
HDAC1: a promising target for cancer treatment: insights from a thorough analysis of tumor functions
Highlight box
Key findings
• From a multitude of perspectives, histone deacetylase 1 (HDAC1) provides prognostic indicators and therapeutic targets.
What is known and what is new?
• There are distinct correlations between the expression of HDAC1 and the diagnosis and prognosis of many kinds of tumors.
• We firstly sought to explore the potential oncogenic roles of HDAC1 in different tumor types using The Cancer Genome Atlas and Gene Expression Omnibus datasets. Targeting HDAC1 significantly influenced angiogenesis, and infiltration of cancer-associated inflammatory cells.
What is the implication, and what should change now?
• HDAC1 functions in different types of tumors that cause cancer, and a targeted approach to treating cancer may be possible by targeting HDAC1.
Introduction
Cancer is a leading factor in global illness and death (1). In recent years, most studies on tumors have focused on a specific type of cancer (2). However, the tumorigenesis and progression of tumors is a comprehensive process, and various genes contribute significantly to promote tumorigenesis in different tumors. Hence, conducting a comprehensive analysis of oncogenes across different types of cancer is of utmost importance to establish its association with clinical tumor prognosis and relevant signaling pathways (3). Oncogenes such as FOXO1 (4), HS6ST2 (5), SND1 (6), TWF1 (7), and DLGAP5 (8) have been evaluated in pan-cancer studies in recent years. The pan-cancer research can be conveniently conducted primarily due to The Cancer Genome Atlas (TCGA) initiative and the easily accessible Gene Expression Omnibus (GEO) (9-11).
Histone deacetylase 1 (HDAC1), an HDAC class I family member, is widely checked in various organs as a significant type of epigenetic enzyme. During the mitotic phase (12,13), the enzyme HDAC1 is crucial for the condensing of chromatin, the formatting of spindles, and the separating of chromosomes. Most of the existing studies on the function of HDAC1 primarily focus on cancerous growths. The multifunctional HDAC1 has been the focus of our research, and we have documented the functional relationship between HDAC1 and liver cancer development and progression (12,13). Previously, HDAC1 has been evaluated only in relation to a few types of cancers, and its role has remained vague in other kinds of tumors. Furthermore, there has been no examination conducted to dig the involvement of HDAC1 in all cancer’s comprehensive analysis.
Using TCGA and GEO databases, our research provided a comprehensive analysis of HDAC1 across various cancer types. We investigated in detail the role of HDAC1 in the pathogenesis and clinic prognoses of cancer by analyzing survival condition, gene expression, protein phosphorylation, gene alterations, immune infiltration, and relevant cellular pathways. The extensive examination uncovered the possible molecular process of HDAC1 in the development and medical outlook of various types of human malignancies. We present this article in accordance with the STROBE reporting checklist (available at https://tcr.amegroups.com/article/view/10.21037/tcr-24-23/rc).
Methods
Gene expression analysis
Tumor IMmune Estimation Resource 2 (TIMER2) was utilized for the examination of HDAC1 gene expression in tumors and nearby normal tissues with the TCGA. Certain tumors, such as skin cutaneous melanoma (SKCM) and testicular germ cell tumors (TGCT), may have minimal or no surrounding healthy tissues; therefore, we used Gene Expression Profiling Interactive Analysis 2 (GEPIA2). We obtained the Genotype Tissue Expression (GTEx) database box plots comparing the level of HDAC1 (14). We acquired violin plots of HDAC1 expression in pathological stages of all cancers.
The University of ALabama at Birmingham CANcer data analysis Portal (UALCAN) tool was used to acquire cancer omics data and protein level analysis was performed in the Clinical Proteomic Tumor Analysis Consortium (CPTAC) dataset (15). Consequently, we proceeded to inquire into the level of the complete protein or phosphoprotein with HDAC1 (NP_004955.2) phosphorylation at the S393, S406, S410, S421S423, S421, and S423 sites in in normal and primary tumor tissues. Six tumor datasets were chosen for the analysis.
Small interfering RNA and conditioned medium
The small interfering RNA (siRNA) for HDAC1 were designed and performed as reported previously (13). Briefly, siRNA targeting HDAC1 (siHDAC1), or an siRNA of a negative control were transfected into HepG2/Huh7 of HCC cell lines. After 48 hours of interference, the medium was spun at 700 rpm for 2 minutes, then filtered.
