features of the ACT and non-ACT groups, the current study used a logistic regression model using propensity scores. The dependent variable was designated as ACT, whereas the covariables were various baseline characteristics. The PSM was carried out in a 1:2 ratio using closest neighbor matching and a caliper of 0.001. To compare baseline characteristics between groups, chi-squared or Fisher’s exact tests were utilized.
Statistical analysis
R version 4 was used for the analyses. For survival analysis, Kaplan-Meier methods and log-rank tests were utilized. Cox proportional hazards regression was used for univariate and multivariate analyses. Variables from the univariate analysis with P<0.05 were selected for multivariate analysis. Differences of P<0.05 were considered statistically significant.
ResultsOther Section
Baseline characteristics
This research involved 2,000 patients aged 70 years or older with stage IB–IIIB NSCLN receiving surgical resection without preoperative chemotherapy. In total, 1,497 patients (74.85%) did not receive ACT, whereas 503 patients (25.15%) did. The patients’ baseline characteristics are displayed in Table 1. Age (P<0.001), pathological type (P=0.004), histological grade (P<0.001), T stage (P<0.001), N stage (P<0.001), and radiation (P<0.001) showed significant differences in the unmatched cohort between the ACT and non-ACT groups. The matched cohort included 206 patients who received ACT and 317 patients who did not. In the matched cohorts, the baseline characteristics were well-balanced between the ACT and non-ACT groups. Figure 2 depicts the propensity score distribution map and histogram before and after matching.
Table 1
| Characteristics | Before PSM | After PSM | |||||
|---|---|---|---|---|---|---|---|
| Non-ACT (n=1,497) | ACT (n=503) | P value | Non-ACT (n=317) | ACT (n=206) | P value | ||
| Age (years), n (%) | <0.001 | 0.76 | |||||
| 70–79 | 1,061 (70.9) | 450 (89.5) | 290 (91.5) | 186 (90.3) | |||
| ≥80 | 436 (29.1) | 53 (10.5) | 27 (8.5) | 20 (9.7) | |||
| Gender, n (%) | 0.52 | >0.99 | |||||
| Female | 768 (51.3) | 249 (49.5) | 165 (52.1) | 108 (52.4) | |||
| Male | 729 (48.7) | 254 (50.5) | 152 (47.9) | 98 (47.6) | |||
| Race, n (%) | 0.50 | 0.47 | |||||
| White | 1,233 (82.4) | 404 (80.3) | 280 (88.3) | 176 (85.4) | |||
| Black | 75 (5.0) | 31 (6.2) | 13 (4.1) | 8 (3.9) | |||
| Other | 189 (12.6) | 68 (13.5) | 24 (7.6) | 22 (10.7) | |||
| Laterality, n (%) | 0.32 | 0.38 | |||||
| Left | 621 (41.5) | 222 (44.1) | 122 (38.5) | 88 (42.7) | |||
| Right | 876 (58.5) | 281 (55.9) | 195 (61.5) | 118 (57.3) | |||
| Tumor site, n (%) | >0.99 | 0.48 | |||||
| Lung lobe | 1,431 (95.6) | 481 (95.6) | 307 (96.8) | 201 (97.6) | |||
| Main bronchus | 6 (0.4) | 2 (0.4) | 0 (0.0) | 1 (0.5) | |||
| Overlapping | 28 (1.9) | 9 (1.8) | 6 (1.9) | 3 (1.5) | |||
| Unknown | 32 (2.1) | 11 (2.2) | 4 (1.3) | 1 (0.5) | |||
| Pathological type, n (%) | 0.004 | 0.79 | |||||
| Adenocarcinoma | 526 (35.1) | 206 (41.0) | 116 (36.6) | 76 (36.9) | |||
| Squamous cell carcinoma | 483 (32.3) | 124 (24.7) | 82 (25.9) | 58 (28.2) | |||
| Other | 488 (32.6) | 173 (34.4) | 119 (37.5) | 72 (35.0) | |||
| Histological grade, n (%) | <0.001 | 0.43 | |||||
