
TLK2 promotes progression of hepatocellular carcinoma through Wnt/β-catenin signaling
Highlight box
Key findings
• TLK2 stimulates hepatocellular carcinoma growth through the activation of the Wnt/β-catenin pathway.
What is known and what is new?
• TLK2 is highly expressed in tumor tissue has been studied.
• TLK2 promotes hepatocellular carcinoma proliferation through β-catenin is a new finding from our study.
What is the implication, and what should change now?
• Our study revealed that TLK2 may act as a new potential factor to promote the proliferation of hepatocellular carcinoma. Previous studies have indicated that TLK2 is associated with tumor proliferation, but no specific mechanism has been proposed. Our study is the first demonstration that TLK2 promotes hepatocellular carcinoma proliferation in association with β-catenin activation.
Introduction
Hepatocellular carcinoma is one of the leading causes of cancer-related deaths worldwide and has a high incidence (1), and the incidence rates of hepatocellular carcinoma in Hispanics have surpassed those of Asians in the United States (2). Chronic liver disease is caused by a vicious cycle of liver damage, inflammation, and regeneration that can last decades, leading to hepatocellular carcinoma (3). The main causes of chronic liver disease and hepatocellular carcinoma include hepatitis B and hepatitis C virus infections, alcoholic liver disease, fatty liver, and schistosome infection (4,5). Although the etiology and pathogenesis of hepatocellular carcinoma are multifaceted, the exact molecular mechanisms underlying its development are not yet completely understood.
Prior research has indicated that the activation of β-catenin, a key signaling pathway involved in cell proliferation and survival, is highly prevalent in hepatocellular carcinoma (6). A comprehensive genomic analysis of hepatocellular carcinoma revealed the presence of gain-of-function mutations in CTNNB1, which encodes β-catenin in approximately 35% of human hepatocellular carcinoma samples (7). Wnt/β-catenin plays a central role in human embryonic development and regulates liver development during the embryonic period via Wnt signaling. During adulthood, the Wnt/β-catenin pathway is typically inactive in the liver. However, abnormal expression of this pathway can occur in certain instances, such as cell renewal, regeneration, pathological conditions, diseases, and various types of cancers (8). In recent years, numerous studies have shown that Wnt/β-catenin protein signaling plays a major role in not only enhancing cancer cell stemness but also maintaining sorafenib resistance in hepatocellular carcinoma (9,10), and also mediates immune escape in hepatocellular carcinoma (11), suggesting that it may play an essential role in drug resistance.
Tousled-like kinases (TLKs) are an evolutionarily conserved family of serine-threonine kinases that are mainly located in the nucleus (12). In mammals, there are two distinct TLK genes, TLK1 and TLK2. TLK2 is an extensively studied protein that is aberrantly expressed in various types of cancer, including breast cancer, lung cancer, ovarian cancer, hepatocellular carcinoma, and melanoma (13). This protein is closely associated with DNA repair, DNA replication, chromatin structure, transcription, viral latency, cell cycle checkpoint control, and chromosome stability in a wide range of organisms (14). TLK2 primarily functions in the cell division cycle, and its expression typically peaks during the S phase (15). TLK2 amplification has been found to induce genomic instability, leading to the development of breast cancer through the impairment of the G2–M checkpoint (16). In particular, ectopic expression of TLK2 has been shown to lead to increased aggressiveness in breast cancer and glioblastoma (17,18). The TLK-ASF1 axis can promote leukemogenesis by affecting cell cycle and DNA damage pathways (19). These studies support that TLK2 may be an important target for the development of novel cancer therapies.
Although the stimulatory effect of β-catenin on hepatocellular carcinoma progression has been established, the relationship between TLK2 and hepatocellular carcinoma is not understood. Herein, we present a new mechanism for promoting hepatocellular carcinoma by regulating β-catenin. The Cancer Genome Atlas (TCGA) database analysis revealed high TLK2 expression in hepatocellular carcinoma. Patients with elevated TLK2 levels had a poorer prognosis. Moreover, TLK2 was found to activate the classical Wnt pathway and was closely associated with β-catenin, thus promoting hepatocellular carcinoma. We present this article in accordance with the MDAR reporting checklist (available at https://tcr.amegroups.com/article/view/10.21037/tcr-23-2264/rc).