Tube formation assay
Corning’s Matrigel matrix (Corning, NY, USA), measuring 70 µL per well (#356234), was introduced into 96-well culture plates. After incubating at 37 ℃ for 30 minutes, human umbilical vein endothelial cells (HUVECs) were resuspended in 100 µL of conditioned medium (CM) and gently placed on 96-well plates coated with Matrigel, with 2×104 cells per well. Well-developed HUVEC tube networks usually form 4–6 hours after the cells are seeded into wells. Cells were imaged and then calculated at 4–6 hours after plating by using a microscope (ZEISS, Oberkochen, Germany) and Image J software (National institutes of Health, Bethesda, MD, USA).
Transwell migration assay
The migratory effect of CM to HUVEC was further detected by using transwell chambers. In short, the cells that were treated with CM were placed in the upper wells, whereas CM collected from siHDAC1 was added to the lower wells. Following a 10-hour incubation period, the cells were fixed for 30 minutes and stained with crystal violet. The Leica microscope and ImageJ were used to obtain the images and cell numbers, respectively.
Analysis of survival prognosis
We used the GEPIA2 to acquire the overall survival (OS) and disease-free survival (DFS) of HDAC1 across all tumors in TCGA project. We categorized the cases into two groups based on the expression.
Analysis of gene alteration
In the ‘Quick select’ section of the cBioPortal website (https://www.cbioportal.org/), we inputted ‘HDAC1’ of the search queries to explore the genetic alteration traits of HDAC1. We obtained changes frequency, mutations types, and copy number alteration (CNA) in all tumor types in TCGA in the section titled ‘Summary of Cancer Types’. With the “Mutations” module, we viewed the mutation site information of HDAC1 as a schematic or three-dimensional (3D) structure. As well as finding the differences in OS, DFS, and progression-free survival (PFS), and respectively for TCGA cases with or without genetically altered HDAC1, we used the “Comparison” module. A schematic diagram of the protein structure was used to show the mutated site details of HDAC1.
Analysis of immune infiltrations
We used TIMER2 to explore the HDAC1 level and immune infiltrates across all kinds of tumors in TCGA. Cancer-associated fibroblast was chosen for further analysis. Many algorithms, such as CIBERSORT, QUANTISEQ, CIBERS-ORT-ABS, Microenvironment Cell Populations-counter (MCPCOUNTER), and Tumor Immune Dysfunction and Exclusion (TIDE), were applicable to predicate the immunological environment. The data were visualized using heatmaps and scatterplots, and the P values and partial correlations (cor) were calculated using the purity-adjusted Spearman’s rank correlation test.
Analysis of gene enrichment related to HDAC1
To identify mutually binding proteins, we utilized the Search Tool for the Retrieval of Interacting Genes/Proteins (STRING) and retrieved 50 HDAC1-binding proteins that had been experimentally determined. To acquire the top 100 genes correlated with HDAC1, we utilized GEPIA2 to obtain these targeting genes and their ‘correlation analysis’. The P and R values were indicated. Furthermore, we utilized the TIMER2 ‘Gene_Corr’ module to create a heatmap for these chosen genes.
To conduct Kyoto Encyclopedia of Genes and Genomes (KEGG) pathways analyses, we combined and refined two datasets: HDAC1-binding analysis, and genes that interacted with each other. Furthermore, we submitted the gene lists to the Database for Annotation, Visualization and Integrated Discovery (DAVID) to obtain the annotation chart. The “tidyr” and “ggplot2” packages were used to visualize and conduct GO analysis. The cnetplot function was used to display the molecular function (MF) data in formation of cnetplots. The statistical significance was determined by two-tailed P<0.05 (16) for this analysis.
Immunohistochemistry (IHC) staining
Tumors sections were subjected to staining using a suitable primary antibody. IHC staining of HDAC1 (no.ab7028, dilution 1:1,000; Abcam, Cambridge, MA, USA) was performed. Cells that had been stained were observed using Pannoramic DESK (3DHISTECH, Budapest, Hungary) to scan and capture an image in a blinded manner at a magnification of 200 times.
