Establishment of a nomogram for potential prediction of lung metastasis in patients with primary limb bone tumors: a study based on the SEER database
Highlight box
Key findings
• The study developed a nomogram for predicting the risk of lung metastasis in patients with primary limb bone tumors, using data from the SEER database. Seven key predicting variables were identified: age, histological type, tumor size, surgery, radiation, chemotherapy, and TNM classification. The nomogram showed good accuracy with an area under the curve of 0.806 in the training cohort and 0.767 in the validation cohort.
What is known and what is new?
• It is known that predicting lung metastasis in patients with primary bone tumors of the limb is challenging due to the complexity of factors involved. Existing models have not specifically addressed this patient population.
• This manuscript introduces a novel predictive tool that translates demographic, clinical, and pathological data into a usable nomogram, improving risk prediction for lung metastasis.
What is the implication, and what should change now?
• The nomogram can enhance clinical decision-making by providing a more accurate risk assessment for lung metastasis in patients with primary limb bone tumors. This tool aids in early detection and timely intervention, potentially improving patient outcomes. Clinicians should integrate this nomogram into their practice to better stratify patients by risk and personalize treatment plans accordingly.
Introduction
In the current 21st century, cancer has been on the rise as a major cause of death, both within China and worldwide (1,2). Cancer currently remains one of the major constraints for expanding life expectancy. In the United States, malignancies of bones and joints have become the third leading causes of death related to cancers in individuals under the age of 20 years (3). According to the newest figures, the one-year overall survival rates for a malignant bone tumor are 74% for the age group of 0–14 years and 69% in those aged 15–19 years (4).
Primary limb bone tumors are widespread, due to the prevalence of different cancer types ranging from bone-forming osteosarcoma (5), chondrosarcoma (6), to Ewing’s sarcoma (7). Typically, the tumors that adversely affect the long bones of adolescents are quite obvious and would not be initially discovered at the spinal column or pelvic bones sites (8).
The Surveillance, Epidemiology, and End Results (SEER) database, developed by the National Cancer Institute (NCI) of the United States, is a very large cancer registry which records and stores comprehensive information about cancer patients. This repository is designed to capture detailed data of the present epidemiological traits of various tumors, including primary limb bone tumors, and permits comprehensive exploration and analysis of these data. Covering approximately 28% of the United States (US) population (9), the database contains valuable data, including fundamental demographics such as age and gender, as well as specific disease attributes including tumor size and location. Data on survival and death rates are also included and are of utmost importance.
Nomograms solely dominate the territory of clinical prognosticating tools and are the unique pillar of such pragmatic types of tools; they amalgamate statistical data and clinical acumen, which helps to determine the true course of cancer progression (10,11). In general, the establishment of a nomogram is usually based on a development cohort and is validated in a validation cohort to further ensure the model’s validity. A handful of nomograms have been established to predict the general prognosis of primary spinal tumors (8,12), and individual nomograms have been created for specific pathological types of tumors, namely, osteosarcoma, chondrosarcoma, and Ewing’s sarcoma (13-16), to address concerns metastatic and survival issues. However, there is currently a noticeable lack of a model that can predict the spread to the lung from primary limb tumors. Herein, we raise several queries: Does the specter of lung metastasis have an important impact on the survival rates of patients with primary limb bone tumors? Which factors may affect the course of the diseases? Can we distill these variables into a nomogram which can predict the risk of lung metastasis accurately? The answers to these questions will help doctors to make more evidence-based decisions, to the benefit of a larger number of patients. We present this article in accordance with the TRIPOD reporting checklist (available at https://tcr.amegroups.com/article/view/10.21037/tcr-24-570/rc).
Methods
Data acquisition
The SEER database, maintained by the NCI, is the source of our study. The research cases for the SEER database, which come from 17 registries, ranging between 2000 and 2020, have been updated to correctly reflect the survival and incidence of cancer patients from different population groups. Data extraction was performed using SEER*Stat software, version 8.4.3 (https://seer.cancer.gov/seerstat/), to ensure that the process and result of our data analysis remained stable and credible. This study was conducted in accordance with the Declaration of Helsinki (as revised in 2013).
Cohort definition
The inclusion criteria were as follows: the group consisted of patients with primary tumors of the limb bones diagnosed between 2010 and 2015 using unified diagnostic criteria; a confirmed diagnosis of lung metastasis; and the primary site was restricted to the limbs.