Methods
Acquisition of single gene expression data from TCGA
Gene expression profile matrix and clinical records of hepatocellular carcinoma were downloaded from TCGA (https://tcga-data.nci.nih.gov/tcga/). We conducted transcriptome profiling on 374 tumor and 50 normal tissue samples, with the primary site being the liver. The gene expression profile matrix was standardized using log2(value +1) (version 3.6.3; http://www.r-project.org). Additionally, TLK2 expression in hepatocellular carcinoma was analyzed using the Integrative Molecular Database for Hepatocellular Carcinoma (HCCDB) (https://ngdc.cncb.ac.cn/). The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013).
Univariate/multivariate Cox hazard regression analyses and nomogram construction
We utilized univariate and multivariate Cox regression analyses to explore the impact of the TLK2 gene and clinical characteristics, such as age, sex, alpha fetoprotein (AFP), pathological stage, and histological grade, on the prognosis of hepatocellular carcinoma. The R package “forestplot” was employed to display the P value, hazard ratio (HR), and 95% confidence interval (CI) of each variable. Additionally, TLK2 expression in hepatocellular carcinoma was evaluated using the HCCDB database. To standardize the gene expression profile matrix, we employed R software (version 3.6.3; http://www.r-project.org). Kaplan-Meier Plotter was used to generate survival curves, including overall survival (OS), progression-free survival (PFS), and disease-specific survival (DSS) using the log-rank test.
Cell lines
The PLC/PRF/5 and HepG2 cell lines used in this study were purchased from the Cell Bank of the Chinese Academy of Sciences in Shanghai, China. These cells were cultured in dulbecco’s modified eagle medium (DMEM) supplemented with 10% fetal bovine serum and maintained at 37 ℃ in a 5% CO2 incubator. PLC/PRF/5 and HepG2 cells were authenticated by short tandem repeats (STRs).
Plasmids and siRNA transfection
To investigate the role of TLK2, we designed three small interfering RNA (siRNA) sequences to suppress its expression. The primers are listed in Table 1. The siRNAs were obtained from Tsingke Biotechnology Co., Ltd. (Beijing, China). The cells used for the transfection experiments were PLC/PRF/5 and HepG2 cells, and Lipofectamine 2000 was used at a concentration of 50 nmol according to the manufacturer’s instructions. The pCDH-GFP-Puro-3xFlag plasmid encoding TLK2 and a control plasmid were transfected into the cells with Lipofectamine 3000 (Invitrogen, Waltham, USA).
Table 1
| Genes primers (5'-3') | Sequences |
|---|---|
| SI-TLK2-1 forward | GCAAGAAACUCCUUAUAGA |
| SI-TLK2-1 reverse | UCUAUAAGGAGUUUCUUGC |
| SI-TLK2-2 forward | GUCCGACCUCACAAUAGAA |
| SI-TLK2-2 reverse | UUCUAUUGUGAGGUCGGAC |
siRNA, small interfering RNA.
Western blot analysis
Thirty micrograms of protein was separated on a 10% sodium dodecyl sulfate - polyacrylamide gel electrophoresis (SDS-PAGE) gel and transferred to a 0.45 µm polyvinylidene difluoride (PVDF) membrane in a wet electron transfer device. Tris buffered saline (TBS) and Tween 20 were used to prepare 0.05% tris buffered saline with Tween 20 (TBST). Five percent skim milk was prepared with TBST to block the membrane for 2 hours at room temperature. After transferring the proteins onto the membrane, the membrane was incubated with primary antibodies overnight at 4 ℃. The primary antibodies used were TLK2 (1:1,000, 13979-1-AP, Proteintech, Wuhan, China) and β-catenin (1:1,000, 51067-2-AP, Proteintech). The membranes were then incubated with horse radish peroxidase (HRP)-conjugated goat anti-rabbit or goat anti-mouse secondary antibodies for 1.5 hours at room temperature.
Methyl thiazolyl tetrazolium (MTT) analysis and colony formation assay
Hepatocellular carcinoma cells (4,000 per well) were seeded into 96-well plates. Cells were collected at 0, 24, 48, and 72 h, and the relative viable cell numbers were quantified using the MTT assay, with absorbance was measured at 490 nm. For the colony formation assay, hepatocellular carcinoma cells were seeded at a density of 500 cells per well on 6-well plates. After 2 weeks, each group of cells was fixed with 4% paraformaldehyde (PFA) for 20 minutes and then stained with 0.1% crystal violet solution for imaging and counting.