| I | 210 (14.0) | 40 (8.0) | 39 (12.3) | 18 (8.7) | |||
| II | 695 (46.4) | 219 (43.5) | 137 (43.2) | 94 (45.6) | |||
| III | 483 (32.3) | 208 (41.4) | 125 (39.4) | 81 (39.3) | |||
| IV | 23 (1.5) | 7 (1.4) | 6 (1.9) | 2 (1.0) | |||
| Unknown | 86 (5.7) | 29 (5.8) | 10 (3.2) | 11 (5.3) | |||
| T stage, n (%) | <0.001 | 0.57 | |||||
| T1 | 64 (4.3) | 81 (16.1) | 23 (7.3) | 20 (9.7) | |||
| T2 | 1,072 (71.6) | 254 (50.5) | 207 (65.3) | 123 (59.7) | |||
| T3 | 285 (19.0) | 125 (24.9) | 73 (23.0) | 52 (25.2) | |||
| T4 | 76 (5.1) | 43 (8.5) | 14 (4.4) | 11 (5.3) | |||
| N stage, n (%) | <0.001 | 0.09 | |||||
| N0 | 1,212 (81.0) | 178 (35.4) | 220 (69.4) | 126 (61.2) | |||
| N1 | 188 (12.6) | 180 (35.8) | 71 (22.4) | 53 (25.7) | |||
| N2 | 97 (6.5) | 145 (28.8) | 26 (8.2) | 27 (13.1) | |||
| Surgery, n (%) | 0.08 | 0.22 | |||||
| Sublobectomy | 261 (17.4) | 71 (14.1) | 33 (10.4) | 19 (9.2) | |||
| Lobe or bilobectomy | 1,187 (79.3) | 408 (81.1) | 281 (88.6) | 181 (87.9) | |||
| Pneumonectomy | 49 (3.3) | 24 (4.8) | 3 (0.9) | 6 (2.9) | |||
| SRLNR, n (%) | 0.17 | 0.54 | |||||
| None | 126 (8.4) | 33 (6.6) | 16 (5.0) | 12 (5.8) | |||
| <4 | 159 (10.6) | 41 (8.2) | 25 (7.9) | 14 (6.8) | |||
| ≥4 | 1,154 (77.1) | 411 (81.7) | 271 (85.5) | 173 (84.0) | |||
| Others | 58 (3.9) | 18 (3.6) | 5 (1.6) | 7 (3.4) | |||
| Radiotherapy, n (%) | <0.001 | 0.34 | |||||
| No | 1,435 (95.9) | 384 (76.3) | 308 (97.2) | 196 (95.1) | |||
| Yes | 62 (4.1) | 119 (23.7) | 9 (2.8) | 10 (4.9) | |||
ACT, adjuvant chemotherapy; PSM, propensity score matching; SRLNR, scope of region lymph node removed.

Survival analysis
Kaplan-Meier analysis revealed no statistically significant difference in the OS of patients who received ACT vs. those who did not (P=0.07) (Figure 3A). The median OS for the ACT and non-ACT groups was 56 months [95% confidence interval (CI): 47–65] and 49 months (95% CI: 45–53), respectively. The ACT and non-ACT groups had 3- and 5-year OS of 62.28% vs. 58.74% and 47.86% vs. 43.66%, respectively. After 1:2 matching, patients who received ACT had a longer OS than non-ACT patients (P=0.044) (Figure 3B). The median OS for the ACT and non-ACT groups was 67 months (95% CI: 56–106) and 48 months (95% CI: 42–71), respectively. The ACT and non-ACT groups’ 3- and 5-year OS were 65.37% vs. 59.38% and 53.66% vs. 45.91%, respectively.

Univariate and multivariate Cox regression analyses after PSM
Age, gender, primary tumor site, histological grade, N stage, radiotherapy, and ACT were all significantly associated with OS (P<0.05) (Table 2). Variables from the univariate analysis with P<0.05 were considered for multivariate analysis. Gender, primary tumor site, histological grade, N stage, and ACT were shown to be independently linked with OS (P<0.05) in the multivariate analysis. In relation to gender, females were used as the control group, while men [hazard ratio (HR) =1.42; 95% CI: 1.13–1.79; P=0.003] were identified as an adverse prognostic factor for OS. N1 (HR =1.72; 95% CI: 1.3–2.26; P<0.001) and N2 (HR =3.33; 95% CI: 2.3–4.82; P<0.001) exhibited a negative prognostic impact on OS in comparison to N0. ACT was linked to a higher life expectancy (P=0.002).