The exclusion criteria were as follows: cases which did not mention the status of surgical intervention, tumor, node, metastasis (TNM) stage and laterality; and failure to provide essential demographic features such as ethnicity.
Clinicopathological variables
Two researchers, X.H. and J.W.G., analyzed the data independently to ensure accuracy. We screened sets of data for further correlation, retrieving demographic details (gender, age, and race) as well as pathological statuses such as lung metastasis, the malignant poly-invasive tumor type, laterality, and tumor size. A coded message was used to indicate the presence of the primary malignant indicator, surgery, radiation, or chemotherapy statuses, and tumor grading according to the TNM stage. These data sources helped us understand the general trends in survival rate metrics, in addition to providing valuable guidance regarding highly correlated metrics. The data used for the analysis are presented in the supplementary material (available at https://cdn.amegroups.cn/static/public/tcr-24-570-1.xlsx) and Table S1.
Statistical analysis
Using the RAND function in Excel to ensure that the basic information between the two groups was the same, we assigned a random number to each case in our dataset. The RAND function generates a random decimal number between 0 and 1. After generating random numbers for all cases, we sorted the entire dataset in ascending order based on the random numbers assigned. Following the sorting, the cohort (containing 1,822 patients) was dichotomized into two groups based on a 3:1 ratio: a training cohort (n=1,364) consisting of the first 75% of cases (those with the smallest random numbers), and the remaining 25% (n=458) were allocated to the validation cohort (which was used to validate the experimental modeling). Chi-squared test was used for categorical outcomes and the Kruskal-Wallis rank sum test was used for continuous outcomes indicating significance level.
Subsequent analyses involved a two-pronged approach: initially, we carried out univariate analysis as well as least absolute shrinkage and selection operator (LASSO) regression on the training cohort to identify significant factors; these then acted as a base for multivariate logistic regression to confirm the prediction factors for inclusion in the nomogram.
This nomogram was subjected to an extensive verification in both cohorts, evaluating not only the areas under receiver operating characteristic (ROC) curve (AUC) (17), but also the AUC metrics, to ascertain its predictive accuracy. Besides the external calibration curves, the model’s fit was assessed, and a decision curve analysis (DCA) (18) was performed to evaluate its net benefit and clinical utility. Kaplan-Meier survival curves provided a visual representation of the survival time differences between the two groups. All statistical assessments were conducted using the software SPSS 26.0 (IBM Corp., Armonk, NY, USA) and R software (version 4.3.2; R Foundation for Statistical Computing, Vienna, Austria). A P value of less than 0.05 was considered statistically significant.
Results
Demographic and baseline characteristics
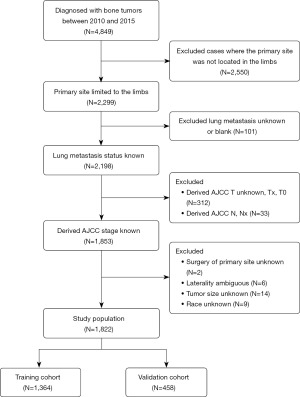
According to the inclusion and exclusion criteria mentioned in the Methods section, we distilled a focused group of 1,822 cases from an initial cohort of 4,849 patients (see Figure 1 for details of the selection process). Eventually, this cohort was divided into two smaller cohorts, a training cohort of 1,364 cases and a validation cohort of 458 individuals. The statistical analysis did not show a significant difference (P>0.05) between the two groups of patients in several attributes (Table 1), for instance, the age, sex, race, and histological type of the given patients. Thus, by using these patients as the training and validation cohorts, we demonstrated their rationality.