Immunofluorescence assay
Cells were grown in confocal dishes at a density of 1,000/well and washed three times with phosphate buffer saline (PBS). The cells were then fixed with 4% PFA for 15 min and washed three times with PBS. The cells were permeabilized with 0.5% Triton X-100 for 15 min at room temperature and washed three times with PBS. Fresh containment solution (1 mg/mL BSA, 10% normal goat serum, 0.1% Tween) was incubated for at least 30 min. The primary antibody (1:200) was diluted in the containment solution and incubated with the cells at room temperature for approximately 2 hours or overnight at 4 ℃. The cells were washed three times with PBS for 5 minutes each and incubated with goat anti-rabbit Alexa-coupled secondary antibody (1:500, 594 nm) obtained from Life Technologies at room temperature for 1 to 2 hours. The cells were stained with 4',6-diamidino-2-phenylindole (DAPI) (5 µg/mL) by adding the stain dropwise for 5 min. After washing the cells twice with PBS, they were observed by confocal microscopy.
Statistical analysis
The results of the experiment were expressed as mean ± standard deviation. SPSS 21.0 and Graphpad Prism 9 were used for statistical analysis. Comparisons between two groups were made using t-test, and one-way analysis of variance (ANOVA) was used for groups of three or more. Kaplan-Meier curve and log-rank test were used to compare OS, PFS, and disease-free survival (DFS) among different patient groups. Differences were considered statistically significant when P<0.05.
Results
TLK2 was highly expressed in hepatocellular carcinoma
We compared the expression levels of TLK2 in cancer samples and normal samples from different cancers using TCGA data. We found that the expression level of TLK2 in hepatocellular carcinoma cells was significantly higher than that in normal samples (Figure 1A). We collected relevant patient clinical information from the TCGA database (Table 2). A boxplot was generated showing that the expression level of TLK2 in hepatocellular carcinoma tissues (n=374) was higher than that in normal tissues (n=50) (Figure 1B). We also analyzed 11 hepatocellular carcinoma study cohorts in the HCCDB database and found that the mRNA expression levels of TLK2 were significantly higher in hepatocellular carcinoma tissues than in adjacent normal tissues (Figure 1C). The above results suggested that TLK2 expression was significantly higher in hepatocellular carcinoma tissues than in normal tissues.
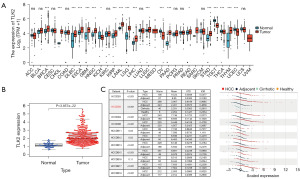
Table 2
| Characteristics | Values (n=418), n (%) |
|---|---|
| Age (years) | |
| <60 | 172 (41.15) |
| ≥60 | 204 (48.80) |
| Unknown | 42 (10.05) |
| Gender | |
| Male | 272 (65.07) |
| Female | 146 (34.93) |
| T | |
| T1 | 204 (48.80) |
| T2 | 107 (25.60) |
| T3 | 90 (21.53) |
| T4 | 14 (3.35) |
| Unknown | 3 (0.72) |
| N | |
| N0 | 290 (69.38) |
| N1 | 8 (1.91) |
| Unknown | 120 (28.71) |
| M | |
| M0 | 303 (72.49) |
| M1 | 8 (1.91) |
| Unknown | 107 (25.60) |
| Stage | |
| Stage I–II | 292 (69.86) |
| Stage III–IV | 102 (24.40) |
| Unknown | 24 (5.74) |
| Histologic grade | |
| G1 | 55 (13.16) |
| G2 | 180 (43.06) |
| G3 | 124 (29.67) |
| G4 | 13 (3.11) |
| Unknown | 46 (11.00) |
| OS event | |
| Alive | 271 (64.83) |
| Dead | 147 (35.17) |
HCC, hepatocellular carcinoma; TCGA, The Cancer Genome Atlas; T, tumor; N, node; M, metastasis; OS, overall survival.
High TLK2 expression was correlated with poor prognosis in hepatocellular carcinoma
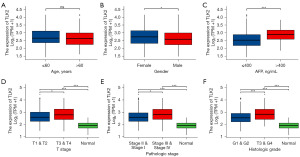
To investigate the clinical significance of TLK2, we used logistic regression analysis to assess the relationship between TLK2 expression and age, sex, AFP, T stage, pathological stage, and histological grade. Our analysis showed that high expression of TLK2 was not associated with age (Figure 2A), but significantly correlated with sex (P=0.03) (Figure 2B), AFP (P<0.001) (Figure 2C), T-stage (P=0.03) (Figure 2D), pathological stage (P=0.01) (Figure 2E) and histological grade (P<0.001) (Figure 2F). These results substantiated that high expression of TLK2 expression was significantly correlated with the prognosis of hepatocellular carcinoma patients.