Study approval
Patient specimens were collected from the Second Affiliated Hospital of Chongqing Medical University (Chongqing, China). Before conducting the surgeries, patients provided their informed consent and confirmed that they had not undergone any treatments. All procedures were conducted in compliance with applicable guidelines and regulations.
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the ethics committee of Chongqing Medical University (No. 2021020). Informed consent was provided by all patients.
Statistical analysis
All statistical data were analyzed and plotted using GraphPad Prism 8 (GraphPad Software, San Diego, CA, USA). Data were represented as mean ± standard deviation (SD) [standard error of the mean (SEM)]. A two-tailed paired t-test was used to make a comparison of two groups. We considered statistical significance to be P value less than 0.05, with significance levels of 0.05, 0.01, and 0.001.
Results
HDAC1 expression analysis
Specifically, this research investigated the function of HDAC1 [messenger RNA (mRNA): NM_004964.3, protein: NP_004955.2, Figure S1A] in various types of cancer in humans. The HDAC1 protein structure is conserved among different species (e.g., H. Sapiens, M. mulatta, C. lupus, etc.) typically including an Arginase HDAC (Accession numbercl17011) domain (Figure S1B). To determine the level of HDAC1 expression in various paracancerous and cancerous tissues of the TCGA repository, we used the TIMER2 algorithm. The mRNA levels of HDAC1 in tumor tissues were significantly elevated compared to the corresponding control tissues (Figure 1A) [P<0.01 for BLCA and P<0.001 for breast invasive carcinoma (BRCA) and cervical squamous cell carcinoma (CESC)].

To assess the difference in HDAC1 expression, we examined the GTEx dataset for cancers that lacked the normal controls in the TIMER2. In comparison to regular tissues, HDAC1 was undoubtedly expressed at a heightened level in lower-grade glioma (LGG), glioblastoma multiforme (GBM), diffuse large B-cell lymphoma (DLBC), and thymoma (THYM) (Figure 1B). Overall, our findings indicated that HDAC1 expression was elevated in most human tumors. Furthermore, apart from evaluating the transcription level, CPTAC proteomic data was also used to analyze HDAC1 at the protein expression level. In breast, ovarian, colon, and clear cell renal cell carcinoma (RCC), endometrium, and lung tumor tissues, the protein expression of HDAC1 was significantly elevated compared to the normal tissue (Figure 1C). Moreover, we employed the GEPIA2 module to reveal a significant association between the level of HDAC1 and the pathology stage of a variety of tumors, such as bladder cancer (BLCA), kidney chromophobe (KICH), liver hepatocellular carcinoma (LIHC), and TGCT. This correlation is shown in Figure 1D and most had a P value of less than 0.05.
To elucidate the disparity in HDAC1 expression between breast, ovarian, colon, clear cell renal cell carcinoma (ccRCC), endometrium, lung, and thyroid cancer tissues and their paracancerous tissues, and to juxtapose these findings with HDAC1 gene expression data from TCGA, IHC was conducted on clinical human samples of the aforementioned six tissue samples gathered from the clinic. In these cancer tissues, the IHC findings indicated a significant rise in the protein levels of HDAC1 (Figure 2).

HDAC1 promotes angiogenic activity
Tumor progression and metastasis are heavily dependent on the ability of angiogenesis. Tumor cells secrete a variety of growth factors to stimulate angiogenesis. We conducted experiments using tube formation assay and transwell assay to evaluate the ability of HUVECs in generating tubes and metastasizing on Matrigel-coated wells and 12-well transwell chambers. HUVECs were incubated with conditioned medium obtained from HepG2/Huh7 cells transfected with siHDAC1 (Figure 3A). HUVECs cultured by the CM from HDAC1-knockdown HepG2/Huh7 caused a marked reduction in the number of tubes and junctions, and in the cell numbers of transwell compared with those treated with CM from siNC-transfected HepG2/Huh7 cells (Figure 3B,3C), which indicated a possible deficiency in the tumor progression and metastasis by knocking down HDAC1.