Table 1
| Characteristics | Total | Training cohort | Validation cohort | P value* |
|---|---|---|---|---|
| N | 1,822 (100.0) | 1,364 (74.9) | 458 (25.1) | |
| Age (years) | 0.06 | |||
| <25 | 894 (49.07) | 676 (49.56) | 218 (47.60) | |
| 25–49 | 416 (22.83) | 310 (22.73) | 106 (23.14) | |
| 50–74 | 403 (22.12) | 287 (21.04) | 116 (25.33) | |
| >74 | 109 (5.98) | 91 (6.67) | 18 (3.93) | |
| Sex | 0.75 | |||
| Male | 1,046 (57.41) | 786 (57.62) | 260 (56.77) | |
| Female | 776 (42.59) | 578 (42.38) | 198 (43.23) | |
| Race | 0.90 | |||
| White | 1,465 (80.41) | 1,094 (80.21) | 371 (81.00) | |
| Black | 201 (11.03) | 151 (11.07) | 50 (10.92) | |
| Others | 156 (8.56) | 119 (8.72) | 37 (8.08) | |
| Lung metastasis | 0.76 | |||
| No | 1,608 (88.25) | 1,202 (88.12) | 406 (88.65) | |
| Yes | 214 (11.75) | 162 (11.88) | 52 (11.35) | |
| Histological type | 0.65 | |||
| Osteosarcoma | 890 (48.85) | 673 (49.34) | 217 (47.38) | |
| Chondrosarcoma | 508 (27.88) | 381 (27.93) | 127 (27.73) | |
| Ewing sarcoma | 211 (11.58) | 158 (11.58) | 53 (11.57) | |
| Others | 213 (11.69) | 152 (11.14) | 61 (13.32) | |
| Laterality | 0.57 | |||
| Right | 874 (47.97) | 649 (47.58) | 225 (49.13) | |
| Left | 948 (52.03) | 715 (52.42) | 233 (50.87) | |
| First malignant primary indicator | 0.56 | |||
| Yes | 1,663 (91.27) | 1,248 (91.50) | 415 (90.61) | |
| No | 159 (8.73) | 116 (8.50) | 43 (9.39) | |
| Surgery | 0.28 | |||
| Yes | 1,628 (89.35) | 1,225 (89.81) | 403 (87.99) | |
| No | 194 (10.65) | 139 (10.19) | 55 (12.01) | |
| Radiation | 0.34 | |||
| No | 1,642 (90.12) | 1,224 (89.74) | 418 (91.27) | |
| Yes | 180 (9.88) | 140 (10.26) | 40 (8.73) | |
| Chemotherapy | 0.89 | |||
| Yes | 1,117 (61.31) | 835 (61.22) | 282 (61.57) | |
| No | 705 (38.69) | 529 (38.78) | 176 (38.43) | |
| Total number of tumors | 0.83 | |||
| 1 | 1,566 (85.95) | 1,171 (85.85) | 395 (86.24) | |
| >1 | 256 (14.05) | 193 (14.15) | 63 (13.76) | |
| T | 0.14 | |||
| T1 | 846 (46.43) | 643 (47.14) | 203 (44.32) | |
| T2 | 936 (51.37) | 696 (51.03) | 240 (52.40) | |
| T3 | 40 (2.20) | 25 (1.83) | 15 (3.28) | |
| N | 0.51 | |||
| N0 | 1,774 (97.37) | 1,330 (97.51) | 444 (96.94) | |
| N1 | 48 (2.63) | 34 (2.49) | 14 (3.06) | |
| M | 0.86 | |||
| M0 | 1,563 (85.78) | 1,169 (85.70) | 394 (86.03) | |
| M1 | 259 (14.22) | 195 (14.30) | 64 (13.97) | |
| Tumor size (mm) | 0.84 | |||
| ≤68 | 616 (33.81) | 462 (33.87) | 154 (33.62) | |
| >68, ≤110 | 617 (33.86) | 457 (33.50) | 160 (34.93) | |
| >110 | 589 (32.33) | 445 (32.62) | 144 (31.44) | |
Data are presented as n (%). *, if it is a continuous variable, it is obtained by the Kruskal-Wallis rank sum test; if it is a count variable with expected count less than 10, it is obtained by the Fisher’s exact test.
Kaplan-Meier survival analysis
Figure 2A shows that at 12 months, patients with lung metastases had a significantly lower survival probability compared to those without (P<0.001). At 30 months, this trend continued, as shown in Figure 2B, with survival probabilities remaining lower for those with lung metastases (P=0.001). These Kaplan-Meier curves highlight the negative impact of lung metastasis on survival outcomes.

Prognostic factor identification
In the training cohort, univariate analysis and LASSO regression analysis were conducted to identify factors significantly affecting lung metastasis. Both analyses utilized 14 factors, ultimately identifying eight significant factors (P<0.05): age, histological type, tumor size, surgery, radiation, chemotherapy, T stage, and N stage (Table 2, Figure 3). These eight factors were then subjected to multivariate logistic regression analysis (detailed in Table 3). The results indicated that seven factors—age, histological type, surgery, radiation, chemotherapy, T stage, and N stage—were independent predictors of lung metastasis.