TLK2 was an independent prognostic factor for hepatocellular carcinoma
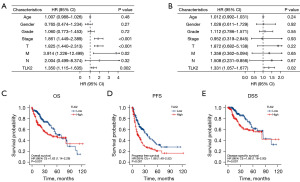
We examined the prognostic relevance of TLK2 in hepatocellular carcinoma to determine whether it consistently correlates with hepatocellular carcinoma patient outcomes. We performed univariate and multivariate Cox regression analyses to analyze the relationship between TLK2 expression, clinical factors (such as age, sex, pT stage, pTNM stage, and histological grade) (Tables 3,4), and OS in hepatocellular carcinoma patients. Univariate cox analysis showed that TLK2 expression (P=0.002), pT stage (P<0.001), and pTNM stage (P<0.001) were significantly correlated with OS in hepatocellular carcinoma (Figure 3A). Multivariate cox regression analysis results suggested that TLK2 expression (P=0.02) was an independent prognostic factor for hepatocellular carcinoma (Figure 3B). The median value of TLK2 expression was used as a cutoff value to divide patients into low-expression and high-expression groups. According to the Kaplan-Meier survival curve illustrated in Figure 3C, patients in the high-expression group had significantly poorer OS, PFS, and DSS than patients in the low-expression group (OS, HR =1.62, 95% CI: 1.14–2.29, P=0.007; PFS, HR =1.88, 95% CI: 1.40–2.52, P<0.001; DSS, HR =1.86, 95% CI: 1.18–2.93, P=0.007) (Figure 3C-3E). These findings indicated that TLK2 expression was an independent predictor of adverse outcomes in patients with hepatocellular carcinoma.
Table 3
| ID | HR | HR.95L | HR.95H | P value |
|---|---|---|---|---|
| Age | 1.007 | 0.988 | 1.026 | 0.48 |
| Gender | 0.765 | 0.474 | 1.234 | 0.27 |
| Grade | 1.060 | 0.773 | 1.453 | 0.72 |
| Stage | 1.861 | 1.449 | 2.389 | <0.001 |
| T | 1.825 | 1.440 | 2.313 | <0.001 |
| M | 3.914 | 1.226 | 12.499 | 0.02 |
| N | 2.044 | 0.499 | 8.374 | 0.32 |
| TLK2 | 1.350 | 1.115 | 1.635 | 0.002 |
HR, hazard ratio; HR.95L, hazard ratio, low 95% CI; HR.95H, hazard ratio, high 95% CI; CI, confidence interval; T, tumor; N, node; M, metastasis.
Table 4
| ID | HR | HR.95L | HR.95H | P value |
|---|---|---|---|---|
| Age | 1.012 | 0.992 | 1.031 | 0.24 |
| Gender | 1.028 | 0.611 | 1.729 | 0.92 |
| Grade | 1.112 | 0.786 | 1.571 | 0.55 |
| Stage | 0.952 | 0.319 | 2.845 | 0.93 |
| T | 1.872 | 0.682 | 5.138 | 0.22 |
| M | 1.358 | 0.362 | 5.094 | 0.65 |
| N | 1.508 | 0.231 | 9.856 | 0.67 |
| TLK2 | 1.331 | 1.057 | 1.677 | 0.02 |
HR, hazard ratio; HR.95L, hazard ratio, low 95% CI; HR.95H, hazard ratio, high 95% CI; CI, confidence interval; T, tumor; N, node; M, metastasis.
TLK2 expression was upregulated in hepatocellular carcinoma cell lines and promoted hepatocellular carcinoma progression
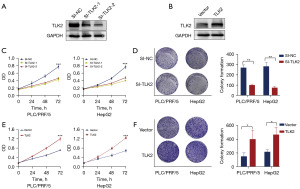
To investigate the role of TLK2 in hepatocellular carcinoma tumor progression, we selected PLC/PRF/5 and HepG2 cells for functional validation. To further investigate the effect of TLK2 on hepatocellular carcinoma proliferation, we designed two types of siRNAs (SI-TLK2-1 and SI-TLK2-2) based on the TLK2 sequence, and SI-TLK2-1 significantly knocked down TLK2 expression (Figure 4A). TLK2 was significantly overexpressed (Figure 4B). MTT assays revealed that knockdown of TLK2 could significantly reduce the proliferation of PLC/PRF/5 and HepG2 cells (Figure 4C). Colony formation assays confirmed that knockdown of TLK2 also inhibited the growth of PLC/PRF/5 and HepG2 cells (Figure 4D). Furthermore, overexpression of TLK2 increased cell proliferation and colony formation ability in PLC/PRF/5 and HepG2 cells (Figure 4E,4F). Taken together, these data indicated that TLK2 was highly expressed in hepatocellular carcinoma and promoted hepatocellular carcinoma proliferation in vitro.