Table 2
| Characteristics | Univariate analysis | Multivariate analysis | |||
|---|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | ||
| Age (years) | |||||
| 70–79 | 1 | 1 | |||
| ≥80 | 1.61 (1.13–2.29) | 0.008 | 1.12 (0.77–1.62) | 0.56 | |
| Gender | |||||
| Female | 1 | 1 | |||
| Male | 1.37 (1.09–1.72) | 0.007 | 1.42 (1.13–1.79) | 0.003 | |
| Race | |||||
| White | 1 | ||||
| Black | 1.09 (0.64–1.86) | 0.76 | |||
| Other | 0.66 (0.42–1.03) | 0.07 | |||
| Laterality | |||||
| Left | 1 | ||||
| Right | 1.03 (0.81–1.3) | 0.82 | |||
| Tumor site | |||||
| Lung lobe | 1 | 1 | |||
| Main bronchus | 7.32 (1.02–52.7) | 0.048 | 13.98 (1.76–111.01) | 0.01 | |
| Overlapping lesion of lung | 1.52 (0.72–3.22) | 0.27 | 1.66 (0.77–3.55) | 0.19 | |
| Unknown | 1.24 (0.4–3.88) | 0.71 | 1.83 (0.58–5.8) | 0.30 | |
| Pathological type | |||||
| Adenocarcinoma | 1 | ||||
| Squamous cell carcinoma | 1.11 (0.84–1.48) | 0.46 | |||
| Other | 0.93 (0.71–1.22) | 0.61 | |||
| Histological grade | |||||
| I | 1 | 1 | |||
| II | 1.04 (0.68–1.58) | 0.86 | 0.88 (0.57–1.35) | 0.55 | |
| III | 1.59 (1.05–2.4) | 0.03 | 1.39 (0.91–2.12) | 0.13 | |
| IV | 5.02 (2.18–11.58) | <0.001 | 5.55 (2.34–13.16) | <0.001 | |
| Unknown | 1.22 (0.61–2.46) | 0.58 | 1.29 (0.64–2.61) | 0.48 | |
| T stage | |||||
| T1 | 1 | ||||
| T2 | 0.88 (0.57–1.35) | 0.55 | |||
| T3 | 1.3 (0.82–2.07) | 0.26 | |||
| T4 | 0.79 (0.39–1.59) | 0.51 | |||
| N stage | |||||
| N0 | 1 | 1 | |||
| N1 | 1.53 (1.17–2) | 0.002 | 1.72 (1.3–2.26) | <0.001 | |
| N2 | 2.96 (2.12–4.14) | <0.001 | 3.33 (2.3–4.82) | <0.001 | |
| Surgery | |||||
| Sublobectomy | 1 | ||||
| Lobe or bilobectomy | 0.8 (0.56–1.16) | 0.24 | |||
| Pneumonectomy | 0.79 (0.31–2.03) | 0.63 | |||
| SRLNR | |||||
| None | 1 | ||||
| <4 | 0.9 (0.49–1.65) | 0.73 | |||
| ≥4 | 0.8 (0.5–1.29) | 0.36 | |||
| Others | 0.85 (0.37–1.95) | 0.70 | |||
| Radiotherapy | |||||
| No | 1 | 1 | |||
| Yes | 2.7 (1.65–4.42) | <0.001 | 1.49 (0.86–2.57) | 0.15 | |
| ACT | |||||
| No | 1 | 1 | |||
| Yes | 0.78 (0.62–0.99) | 0.044 | 0.68 (0.53–0.86) | 0.002 | |
OS, overall survival; PSM, propensity score matching; HR, hazard ratio; CI, confidence interval; SRLNR, scope of regional lymph node removed; ACT, adjuvant chemotherapy.