Table 2
| Variable | Univariate analysis | |
|---|---|---|
| OR (95% CI) | P value | |
| Age (years) | ||
| <25 | 1 | |
| 25–49 | 0.34 (0.20, 0.57) | <0.001 |
| 50–74 | 0.57 (0.36, 0.89) | 0.01 |
| >74 | 0.92 (0.49, 1.71) | 0.78 |
| Sex | ||
| Male | 1 | |
| Female | 0.78 (0.55, 1.09) | 0.14 |
| Race | ||
| White | 1 | |
| Black | 0.86 (0.49, 1.48) | 0.58 |
| Others | 0.89 (0.48, 1.62) | 0.69 |
| Histological type | ||
| Osteosarcoma | 1 | |
| Chondrosarcoma | 0.29 (0.17, 0.48) | <0.001 |
| Ewing sarcoma | 0.99 (0.61, 1.61) | 0.97 |
| Others | 0.65 (0.37, 1.14) | 0.13 |
| Laterality | ||
| Right | 1 | |
| Left | 0.80 (0.58, 1.11) | 0.19 |
| Tumor size (mm) | ||
| ≤68 | 1 | |
| >68, ≤110 | 1.97 (1.24, 3.14) | 0.004 |
| >110 | 3.01 (1.93, 4.70) | <0.001 |
| First malignant primary indicator | ||
| Yes | 1 | |
| No | 1.11 (0.63, 1.96) | 0.71 |
| Surgery | ||
| Yes | 1 | |
| No | 5.36 (3.60, 7.98) | <0.001 |
| Radiation | ||
| No | 1 | |
| Yes | 3.94 (2.62, 5.93) | <0.001 |
| Chemotherapy | ||
| Yes | 1 | |
| No | 0.15 (0.09, 0.25) | <0.001 |
| Total number of tumors | ||
| 1 | 1 | |
| >1 | 0.63 (0.37, 1.09) | 0.10 |
| T | ||
| T1 | 1 | |
| T2 | 2.56 (1.77, 3.72) | <0.001 |
| T3 | 10.96 (4.69, 25.60) | <0.001 |
| N | ||
| N0 | 1 | |
| N1 | 11.95 (5.91, 24.18) | <0.001 |
| M | ||
| M0 | 1 | |
| M1 | inf. (0.00, inf) | 0.98 |
CI, confidence interval; OR, odds ratio.

Table 3
| Variable | Multivariate analysis | |
|---|---|---|
| OR (95% CI) | P value | |
| Age (years) | ||
| <25 | 1 | |
| 25–49 | 0.58 (0.35, 0.96) | 0.03 |
| 50–74 | 1.17 (0.69, 1.96) | 0.56 |
| >74 | 2.25 (0.96, 5.26) | 0.06 |
| Histological type | ||
| Osteosarcoma | 1 | |
| Chondrosarcoma | 0.90 (0.47, 1.72) | 0.75 |
| Ewing sarcoma | 0.41 (0.24, 0.70) | 0.001 |
| Others | 0.81 (0.44, 1.50) | 0.50 |
| Tumor size (mm) | ||
| ≤68 | 1 | |
| >68, ≤110 | 1.02 (0.54, 1.90) | 0.96 |
| >110 | 1.36 (0.68, 2.74) | 0.39 |
| Surgery | ||
| Yes | 1 | |
| No | 4.68 (3.02, 7.26) | <0.001 |
| Radiation | ||
| No | 1 | |
| Yes | 2.06 (1.25, 3.40) | 0.005 |
| Chemotherapy | ||
| Yes | 1 | |
| No | 0.15 (0.08, 0.28) | <0.001 |
| T | ||
| T1 | 1 | |
| T2 | 1.66 (0.93, 2.95) | 0.09 |
| T3 | 4.97 (2.01, 12.25) | 0.001 |
| N | ||
| N0 | 1 | |
| N1 | 4.98 (2.49, 9.94) | <0.001 |
CI, confidence interval; OR, odds ratio.