HDAC1-linked cancer survival
In order to examine the effect of HDAC1 expression on the individual’s survival with cancers, we analyzed the survival prognoses for patients who had been categorized into high and low HDAC1 level groups. Our next step was to examine whether HDAC1 expression and prognosis were correlated in TCGA and GEO datasets for diverse tumors. Tumors of KICH, LGG, and LIHC with increased HDAC1 expression had a worse prognosis of OS (Figure 4A). The analysis of DFS suggested a consistent association between elevated HDAC1 and unfavorable prognoses in TCGA of KICH and LGG (Figure 4B). In contrast, a low level of HDAC1 was related to worse OS and DFS prognosis outcomes for THYM (Figure 4A,4B).

In addition, Kaplan-Meier (KM) analysis (17) revealed a correlation between decreased HDAC1 expression and an unfavorable distant metastasis-free survival (DMFS) and post-progression survival (PPS) for breast cancer patients (Figure S2A). Furthermore, low HDAC1 levels were involved in worse first progression (FP), OS, and PPS prognoses for gastric tumors (Figure S2B). Then, lower HDAC1 expression level was related to poorer FP for lung tumor (Figure S2C). Meanwhile, in ovarian cancer, increased levels of HDAC1 had worse FP, PFS, and PPS outcomes (Figure S2D). Liver cancer cases had poorer OS, PFS, and relapse-free survival (RFS) prognoses with a low HDAC1 level (Figure S2E). The above information suggests that HDAC1 level is differentially associated with the prognosis of tumor cases that the expression of HDAC1 is decreased in most cancer types, which can lead to a poorer prognosis.
Gene alterations of HDAC1 in pan-cancer investigation
We examined the mutations of HDAC1 in TCGA, encompassing various tumors, to investigate its potential role in carcinogenesis. Variations in HDAC1 can occur through amplifications, deep deletion, mutations, fusions, and multiple alterations. The form-change most commonly encountered are amplifications and mutations, primarily found in ovarian cancer, uterine corpus endometrial carcinoma (UCEC), stomach cancer, and sarcoma. Notably, ovarian cancer exhibited the highest occurrence of the ‘amplification’ form of CNA, accounting for approximately 3% in terms of frequency (Figure 5A).

The main genetic alterations on the case number, locations, and variations identified were a missense mutation in HDAC1, and an E455del alteration in the intron region in 2 cases of stomach adenocarcinoma (STAD) and 1 case of UCEC (Figure 5B). According to the data presented in Figure 5C, STAD cases that had HDAC1 alteration showed a more favorable prognosis in terms of disease-specific survival (DSS), and OS, but not DFS and PFS compared to individuals without HDAC1 alterations. These results suggest that high expression correlates with outcomes whereas mutations and alterations are too low to make any associations.
Protein phosphorylation levels of HDAC1 in cancers
In order to explore the effect of HDAC1 on tumors via protein phosphorylation, we examined the phosphorylation levels of HDAC1 in both normal and tumor tissues. We analyzed the phosphorylation levels of six tumor types in breast, colon, ccRCC, lung, ovary, and endometrium in CPTAC. In nearly all primary tumor tissues, except for ccRCC, the phosphorylation locus of the S421 of HDAC1 was higher compared to that in normal tissue (Figure 6A-6G). Additionally, breast cancer (Figure 6B), colon cancer (Figure 6D), and lung adenocarcinoma (LUAD) (Figure 6G) exhibited increased phosphorylation levels of the S393 locus. Meanwhile, the S393 site showed a decrease in phosphorylation in ccRCC (Figure 6E) and UCEC (Figure 6F). Further molecular assays are warranted to investigate the underlying involvement of S421 phosphorylation in tumor formation.