Subgroup analysis after PSM
Subgroup analyses stratified by age and N stage were performed using Kaplan-Meier. When the age range was 70–79 years, the ACT group had a longer OS than the non-ACT group. The 3- and 5-year OS of the ACT and non-ACT groups was 67.03% vs. 60.8% and 55.27% vs. 47.1%, respectively (P=0.03) (Figure 4A). There was not a significant difference in the OS of the ACT and non-ACT groups when the age was ≥80 years. The 3- and 5-year OS of the ACT and non-ACT groups was 50% vs. 44.44% and 38.6% vs. 33.33%, respectively (P=0.96) (Figure 4B). In patients with N0, there was no significant difference in OS between the ACT and non-ACT groups, with 3-year OS rates of 70.41% vs. 68.55% and 5-year OS rates of 57.74% vs. 55.24% (P=0.58) (Figure 5A). Conversely, among patients with N1, the ACT group had a 3- and 5-year OS of 71.7% and 57.97%, respectively, compared to 44.43% and 28.51% in the non-ACT group (P<0.001) (Figure 5B). Furthermore, for patients with N2, although there was a trend toward better OS in the ACT group, the difference was not statistically significant, with respective 3-year OS of 29.63% vs. 23.08% and 5-year OS of 25.93% vs. 15.38% (P=0.08) (Figure 5C). Cox proportional hazard analysis was used to investigate the survival benefit of ACT for patients in various categories. ACT improved the OS of patients who were 70–79 years of age (HR =0.76; 95% CI: 0.59–0.98; P=0.03), male (HR =0.72; 95% CI: 0.52–1; P=0.048), lung lobes (HR =0.78; 95% CI: 0.61–0.99; P=0.04), N1 stage (HR =0.42; 95% CI: 0.26–0.68; P<0.001), and non-radiotherapy (HR =0.75; 95% CI: 0.59–0.96; P=0.02) (Figure 6).



DiscussionOther Section
Randomized controlled studies of patients with stage I–III NSCLC have shown that postoperative cisplatin-based treatment considerably lowers the risk of mortality, particularly in stage II and III cancer (12,13). ACT with a cisplatin-based combination regimen is the present treatment standard in stage II and IIIA NSCLC following surgical resection (14,15). Postoperative ACT is also recommended by the National Comprehensive Cancer Network (NCCN) for stage IB patients with high-risk criteria such as poorly differentiated tumors, vascular invasion, wedge resection, visceral pleural invasion (VPI), and uncertain lymph node status (16). Nonetheless, the applicability of ACT to the elderly has been questioned due to their shorter life expectancy. It is critical for older patients to examine both the long-term advantages of ACT and the hazards associated with short-term toxicity. These dangers make deciding whether ACT is beneficial challenging (17). According to one study, early mortality with ACT following full resection of NSCLC was greater in individuals over the age of 70 years (18). For these reasons, it is critical to investigate the effect of ACT on the OS of elderly stage IB–IIIB NSCLC patients.
A meta-analysis of randomized trials found that patients over the age of 70 with NSCLC benefited from ACT in the same way as their younger counterparts (19). Another recent trial found that ACT enhanced the prognosis following routine lung cancer surgery in people over the age of 75 years with stage IB–IIIA NSCLC (17). Before PSM, our investigation found that ACT did not substantially enhance the OS of patients aged 70 years or older with stage IB–IIIB NSCLC. Differences in clinicopathological parameters, such as age, gender, race, tumor location, pathological type, histological grade, T stage, N stage, surgical procedures, SRLNR, and radiotherapy, must be considered. In the adjusted cohorts, baseline characteristics were identical across the ACT and non-ACT groups after PSM, and patients with ACT had a longer OS than those without. Subgroup analysis stratified by age revealed that ACT enhanced the OS of those 70–79 years old with stage IB–IIIB NSCLC. When the participants were over 80 years old, there was no significant difference in OS between the ACT and non-ACT groups. As a result, the maximum age for ACT is may be 80 years old.