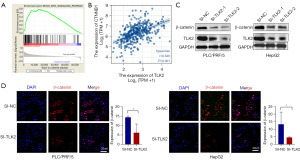
TLK2 promoted hepatocellular carcinoma proliferation through the Wnt/β-catenin pathway
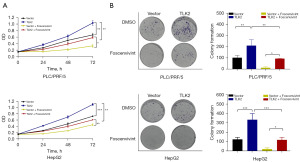
To comprehend the biological importance of TLK2 in hepatocellular carcinoma, gene set enrichment analysis (GSEA) was carried out. The results revealed that TLK2 was predominantly linked to oocyte-meiosis, cell cycle, and ubiquitin-mediated proteolysis pathways in hepatocellular carcinoma. In contrast, activities related to fatty acid metabolism, retinol metabolism, and primary bile acid biosynthesis were significantly suppressed (Figure S1). TLK2 was significantly enriched in pathways linked to cell proliferation, revealing a clear association between TLK2 and the Wnt signaling pathway. Our results showed that TLK2 was involved in the cell proliferation pathway, and among them, the Wnt pathway, which is associated with cell proliferation, was significantly enriched (Figure 5A). Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway enrichment analysis revealed that TLK2 was significantly enriched in the Wnt pathway. We then assessed the correlation between CTNNB1 and TLK2 and found that TLK2 was significantly correlated with CTNNB1, a key factor in the Wnt pathway (Figure 5B). Moreover, we verified the relationship between TLK2 and β-catenin in hepatocellular carcinoma cells by Western blot and immunofluorescence assays. As shown in Figure 5C, β-catenin expression was significantly decreased in SI-TLK2 cells compared to control cells (Figure 5C), consistent with the results of the immunofluorescence assay (Figure 5D). These findings suggested that TLK2 can promote the proliferation of hepatocellular carcinoma cells by increasing the expression of β-catenin. We further validated that TLK2 promoted the proliferation of hepatocellular carcinoma cells through β-catenin by MTT and colony formation assays. As shown in the results, TLK2 was able to promote the proliferation of hepatocellular carcinoma cells, and the β-catenin inhibitor Foscenvivint significantly inhibited the proliferation of hepatocellular carcinoma cells (Figure 6A,6B). However, inhibition of β-catenin after TLK2 overexpression rescued the inhibitory effect of β-catenin on hepatocellular carcinoma cells (Figure 6A,6B).
Discussion
Hepatocellular carcinoma is a highly aggressive type of hepatocellular carcinoma (20). Several molecular pathways have been implicated in hepatocellular carcinoma development and progression, but the role of TLK2 in hepatocellular carcinoma is not fully understood. Current evidence suggests that the Wnt/β-catenin pathway is dysregulated in many types of cancer, including hepatocellular carcinoma, and has been shown to play a critical role in tumor initiation and progression (21). It is widely thought that β-catenin is a crucial driver of tumorigenesis in hepatocellular carcinoma, with aberrant activation of the β-catenin signaling pathway being a common feature of this type of cancer (22). Study revealed that GINS1 promotes ZEB1-mediated tumor metastasis in hepatocellular carcinoma through β-catenin signaling (23). MiR-557 blocked HCC progression through the Wnt/β-catenin pathway by targeting RAB10 (24). These studies have demonstrated the importance of β-catenin in the development of hepatocellular carcinoma. It has been shown that frequent upregulation of TLK2 is associated with poor patient prognosis in several types of cancer and that depletion of TLK2 activity leads to increased replication stress and DNA damage in cancer cells (25). Overwhelming evidence substantiates that the absence or abnormal expression of TLK2 in DNA repair can lead to the accumulation of DNA damage and reduced DNA repair capacity, which increases the risk of cancer development (26). Meanwhile, aberrant expression of TLK2 in cancer cells leads to cell cycle disruption and chromosomal instability, increasing the risk of cancer development and immune evasion (27). Therefore, TLK2 may play a crucial role in cancer development and progression via β-catenin.