Expression of HDAC1 and immune infiltration
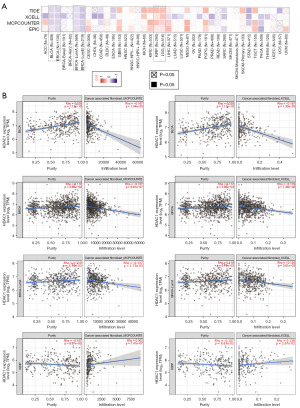
Infiltrating immune cells perform the predominant roles of microenvironment in tumorigenesis and development or metastasis. It is widely known that tumor-associated fibroblasts exert a controlling influence on the function of different immune cells that infiltrate cancer. Consequently, our objective was to examine the possible correlation in the immune cell infiltration and HDAC1 expression in various genres of tumors. To accomplish this, we utilized a range of algorithms including CIBERSORT, TIMER, QUANTISEQ, CIBERS-ORT-ABS, XCELL, MCPCOUNTER, TIDE, and Estimating the Proportion of Immune and Cancer cells (EPIC) (16,18). Surprisingly, a negative correlation was observed between the expression of HDAC1 and the estimated level of infiltration of cancer-associated fibroblasts (CAFs) in STAD and TGCT cases (as shown in Figure 7). Further, in the TCGA tumor samples of kidney renal papillary cell carcinoma (KIRP) and LGG, HDAC1 expression was significantly correlated with estimated levels of CAF infiltration. However, we observed a negative correlation in cases of BLCA, BRCA, BRCA-LumA, and BRCA-LumB (Figure 7A). Figure 7B presents the scatterplot data produced using four algorithms for the above tumors. On the basis of EPIC, MCPCOUNTER, XCELL, and TIDE, HDAC1 expression levels were shown to be positively correlated with the level of infiltration of CAFs in KIRP and LGG (Figure 7B). Additionally, we assessed the relationship between HDAC1 and CD8+ T cell and macrophage infiltration (as shown in Figure S3A,S3B).
HDAC1-related pathway enrichment analysis
For pathway enrichment analysis, we selected genes associated with HDAC1 expression and HDAC1-combining proteins to gain a better understanding of the molecular mechanism of HDAC1 function in tumor development. By utilizing the STRING tool, we acquired the interaction network of a total 50 proteins binding to HDAC1 (as displayed in Figure 8A). By utilizing GEPIA2, we merged the entirety of TCGA tumor expression and acquired the top 100 genes correlated with HDAC1. HDAC1 expression showed a positive correlation with with PTBP1, HNRNPL, HNRNPA, EIF3, HNRNPF, SNRNP4, SRSF9, PTMA, KHDRBS1, and TMEM69 (Figure 8B, Figure S4). Figure 8C displays a positive relationship between HDAC1 and PTBP1, HNRNPL, HNRNPA3, EIF3I, and HNRNPF according to the relative heatmap data. Through analyzing the intersection of the aforementioned two groups, we identified four shared members: Yy1, RBBP4, MTA2, and SUDS3 (as shown in Figure 8D).

We combined these two datasets to operate KEGG/ Gene Ontology (GO) enrichment experiments. The KEGG data suggest that HDAC1 might participate in “Viral carcinogenesis” and “Splicesome” in terms of tumor progress (Figure 8E). According to the GO data, it was revealed that for the most part, these genes are associated with DNA or epigenetics function, including DNA attachment, chromatin DNA attachment, nucleosome attachment, histone deacetylase attachment, and more (Figure 8F).
Discussion
HDAC1 promotes the condensation of chromatin by eliminating the acetyl group, leading to the strong binding of the positively charged lysine to the negatively charged DNA (19). Prior research has shown that the versatile HDAC1 protein is involved in various biological processes, including liver regeneration (20), red blood cell production (21), formation of new blood vessels (22), programmed cell death (23), and regulation of cell division (24). An increasing number of studies have been paying close attention to examining the physiology and pathology role of HDAC1 in multitudinous diseases, particularly in cancer (13). The impact of HDAC1 in the progression of most tumors is still unknown, as it regulates specific molecular signaling pathways that have yet to be determined. Thus, we examined HDAC1 in a pan-cancer context.
However, it is still obscure whether HDAC1 is instrumental in the tumorigenesis or has a significant role in conventional pathways that promote tumor pathogenesis. An extensive review of the literature revealed that HDAC1 has not been investigated holistically from a tumor perspective in any available publication. With that being said, our objective was to comprehensively analyze the expression level, genetic modification, and gene functionality of HDAC1 in 33 diverse tumors using information from the CPTAC, TCGA, and GTEx datasets.