A lobectomy with mediastinal lymph node dissection is the usual treatment modality for lung cancer. Because of its high local control, the operation has been demonstrated to be better than a sublobar resection such as a wedge resection or a segmentectomy (20,21). The NCCN classifies sublobar resection as a high-risk characteristic and a sign of ACT (16). Patients receiving sublobar resection have a higher risk of insufficient lymphadenectomy and positive margins (22). Lymph node assessment is critical for appropriate staging and therapy. The key to effective adjuvant therapy administration is precise staging. ACT, for example, is advised for NSCLC patients who have any evidence of lymph node metastasis, and the advantages of ACT in patients with node-positive NSCLC have been thoroughly documented. Furthermore, the therapeutic benefits of ACT on any undetected residual cancer may contribute to reduced recurrence risk and enhanced survival (23). According to a recent study, ACT was not advantageous to patients with stage T1a to T1c tumors with insufficient nodal evaluation, but it might be used as a supplemental treatment for patients with stage T2a tumors who were stated to have node-negative cancer but were probably understaged (24). In our study, however, the surgical approach and extent of lymph node dissection had no influence on the prognosis of patients aged 70 years or older with stage IB–IIIB NSCLC. In our research, neither sublobectomy nor insufficient lymph node dissection were associated with ACT benefits. As a result, we hypothesize that sublobectomy and insufficient lymph node dissection are unimportant factors in predicting ACT for older NSCLC patients and that R0 resection may be the most significant consideration for elderly NSCLC patients.
In NSCLC patients, tumor stage has been routinely utilized to predict prognosis and guide ACT (25,26). A high T stage always indicates a poor prognosis and may necessitate adjuvant treatment. In individuals with node-negative NSCLC, a tumor size greater than 4 cm is regarded as an indication for ACT (27). We discovered no ACT benefit in patients with high T. In our study, patients with N1 had greater OS after adjuvant treatment. Toubat et al. discovered that N1 NSCLC patients treated with ACT had a 14% 5-year survival advantage over those receiving surgery alone, indicating that N1 NSCLC patients may benefit more than previously thought from ACT (28,29).
Many studies found that women with NSCLC had considerably superior OS (30,31). We also found a comparable effect of gender on OS in individuals aged 70 years or older with stage IB–IIIB NSCLC. On the contrary, male patients in our research had an improved OS as a result of ACT, but female patients did not. Previous research has found that postoperative radiation is ineffective and appears to be related to higher toxicity in older individuals with early-stage NSCLC (32). In our study, those who had radiation had a poorer prognosis, and ACT did not enhance the prognosis. Patients who did not receive radiation had a superior outcome after ACT.
There are various limitations to our study. First, because this is retrospective research, the population selection is bound to be biased, and it cannot control for confounding factors as rigorously as prospective studies. Although we performed the PSM to lessen the potential bias, there may be an undiscovered bias that the PSM did not correct (33). Following that, it is unclear how patients in the SEER database are chosen for various therapies. Finally, the SEER database is unable to give more precise information on performance status, surgical margin status, chemotherapy regimens and cycles, and additional treatment after recurrence, so we must proceed with caution. As a result, additional prospective randomized controlled studies are required to confirm our findings.
ConclusionsOther Section
Finally, our study discovered that ACT may give survival advantages to elderly stage IB–IIIB NSCLC patients. Consideration may be given to a potential upper age limit of 80 years for ACT. ACT is more beneficial to patients at the N1 stage. ACT benefits male patients more than female ones. T stage, pathological type, histological grade, surgical approach, and SRLNR, on the other hand, may not be significant in identifying whether ACT was beneficial. Radiotherapy is not advised for older NSCLC patients who are undergoing surgery. Clinicians should carefully assess if ACT is helpful for elderly patients with advanced NSCLC.
AcknowledgmentsOther Section
We appreciated the National Cancer Institute’s decision to make SEER data available to the general population.
Funding: None.