In this study, we first evaluated the expression levels of TLK2 in cancer tissues and adjacent cells in each tissue and found that TLK2 was highly expressed in hepatocellular carcinoma. We further analyzed TLK2 expression levels in hepatocellular carcinoma and adjacent tissues and found that TLK2 was abnormally highly expressed in hepatocellular carcinoma. Western blotting also confirmed that TLK2 expression was higher in hepatocellular carcinoma than in normal hepatocytes. In recent study, it has been shown that inhibition of TLK2 expression suppresses the progression of gastric cancer by inducing changes in the mTOR/ASNS axis (28). Our results implied that TLK2 may represent a promising target for diagnosing and treating hepatocellular carcinoma. To verify the clinical relevance of TLK2 high expression, we collected 374 tumor tissues and 50 normal tissues from TCGA. Our results showed that TLK2 was significantly associated with sex, AFP, and clinical tumor stage. The correlation between TLK2 and the progression of hepatocellular carcinoma was further strengthened.
On the basis of the clinical relevance of TLK2, we conducted multivariate and univariate analyses and found that TLK2 could serve as an independent prognostic factor to predict the prognosis of hepatocellular carcinoma. The survival curve also indicated that patients with high TLK2 expression had a significantly lower five-year survival rate than those with low TLK2 expression, further highlighting the potential of TLK2 as an independent prognostic factor for predicting hepatocellular carcinoma prognosis. Recent research has shown that TLK2 plays a crucial role in promoting the development of breast cancer and glioblastoma. Kim et al. reported that TLK2 amplifies and damages the G2-M checkpoint, leading to a defective G2-M checkpoint, delayed DNA repair process, and accumulated damage, promoting breast cancer development (16). Lin et al. found that TLK2 overexpression via the SRC signaling pathway is a critical driver of glioblastoma (18). To investigate the role of TLK2 in hepatocellular carcinoma progression, we carried out TLK2 silencing and overexpression experiments and demonstrated that TLK2 knockdown significantly suppressed hepatocellular carcinoma cell proliferation in vitro.
Numerous studies have shown that certain genetic mutations, such as TP53 (29), PTEN (30) and CTNNB1 (31) can disrupt cell growth and lead to uncontrolled growth tumor formation in hepatocellular carcinoma. Additionally, epigenetic alterations, including dysregulation of DNA methylation and histone modifications, may be involved in tumor suppression, cell cycle regulation, and expression of DNA repair genes that contribute to the development of hepatocellular carcinoma (32). To elucidate the molecular mechanism of TLK2 knockdown-mediated hepatoma cell suppression, we first performed pathway enrichment analysis using TCGA data. We observed strong correlation between TLK2 and cell cycle regulation, endocytosis, and deceleration. Notably, our analysis revealed a significant correlation between TLK2 and the Wnt pathway, a crucial pathway involved in cell proliferation. It has been established that in healthy liver tissue, the Wnt/β-catenin pathway is typically inactive but can be reactivated during cell regeneration and in the context of various diseases and cancerous conditions, including hepatocellular carcinoma (8). Studies in recent years have identified the roles of target genes in promoting the development of hepatocellular carcinoma through the Wnt signaling pathway (33,34). Our study further revealed a strong correlation between TLK2 and β-catenin. MTT and colony formation assays further validated that TLK2 promotes the proliferation of hepatocellular carcinoma through β-catenin.
This study’s findings have several implications for hepatocellular carcinoma research and therapy. First, TLK2 and the Wnt/β-catenin pathway represent promising targets for the development of new hepatocellular carcinoma treatments. Inhibition of TLK2 or the Wnt/β-catenin pathway could slow down or halt hepatocellular carcinoma growth, providing a new avenue for drug development. Second, the study highlights the importance of understanding the molecular mechanisms underlying hepatocellular carcinoma growth, which could help identify new biomarkers for early diagnosis and prognosis. However, there are some limitations to the study that should be acknowledged. For example, the experiments were performed using only two hepatocellular carcinoma cell lines, and it is not clear if TLK2 plays the same role in other hepatocellular carcinoma subtypes. Additionally, the study did not investigate the potential role of TLK2 in hepatocellular carcinoma metastasis, which is a major contributor to hepatocellular carcinoma mortality.