During this study, various types of malignancies showed elevated expression of HDAC1 in the tumor tissues exceeding those of the corresponding control tissues in our findings. In GBM, LGG, DLBC, and THYM, HDAC1 is undeniably expressed at a greater magnitude in tumor cells compared to the normal tissues. Moreover, the level of HDAC1 is notably elevated in six types of cancer tissues, as observed in clinical human samples using IHC staining (Figure 2A-2F). In addition to DLBC, GBM, LGG, and THYM, aberrant expression of HDAC1 has also been found in asbestos carcinogenesis and mesothelioma. HDAC1 can be detected in the nucleus of HCC cancer patients by IHC method, and its level is significantly correlated with cancer pathological stage (25).
In addition, increased levels of HDAC1 are associated with poor prognoses for OS in LGG, KICH, and LIHC (Figure 3A). Meanwhile, the examination of KM plotter revealed contrasting outcomes. Specifically, decreased HDAC1 levels are correlated with unfavorable DMFS and PPS prognoses in breast cancer, as well as worse OS, FP, and PPS prognoses in gastric cancer. Additionally, it was related to poorer FP prognosis in lung cancer, and inferior PFS, OS, and RFS prognoses in liver cancer (Figure S2A-S2E). The results suggest that HDAC1 has the potential to be a reliable indicator for forecasting the prognosis of individuals with tumors.
Glioma (26) has been reported to exhibit an increased HDAC1 level, which was associated with an unfavorable prognosis. HDAC1 and HDAC2 was the most upregulated histone deacetylases (HDACs) by GEPIA in glioblastoma (27). Blood vessel invasion is essential for tumor development and metastasis during angiogenesis, such as HUVECs proliferating as vessel tubes. Regulators of the cell cycle expressed in the body (28) are controlled by HDAC1, which in turn regulates this proliferation. However, how HDAC1 regulates cancer cells to impact vessel information is unknown. By knocking down HDAC1, we found that the CM of HCC cell lines were less able to form tubes of HUVECs, which indicated that HDAC1 promotes angiogenic activity.
Afterwards, we utilized the CPTAC to examine the molecular pathways of the protein of HDAC1 in breast cancer, colon cancer, ccRCC, LUAD, ovarian cancer, and UCEC in respect of overall protein and phosphoprotein levels. This research shows that there was a high level of HDAC1 total protein expression and phosphorylation in the primary tumors, except for ccRCC, compared to normal controls at the S421 locus within the intron (Figure 6A-6G). Nevertheless, further investigations are necessary to assess the potential impact of HDAC1 phosphorylation at the S421 location on the development of tumors.
Next, we proceeded with KEGG/GO enrichment analyses to combine the proteins that bind to HDAC1 and the genes related to HDAC1 expression in all tumors. This was followed by an enrichment experiment that revealed the significance of ‘Viral carcinogenesis’ and ‘Splicesome’ in the development and progression of cancer. According to the TCGA tumor samples of KIRP and LGG, expression of HDAC1 was positively correlated with infiltration of CAFs. Inversely, for BLCA, BRCA, BRCA-LumA, and BRCA-LumB samples, HDAC1 was negatively correlated with fibroblasts (Figure 7A). Therefore, a possible role for HDAC1 in CAF and immune cells infiltration needs to be investigated further.
Furthermore, HDAC1 increases Ki-67 level, and plays a pivotal role in the proliferation of ovarian tumors (29). HDAC1 leads to increase p53 acetylation, which involves in blocking cell cycle arrest and apoptosis (23). HDAC1 plays a dual role in tumor development. On one hand, several studies have reported that HDAC1 enhances oncogene expression. HDAC1 regulates YY1 and promotes its transcriptional regulation of METTL3 (30). HDAC1 and CK2 are confirmed to be involved in cancer development (31). On the other hand, HDAC1 suppresses the expression of the tumor suppressor gene PTEN (32).
According to our comprehensive analysis of HDAC1 expression in various human cancers, HDAC1 is associated with clinical outcomes, protein phosphorylation, immunological cells infiltration, and epigenetics. It is useful to understand the role of HDAC1 holistically. HDAC1 provides prognostic markers and therapeutic targets on tumorigenesis from a multitude of angles. However, our studies still have some limitations. In the future, we will conduct basic experiments to validate these obtained data and potential mechanisms.
Conclusions
This study focused on the cancer-causing functions of HDAC1 in different types of tumors, emphasizing the potential use of HDAC1 as a targeted approach in cancer treatment.