FootnoteOther Section
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://tcr.amegroups.com/article/view/10.21037/tcr-24-2/rc
Peer Review File: Available at https://tcr.amegroups.com/article/view/10.21037/tcr-24-2/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tcr.amegroups.com/article/view/10.21037/tcr-24-2/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
ReferencesOther Section
- Thai AA, Solomon BJ, Sequist LV, et al. Lung cancer. Lancet 2021;398:535-54. [Crossref] [PubMed]
- Yang ZR, Liu MN, Yu JH, et al. Treatment of stage III non-small cell lung cancer in the era of immunotherapy: pathological complete response to neoadjuvant pembrolizumab and chemotherapy. Transl Lung Cancer Res 2020;9:2059-73. [Crossref] [PubMed]
- Tozuka T, Noro R, Seike M, et al. Benefits from Adjuvant Chemotherapy in Patients with Resected Non-Small Cell Lung Cancer: Possibility of Stratification by Gene Amplification of ACTN4 According to Evaluation of Metastatic Ability. Cancers (Basel) 2022;14:4363. [Crossref] [PubMed]
- Zhang Y, Chen H. Neoadjuvant or adjuvant chemotherapy for non-small-cell lung cancer: Does the timing matter? J Thorac Cardiovasc Surg 2019;157:756-7. [Crossref] [PubMed]
- Wakelee H, Chhatwani L. Adjuvant chemotherapy for resected non-small cell lung cancer. Semin Thorac Cardiovasc Surg 2008;20:198-203. [Crossref] [PubMed]
- O'Brien M, Paz-Ares L, Marreaud S, et al. Pembrolizumab versus placebo as adjuvant therapy for completely resected stage IB-IIIA non-small-cell lung cancer (PEARLS/KEYNOTE-091): an interim analysis of a randomised, triple-blind, phase 3 trial. Lancet Oncol 2022;23:1274-86. [Crossref] [PubMed]
- Pignon JP, Tribodet H, Scagliotti GV, et al. Lung adjuvant cisplatin evaluation: a pooled analysis by the LACE Collaborative Group. J Clin Oncol 2008;26:3552-9. [Crossref] [PubMed]
- Passiglia F, Bertaglia V, Reale ML, et al. Major breakthroughs in lung cancer adjuvant treatment: Looking beyond the horizon. Cancer Treat Rev 2021;101:102308. [Crossref] [PubMed]
- Mascaux C, Shepherd FA. Adjuvant chemotherapy after pulmonary resection for lung cancer. Thorac Surg Clin 2013;23:401-10. [Crossref] [PubMed]
- Komiya T, Powell E, Guddati AK. Recent trends in use of adjuvant chemotherapy in elderly stage II-III non-small cell lung cancer. Transl Lung Cancer Res 2020;9:1180-6. [Crossref] [PubMed]
- Yang WY, He Y, Hu Q, et al. Survival benefit of thermal ablation therapy for patients with stage II-III non-small cell lung cancer: A propensity-matched analysis. Front Oncol 2022;12:984932. [Crossref] [PubMed]
- Chaft JE, Rimner A, Weder W, et al. Evolution of systemic therapy for stages I-III non-metastatic non-small-cell lung cancer. Nat Rev Clin Oncol 2021;18:547-57. [Crossref] [PubMed]
- Bradbury P, Sivajohanathan D, Chan A, et al. Postoperative Adjuvant Systemic Therapy in Completely Resected Non-Small-Cell Lung Cancer: A Systematic Review. Clin Lung Cancer 2017;18:259-273.e8. [Crossref] [PubMed]
- Pisters KM, Evans WK, Azzoli CG, et al. Cancer Care Ontario and American Society of Clinical Oncology adjuvant chemotherapy and adjuvant radiation therapy for stages I-IIIA resectable non small-cell lung cancer guideline. J Clin Oncol 2007;25:5506-18. [Crossref] [PubMed]
- Szeto CH, Shalata W, Yakobson A, et al. Neoadjuvant and Adjuvant Immunotherapy in Early-Stage Non-Small-Cell Lung Cancer, Past, Present, and Future. J Clin Med 2021;10:5614. [Crossref] [PubMed]
- Ettinger DS, Wood DE, Aisner DL, et al. NCCN Guidelines Insights: Non-Small Cell Lung Cancer, Version 2.2021. J Natl Compr Canc Netw 2021;19:254-66. [Crossref] [PubMed]