Nomogram development and external validation
Using the seven factors selected by multivariate logistic regression, we constructed a nomogram to predict the risk of lung metastasis (Figure 4). Every predictor was assigned an individualized score, which was then amalgamated into a composite score to forecast the likelihood of lung metastasis. We plotted the ROC curve, which unveiled an AUC of 0.806 [95% confidence interval (CI): 0.7754–0.8375] for the training cohort and 0.767 (95% CI: 0.7067–0.8279) for the validation (Figure 5). There was no significant difference in AUC between the training and validation groups.


External calibration curves further demonstrated the nomogram’s repeatability in predicting probabilities of lung metastasis, with a logistic calibration curve approaching the ideal (Figure 6). This consistency between the training and validation cohorts accentuates the model’s stability.

Clinical utility
Based on the training cohorts’ data, the DCA assessed the nomogram’s practical applicability and underscored its substantial clinical benefit. As shown in Figure 7, the model decision curve for the training dataset (model for TD) demonstrated a higher net benefit across various threshold probabilities compared to the “Treat none” and “Treat all” decisions. This indicated that the model performed well on the training dataset. Similarly, the model decision curve for the validation dataset (model for VD) also exhibited a higher net benefit, confirming the model’s reliability and generalizability on an independent dataset (Figure 7).

Discussion
We endeavored to create a nomogram to show the risk of lung metastasis derived from data of the SEER database which have not been previously reported. The calculations and the analysis will be conducted among patients with primary bone limb tumor.
The Kaplan-Meier curves (Figure 2) illustrate that lung metastasis significantly reduces survival probabilities at both 12 and 30 months (P<0.001 and P=0.001, respectively). This underscores the severe impact of lung metastasis on patient survival, emphasizing the need for early prediction and intervention. The nomogram we developed can aid in this by accurately predicting lung metastasis risk, thereby improving clinical decision-making and patient outcomes. The prediction model contains seven different factors including age, histopathological type, surgery, radiation therapy, chemotherapy, T stage, and N stage. Interestingly, we observed a paradoxical fact: patients who accept chemotherapy or radiation therapy may have an increased risk of lung metastasis. In Zhang et al.’s study (19), although this phenomenon was not discussed separately, the nomogram showed similar results, indicating that radiotherapy increased the probability of pancreatic cancer bone metastasis. This phenomenon may suggest a more aggressive disease phenotype or a selection bias towards non-adjuvant therapies in early-stage tumors, warranting further investigation.
The AUC values reflect a reliable prognostic tool for clinical use, supporting the predictive accuracy of our model. The external calibration curves demonstrate the repeatability of our model, indicating that this nomogram can improve patient outcomes and overall health.
The clinical effectiveness of our nomogram, as shown by the DCA, demonstrates that using it provides more benefit than a treat-all or treat-none approach. Due to the radioactive or invasive nature of computed tomography (CT) and needle biopsy, these methods are not usually used for routine screening of lung metastasis. Lung metastases are typically found by chest CT scanning; however, in previous studies, up to 36% of lung metastases were not detected by CT (20-22). Therefore, our predictive model shows good clinical benefit. This predictive tool will aid in the early detection of metastatic disease, facilitating timely and targeted interventions.
Our study had some limitations. It would undoubtedly be more reliable to verify the results using data from another independent medical institution and the reliance on a single database may have introduced biases as a result of the dataset’s demographic and geographic constraints. Moreover, future research has the potential for enhancement due to the deficiency of genomic characteristics in the SEER database.