Conclusions
In summary, the study provides compelling evidence that TLK2 stimulates hepatocellular carcinoma growth through the activation of the Wnt/β-catenin pathway. These findings have important implications for hepatocellular carcinoma research and therapy and underscore the need for further investigations into the molecular mechanisms underlying hepatocellular carcinoma development and progression.
Acknowledgments
TCGA is a public database. Users can download relevant data for free to conduct research and publish related articles. We thank the TCGA database for providing the platform and to contributors for uploading their meaningful datasets.
Funding: This study was supported by grants from
Footnote
Reporting Checklist: The authors have completed the MDAR reporting checklist. Available at https://tcr.amegroups.com/article/view/10.21037/tcr-23-2264/rc
Data Sharing Statement: Available at https://tcr.amegroups.com/article/view/10.21037/tcr-23-2264/dss
Peer Review File: Available at https://tcr.amegroups.com/article/view/10.21037/tcr-23-2264/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tcr.amegroups.com/article/view/10.21037/tcr-23-2264/coif). The authors have no conflicts of interest to declare.
Ethical Statement:
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Chidambaranathan-Reghupaty S, Fisher PB, Sarkar D. Hepatocellular carcinoma (HCC): Epidemiology, etiology and molecular classification. Adv Cancer Res 2021;149:1-61. [Crossref] [PubMed]
- Kulik L, El-Serag HB. Epidemiology and Management of Hepatocellular Carcinoma. Gastroenterology 2019;156:477-491.e1. [Crossref] [PubMed]
- Kang Y, Cai Y, Yang Y. The Gut Microbiome and Hepatocellular Carcinoma: Implications for Early Diagnostic Biomarkers and Novel Therapies. Liver Cancer 2021;11:113-25. [Crossref] [PubMed]
- Kim HS, Yu X, Kramer J, et al. Comparative performance of risk prediction models for hepatitis B-related hepatocellular carcinoma in the United States. J Hepatol 2022;76:294-301. [Crossref] [PubMed]
- Huang DQ, Mathurin P, Cortez-Pinto H, et al. Global epidemiology of alcohol-associated cirrhosis and HCC: trends, projections and risk factors. Nat Rev Gastroenterol Hepatol 2023;20:37-49. [Crossref] [PubMed]
- Ng CKY, Dazert E, Boldanova T, et al. Integrative proteogenomic characterization of hepatocellular carcinoma across etiologies and stages. Nat Commun 2022;13:2436. [Crossref] [PubMed]
- Xu C, Xu Z, Zhang Y, et al. β-Catenin signaling in hepatocellular carcinoma. J Clin Invest 2022;132:e154515. [Crossref] [PubMed]
- Perugorria MJ, Olaizola P, Labiano I, et al. Wnt-β-catenin signalling in liver development, health and disease. Nat Rev Gastroenterol Hepatol 2019;16:121-36. [Crossref] [PubMed]
- Wang J, Yu H, Dong W, et al. N6-Methyladenosine-Mediated Up-Regulation of FZD10 Regulates Liver Cancer Stem Cells' Properties and Lenvatinib Resistance Through WNT/β-Catenin and Hippo Signaling Pathways. Gastroenterology 2023;164:990-1005. [Crossref] [PubMed]
- Leung HW, Leung CON, Lau EY, et al. EPHB2 Activates β-Catenin to Enhance Cancer Stem Cell Properties and Drive Sorafenib Resistance in Hepatocellular Carcinoma. Cancer Res 2021;81:3229-40. [Crossref] [PubMed]
- Ruiz de Galarreta M, Bresnahan E, Molina-Sánchez P, et al. β-Catenin Activation Promotes Immune Escape and Resistance to Anti-PD-1 Therapy in Hepatocellular Carcinoma. Cancer Discov 2019;9:1124-41. [Crossref] [PubMed]
- Silljé HH, Nigg EA. Identification of human Asf1 chromatin assembly factors as substrates of Tousled-like kinases. Curr Biol 2001;11:1068-73. [Crossref] [PubMed]
- Segura-Bayona S, Knobel PA, González-Burón H, et al. Differential requirements for Tousled-like kinases 1 and 2 in mammalian development. Cell Death Differ 2017;24:1872-85. [Crossref] [PubMed]
- Ransom M, Dennehey BK, Tyler JK. Chaperoning histones during DNA replication and repair. Cell 2010;140:183-95. [Crossref] [PubMed]
- Ehsan H, Reichheld JP, Durfee T, et al. TOUSLED kinase activity oscillates during the cell cycle and interacts with chromatin regulators. Plant Physiol 2004;134:1488-99. [Crossref] [PubMed]
- Kim JA, Anurag M, Veeraraghavan J, et al. Amplification of TLK2 Induces Genomic Instability via Impairing the G2-M Checkpoint. Mol Cancer Res 2016;14:920-7. [Crossref] [PubMed]
- Kim JA, Tan Y, Wang X, et al. Comprehensive functional analysis of the tousled-like kinase 2 frequently amplified in aggressive luminal breast cancers. Nat Commun 2016;7:12991. [Crossref] [PubMed]
- Lin M, Yao Z, Zhao N, et al. TLK2 enhances aggressive phenotypes of glioblastoma cells through the activation of SRC signaling pathway. Cancer Biol Ther 2019;20:101-8. [Crossref] [PubMed]
- Lin HY, M Hosseini M, McClatchy J, et al. The TLK-ASF1 histone chaperone pathway plays a critical role in IL-1b-mediated AML progression. Blood 2024;blood.2023022079.