- Yamanashi K, Okumura N, Yamamoto Y, et al. Adjuvant chemotherapy for elderly patients with non-small-cell lung cancer. Asian Cardiovasc Thorac Ann 2017;25:371-7. [Crossref] [PubMed]
- Morgensztern D, Samson PS, Waqar SN, et al. Early Mortality in Patients Undergoing Adjuvant Chemotherapy for Non-Small Cell Lung Cancer. J Thorac Oncol 2018;13:543-9. [Crossref] [PubMed]
- Früh M, Rolland E, Pignon JP, et al. Pooled analysis of the effect of age on adjuvant cisplatin-based chemotherapy for completely resected non-small-cell lung cancer. J Clin Oncol 2008;26:3573-81. [Crossref] [PubMed]
- Chida M, Minowa M, Karube Y, et al. Worsened long-term outcomes and postoperative complications in octogenarians with lung cancer following mediastinal lymph-node dissection. Interact Cardiovasc Thorac Surg 2009;8:89-92. [Crossref] [PubMed]
- Ginsberg RJ, Rubinstein LV. Randomized trial of lobectomy versus limited resection for T1 N0 non-small cell lung cancer. Lung Cancer Study Group. Ann Thorac Surg 1995;60:615-22; discussion 622-3. [Crossref] [PubMed]
- Khullar OV, Liu Y, Gillespie T, et al. Survival After Sublobar Resection versus Lobectomy for Clinical Stage IA Lung Cancer: An Analysis from the National Cancer Data Base. J Thorac Oncol 2015;10:1625-33. [Crossref] [PubMed]
- Liang W, He J, Shen Y, et al. Impact of Examined Lymph Node Count on Precise Staging and Long-Term Survival of Resected Non-Small-Cell Lung Cancer: A Population Study of the US SEER Database and a Chinese Multi-Institutional Registry. J Clin Oncol 2017;35:1162-70. [Crossref] [PubMed]
- Dai J, Liu M, Yang Y, et al. Optimal Lymph Node Examination and Adjuvant Chemotherapy for Stage I Lung Cancer. J Thorac Oncol 2019;14:1277-85. [Crossref] [PubMed]
- Ettinger DS, Wood DE, Aisner DL, et al. Non-Small Cell Lung Cancer, Version 3.2022, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw 2022;20:497-530. [Crossref] [PubMed]
- Li DH, He YQ, Wang TM, et al. Development and validation of a polygenic hazard score to predict prognosis and adjuvant chemotherapy benefit in early-stage non-small cell lung cancer. Transl Lung Cancer Res 2022;11:1809-22. [Crossref] [PubMed]
- Pathak R, Goldberg SB, Canavan M, et al. Association of Survival With Adjuvant Chemotherapy Among Patients With Early-Stage Non-Small Cell Lung Cancer With vs Without High-Risk Clinicopathologic Features. JAMA Oncol 2020;6:1741-50. [Crossref] [PubMed]
- Toubat O, Atay SM, Kim AW, et al. Disparities in Guideline-Concordant Treatment for Pathologic N1 Non-Small Cell Lung Cancer. Ann Thorac Surg 2020;109:1512-20. [Crossref] [PubMed]
- Toubat O, Ding L, Ding K, et al. Benefit of adjuvant chemotherapy for resected pathologic N1 non-small cell lung cancer is unrecognized: A subgroup analysis of the JBR10 trial. Semin Thorac Cardiovasc Surg 2022; Epub ahead of print. [Crossref] [PubMed]
- Hanagiri T, Sugio K, Uramoto H, et al. Gender difference as a prognostic factor in patients undergoing resection of non-small cell lung cancer. Surg Today 2007;37:546-51. [Crossref] [PubMed]
- Wang ZH, Deng L. Establishment and Validation of a Predictive Nomogram for Postoperative Survival of Stage I Non-Small Cell Lung Cancer. Int J Gen Med 2022;15:7287-98. [Crossref] [PubMed]
- Veluswamy RR, Mhango G, Bonomi M, et al. Adjuvant treatment for elderly patients with early-stage lung cancer treated with limited resection. Ann Am Thorac Soc 2013;10:622-8. [Crossref] [PubMed]
- Ning J, Ge T, Zhu S, et al. The role of surgery in older patients with T1-2N0M0 small cell lung cancer: A propensity score matching analysis. Front Oncol 2022;12:958187. [Crossref] [PubMed]