Conclusions
This study developed a robust nomogram using SEER database data to predict lung metastasis in primary limb bone tumor patients. Incorporating seven key variables, the model demonstrated strong predictive accuracy with AUC values of 0.806 and 0.767 in the training and validation cohorts, respectively. This tool enhances clinical decision-making, enabling timely and personalized interventions. Although further validation in diverse settings is needed due to the SEER database’s limitations, this nomogram marks a significant advancement in personalized oncology, improving risk stratification and patient outcomes.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the TRIPOD reporting checklist. Available at https://tcr.amegroups.com/article/view/10.21037/tcr-24-570/rc
Peer Review File: Available at https://tcr.amegroups.com/article/view/10.21037/tcr-24-570/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tcr.amegroups.com/article/view/10.21037/tcr-24-570/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018;68:394-424. [Crossref] [PubMed]
- Cao B, Bray F, Ilbawi A, et al. Effect on longevity of one-third reduction in premature mortality from non-communicable diseases by 2030: a global analysis of the Sustainable Development Goal health target. Lancet Glob Health 2018;6:e1288-96. [Crossref] [PubMed]
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin 2019;69:7-34. [Crossref] [PubMed]
- Siegel RL, Miller KD, Wagle NS, et al. Cancer statistics, 2023. CA Cancer J Clin 2023;73:17-48. [Crossref] [PubMed]
- Meltzer PS, Helman LJ. New Horizons in the Treatment of Osteosarcoma. N Engl J Med 2021;385:2066-76. [Crossref] [PubMed]
- Weinschenk RC, Wang WL, Lewis VO. Chondrosarcoma. J Am Acad Orthop Surg 2021;29:553-62. [PubMed]
- Balamuth NJ, Womer RB. Ewing's sarcoma. Lancet Oncol 2010;11:184-92. [Crossref] [PubMed]
- Fan Y, Cai M, Xia L. Distinction and Potential Prediction of Lung Metastasis in Patients with Malignant Primary Osseous Spinal Neoplasms. Spine (Phila Pa 1976) 2020;45:921-9. [Crossref] [PubMed]
- Doll KM, Rademaker A, Sosa JA. Practical Guide to Surgical Data Sets: Surveillance, Epidemiology, and End Results (SEER) Database. JAMA Surg 2018;153:588-9. [Crossref] [PubMed]
- Balachandran VP, Gonen M, Smith JJ, et al. Nomograms in oncology: more than meets the eye. Lancet Oncol 2015;16:e173-80. [Crossref] [PubMed]
- Park SY. Nomogram: An analogue tool to deliver digital knowledge. J Thorac Cardiovasc Surg 2018;155:1793. [Crossref] [PubMed]
- Zhou L, Huang R, Wei Z, et al. The Clinical Characteristics and Prediction Nomograms for Primary Spine Malignancies. Front Oncol 2021;11:608323. [Crossref] [PubMed]
- Chen B, Zeng Y, Liu B, et al. Risk Factors, Prognostic Factors, and Nomograms for Distant Metastasis in Patients With Newly Diagnosed Osteosarcoma: A Population-Based Study. Front Endocrinol (Lausanne) 2021;12:672024. [Crossref] [PubMed]
- Li W, Dong S, Wang B, et al. The Construction and Development of a Clinical Prediction Model to Assess Lymph Node Metastases in Osteosarcoma. Front Public Health 2022;9:813625. [Crossref] [PubMed]
- Li W, Hong T, Xu C, et al. Development and Validation of a Novel Clinical Prediction Model to Predict the Risk of Lung Metastasis from Ewing Sarcoma for Medical Human-Computer Interface. Comput Intell Neurosci 2022;2022:1888586. [Crossref] [PubMed]
- Wang J, Zhanghuang C, Tan X, et al. A Nomogram for Predicting Cancer-Specific Survival of Osteosarcoma and Ewing's Sarcoma in Children: A SEER Database Analysis. Front Public Health 2022;10:837506. [Crossref] [PubMed]
- Kerr KF, Brown MD, Zhu K, et al. Assessing the Clinical Impact of Risk Prediction Models With Decision Curves: Guidance for Correct Interpretation and Appropriate Use. J Clin Oncol 2016;34:2534-40. [Crossref] [PubMed]
- Fitzgerald M, Saville BR, Lewis RJ. Decision curve analysis. JAMA 2015;313:409-10. [Crossref] [PubMed]
- Zhang W, Ji L, Wang X, et al. Nomogram Predicts Risk and Prognostic Factors for Bone Metastasis of Pancreatic Cancer: A Population-Based Analysis. Front Endocrinol (Lausanne) 2022;12:752176. [Crossref] [PubMed]
- Christe A, Leidolt L, Huber A, et al. Lung cancer screening with CT: evaluation of radiologists and different computer assisted detection software (CAD) as first and second readers for lung nodule detection at different dose levels. Eur J Radiol 2013;82:e873-8. [Crossref] [PubMed]
- Ludwig C, Cerinza J, Passlick B, et al. Comparison of the number of pre-, intra- and postoperative lung metastases. Eur J Cardiothorac Surg 2008;33:470-2. [Crossref] [PubMed]
- Heaton TE, Hammond WJ, Farber BA, et al. A 20-year retrospective analysis of CT-based pre-operative identification of pulmonary metastases in patients with osteosarcoma: A single-center review. J Pediatr Surg 2017;52:115-9. [Crossref] [PubMed]