Acknowledgments
It is our pleasure to acknowledge the TCGA, GEO, and GTEx researchers who contributed to our research.
Funding: This research was funded by research grants from
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://tcr.amegroups.com/article/view/10.21037/tcr-24-23/rc
Data Sharing Statement: Available at https://tcr.amegroups.com/article/view/10.21037/tcr-24-23/dss
Peer Review File: Available at https://tcr.amegroups.com/article/view/10.21037/tcr-24-23/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tcr.amegroups.com/article/view/10.21037/tcr-24-23/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the ethics committee of Chongqing Medical University (No. 2021020). All patients provided written informed consent.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Global Burden of Disease 2019 Cancer Collaboration; Kocarnik JM, Compton K, et al. Cancer Incidence, Mortality, Years of Life Lost, Years Lived With Disability, and Disability-Adjusted Life Years for 29 Cancer Groups From 2010 to 2019: A Systematic Analysis for the Global Burden of Disease Study 2019. JAMA Oncol 2022;8:420-44.
- Jia Y, Tian Q, Yang K, et al. A Pan-Cancer Analysis of Clinical Prognosis and Immune Infiltration of CKS1B in Human Tumors. Biomed Res Int 2021;2021:5862941. [Crossref] [PubMed]
- Zhang C, Shen Q, Gao M, et al. The role of Cyclin Dependent Kinase Inhibitor 3 (CDKN3) in promoting human tumors: Literature review and pan-cancer analysis. Heliyon 2024;10:e26061. [Crossref] [PubMed]
- Li J, Wang C, Xu X, et al. An extensive analysis of the prognostic and immune role of FOXO1 in various types of cancer. Braz J Med Biol Res 2024;57:e13378. [Crossref] [PubMed]
- Huang L, Irshad S, Sultana U, et al. Pan-cancer analysis of HS6ST2: associations with prognosis, tumor immunity, and drug resistance. Am J Transl Res 2024;16:873-88. [Crossref] [PubMed]
- Cui X, Zhang X, Liu M, et al. A pan-cancer analysis of the oncogenic role of staphylococcal nuclease domain-containing protein 1 (SND1) in human tumors. Genomics 2020;112:3958-67. [Crossref] [PubMed]
- Huo G, Wang Y, Chen J, et al. A Pan-Cancer Analysis of the Oncogenic Role of Twinfilin Actin Binding Protein 1 in Human Tumors. Front Oncol 2021;11:692136. [Crossref] [PubMed]
- Tang N, Dou X, You X, et al. Pan-cancer analysis of the oncogenic role of discs large homolog associated protein 5 (DLGAP5) in human tumors. Cancer Cell Int 2021;21:457. [Crossref] [PubMed]
- Tomczak K, Czerwińska P, Wiznerowicz M. The Cancer Genome Atlas (TCGA): an immeasurable source of knowledge. Contemp Oncol (Pozn) 2015;19:A68-77. [Crossref] [PubMed]
- Clough E, Barrett T. The Gene Expression Omnibus Database. Methods Mol Biol 2016;1418:93-110. [Crossref] [PubMed]
- Blum A, Wang P, Zenklusen JC. SnapShot: TCGA-Analyzed Tumors. Cell 2018;173:530. [Crossref] [PubMed]
- Xia J, Zhou Y, Ji H, et al. Loss of histone deacetylases 1 and 2 in hepatocytes impairs murine liver regeneration through Ki67 depletion. Hepatology 2013;58:2089-98. [Crossref] [PubMed]
- Zhou H, Cai Y, Liu D, et al. Pharmacological or transcriptional inhibition of both HDAC1 and 2 leads to cell cycle blockage and apoptosis via p21(Waf1/Cip1) and p19(INK4d) upregulation in hepatocellular carcinoma. Cell Prolif 2018;51:e12447. [Crossref] [PubMed]
- Tang Z, Kang B, Li C, et al. GEPIA2: an enhanced web server for large-scale expression profiling and interactive analysis. Nucleic Acids Res 2019;47:W556-60. [Crossref] [PubMed]