- Llovet JM, Kelley RK, Villanueva A, et al. Hepatocellular carcinoma. Nat Rev Dis Primers 2021;7:6. [Crossref] [PubMed]
- Yu F, Yu C, Li F, et al. Wnt/β-catenin signaling in cancers and targeted therapies. Signal Transduct Target Ther 2021;6:307. [Crossref] [PubMed]
- He S, Tang S. WNT/β-catenin signaling in the development of liver cancers. Biomed Pharmacother 2020;132:110851. [Crossref] [PubMed]
- Liang J, Yao N, Deng B, et al. GINS1 promotes ZEB1-mediated epithelial-mesenchymal transition and tumor metastasis via β-catenin signaling in hepatocellular carcinoma. J Cell Physiol 2024;239:e31237. [Crossref] [PubMed]
- Cheng X, Wu C, Xu H, et al. miR-557 inhibits hepatocellular carcinoma progression through Wnt/β-catenin signaling pathway by targeting RAB10. Aging (Albany NY) 2024;16:3716-33. [Crossref] [PubMed]
- Lee SB, Segura-Bayona S, Villamor-Payà M, et al. Tousled-like kinases stabilize replication forks and show synthetic lethality with checkpoint and PARP inhibitors. Sci Adv 2018;4:eaat4985. [Crossref] [PubMed]
- Bruinsma W, van den Berg J, Aprelia M, et al. Tousled-like kinase 2 regulates recovery from a DNA damage-induced G2 arrest. EMBO Rep 2016;17:659-70. [Crossref] [PubMed]
- Segura-Bayona S, Villamor-Payà M, Attolini CS, et al. Tousled-Like Kinases Suppress Innate Immune Signaling Triggered by Alternative Lengthening of Telomeres. Cell Rep 2020;32:107983. [Crossref] [PubMed]
- Wang M, Li J, Yang X, et al. Targeting TLK2 inhibits the progression of gastric cancer by reprogramming amino acid metabolism through the mTOR/ASNS axis. Cancer Gene Ther 2023;30:1485-97. [Crossref] [PubMed]
- Qian Z, Liang J, Huang R, et al. HBV integrations reshaping genomic structures promote hepatocellular carcinoma. Gut 2024;73:1169-82. [Crossref] [PubMed]
- Huang F, Guo J, Zhao N, et al. PTEN deficiency potentiates HBV-associated liver cancer development through augmented GP73/GOLM1. J Transl Med 2024;22:254. [Crossref] [PubMed]
- Gao Q, Zhu H, Dong L, et al. Integrated Proteogenomic Characterization of HBV-Related Hepatocellular Carcinoma. Cell 2019;179:561-577.e22. [Crossref] [PubMed]
- Nagaraju GP, Dariya B, Kasa P, et al. Epigenetics in hepatocellular carcinoma. Semin Cancer Biol 2022;86:622-32. [Crossref] [PubMed]
- Lou YX, Gu J, Zhu L, et al. TC2N Promotes Cell Proliferation and Metastasis in Hepatocellular Carcinoma by Targeting the Wnt/β-Catenin Signaling Pathway. Lab Invest 2023;103:100260. [Crossref] [PubMed]
- Hao X, Li J, Liu B, et al. Cavin1 activates the Wnt/β-catenin pathway to influence the proliferation and migration of hepatocellular carcinoma. Ann Hepatol 2024;29:101160. [Crossref] [PubMed]