- Chen F, Chandrashekar DS, Varambally S, et al. Pan-cancer molecular subtypes revealed by mass-spectrometry-based proteomic characterization of more than 500 human cancers. Nat Commun 2019;10:5679. [Crossref] [PubMed]
- Steven A, Seliger B. The Role of Immune Escape and Immune Cell Infiltration in Breast Cancer. Breast Care (Basel) 2018;13:16-21. [Crossref] [PubMed]
- Menyhárt O, Nagy Á, Győrffy B. Determining consistent prognostic biomarkers of overall survival and vascular invasion in hepatocellular carcinoma. R Soc Open Sci 2018;5:181006. [Crossref] [PubMed]
- Fridman WH, Galon J, Dieu-Nosjean MC, et al. Immune infiltration in human cancer: prognostic significance and disease control. Curr Top Microbiol Immunol 2011;344:1-24. [PubMed]
- Song Y, Dagil L, Fairall L, et al. Mechanism of Crosstalk between the LSD1 Demethylase and HDAC1 Deacetylase in the CoREST Complex. Cell Rep 2020;30:2699-2711.e8. [Crossref] [PubMed]
- Ko S, Russell JO, Tian J, et al. Hdac1 Regulates Differentiation of Bipotent Liver Progenitor Cells During Regeneration via Sox9b and Cdk8. Gastroenterology 2019;156:187-202.e14. [Crossref] [PubMed]
- Kim MY, Yan B, Huang S, et al. Regulating the Regulators: The Role of Histone Deacetylase 1 (HDAC1) in Erythropoiesis. Int J Mol Sci 2020;21:8460. [Crossref] [PubMed]
- Ding X, Xu J, Wang C, et al. Suppression of the SAP18/HDAC1 complex by targeting TRIM56 and Nanog is essential for oncogenic viral FLICE-inhibitory protein-induced acetylation of p65/RelA, NF-κB activation, and promotion of cell invasion and angiogenesis. Cell Death Differ 2019;26:1970-86. [Crossref] [PubMed]
- Bahl S, Ling H, Acharige NPN, et al. EGFR phosphorylates HDAC1 to regulate its expression and anti-apoptotic function. Cell Death Dis 2021;12:469. [Crossref] [PubMed]
- Ma P, Schultz RM. HDAC1 and HDAC2 in mouse oocytes and preimplantation embryos: Specificity versus compensation. Cell Death Differ 2016;23:1119-27. [Crossref] [PubMed]
- Novelli F, Bononi A, Wang Q, et al. BAP1 forms a trimer with HMGB1 and HDAC1 that modulates gene × environment interaction with asbestos. Proc Natl Acad Sci U S A 2021;118:e2111946118. [Crossref] [PubMed]
- Cui K, Wu X, Gong L, et al. Comprehensive Characterization of Integrin Subunit Genes in Human Cancers. Front Oncol 2021;11:704067. [Crossref] [PubMed]
- Vengoji R, Atri P, Macha MA, et al. Differential gene expression-based connectivity mapping identified novel drug candidate and improved Temozolomide efficacy for Glioblastoma. J Exp Clin Cancer Res 2021;40:335. [Crossref] [PubMed]
- Dunaway LS, Pollock JS. HDAC1: an environmental sensor regulating endothelial function. Cardiovasc Res 2022;118:1885-903. [Crossref] [PubMed]
- Hayashi A, Horiuchi A, Kikuchi N, et al. Type-specific roles of histone deacetylase (HDAC) overexpression in ovarian carcinoma: HDAC1 enhances cell proliferation and HDAC3 stimulates cell migration with downregulation of E-cadherin. Int J Cancer 2010;127:1332-46. [Crossref] [PubMed]
- Li M, Li M, Xia Y, et al. HDAC1/3-dependent moderate liquid-liquid phase separation of YY1 promotes METTL3 expression and AML cell proliferation. Cell Death Dis 2022;13:992. [Crossref] [PubMed]
- Pflum MK, Tong JK, Lane WS, et al. Histone deacetylase 1 phosphorylation promotes enzymatic activity and complex formation. J Biol Chem 2001;276:47733-41. [Crossref] [PubMed]
- Si W, Liu X, Wei R, et al. MTA2-mediated inhibition of PTEN leads to pancreatic ductal adenocarcinoma carcinogenicity. Cell Death Dis 2019;10:206. [Crossref] [PubMed]


