
Elevated serum carcinoembryonic antigen and CA15-3 levels and the risk of site-specific metastases in metastatic breast cancer
Introduction
The incidence of breast cancer in the United States and China has increased continuously over time, with more than 200,000 females newly diagnosed with the disease every year (1-3). Although significant progress has been achieved in the development of comprehensive therapy of breast cancer (4), distant metastases are still observed in about 20–30% of patients (5-7), resulting in approximately 40,000 deaths each year (1,3). Although the vast majority of breast cancer related deaths occur in patients with distant metastases, the unique patterns of distant metastases and mechanisms of disease progression have not been clearly elucidated.
Serum carcinoembryonic antigen (CEA) and cancer antigen 15-3 (CA15-3) are two of the most widely investigated tumor markers in breast cancer. CEA and CA15-3 are of limited use in the early diagnosis due to a lack of specificity and sensitivity (8,9). However, several studies have reported that elevated preoperative levels of CEA and CA15-3 can predict poor survival of breast cancer patients (10-12). The European School of Oncology-European Society for Medical Oncology (ESO-ESMO) suggests that the use of tumor markers for monitoring treatment response is reasonable for advanced breast cancer (13,14). However, data regarding differences in the risk of site-specific metastases between breast cancer serum tumor markers are limited and conflicting. In this study, we sought to clarify the possible relationship between the risk of site-specific metastases and serum CEA and CA15-3 levels in Chinese women with advanced breast cancer at two cancer centers.
Methods
Patients
We performed a retrospective analysis of breast cancer patients admitted to Sun Yat-sen University Cancer Center (SYSUCC) and the First Affiliated Hospital of Xiamen University [Xiamen Cancer Hospital (XMCH)] during the period from March 1998 to January 2013. The inclusion criteria for study enrollment were as follows: (I) female, unilateral invasive breast carcinoma, without distant metastases at the initial breast cancer diagnosis; (II) underwent surgical treatment (mastectomy or breast-conserving therapy) and axillary lymph node dissection; (III) complete resection of tumor without residual tumor observed in pathological examination; (IV) definite distant metastatic disease was found during the follow up period, with complete results of serum CEA and CA15-3 levels when confirmed with metastatic disease. The study was approved by the ethics committee of the First Affiliated Hospital of Xiamen University and SYSUCC (approval number of institutional review board, 2013B021800157).
Serum CEA and CA15-3 levels measurement and clinicopathologic parameters
Clinicopathologic characteristics including age, menopausal status, tumor size, nodal stage, estrogen receptor (ER) status, progesterone receptor (PR) status, human epidermal growth factor receptor 2 (HER2) status, breast cancer subtype (BCS), serum CEA and CA15-3 levels were used to assess the risk of site-specific metastases. Hormone receptor (HR) positivity was defined as greater than 1% of cells demonstrating positive staining by ER or PR immunohistochemistry. HER2 positivity was defined as an immunohistochemical grade of 3+ before 2003, or 2+ with a fluorescence in situ hybridization test after 2003. Due to the majority of patients lacking Ki-67 data, we did not define the BCS according to the 14th St. Gallen International Breast Cancer Conference in 2015 (15), but instead defined four intrinsic BCS (16-18): HR+/HER2− (ER+ and/or PR+, HER2−), HR+/HER2+ (ER+ and/or PR+, HER2+), HR−/HER2+ (ER−, PR−, and HER2+) and HR−/HER2− (ER−, PR−, and HER2−).
Detection of CEA and CA15-3 was performed as described in our previous studies (10,19). The diagnostic cut-off point for serum CEA and CA15-3 levels was 5 ng/mL and 25 U/mL, respectively.
Sites of distant metastases
The sites of distant metastases in breast cancer patients were divided into seven regions according to a previous study (20), including abdomen/pelvis (liver, adrenal gland, lymph nodes, and other abdominopelvic organs), lung/mediastina (lung or pulmonary lymphangitic spread), brain, bone (skeletal system), pleura (pleura and/or pericardial effusion, pleural effusion and/or pleural effusion), axillary and/or neck lymph nodes, and other distant soft tissue.
Statistical analysis
Statistical analysis was performed using the SPSS version 16.0 (SPSS Inc., Chicago, IL, USA). A chi-square test or Fisher’s exact test were performed to determine the differences between groups for categorical variables. Univariate and multivariate logistic regression analyzes were performed to assess the relationship of patient clinicopathologic characteristics and serum CEA and CA15-3 levels for the risk of site-specific metastases. A P value <0.05 was considered significant in all analyzes.
Results
Patient and tumor characteristics
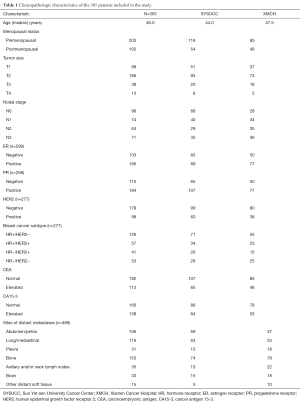
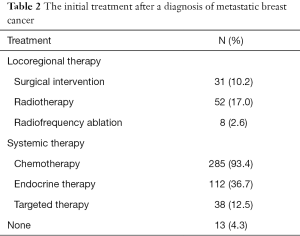
Patient clinicopathologic characteristics are summarized in Table 1. A total of 305 patients were identified in this study. Of these, 56.4% (172/305) of patients were from SYSUCC and 43.6% (133/305) of patients were from XMCH. The median age was 46.0 (range, 27–84) years at the initial breast cancer diagnosis. There were 113 (37.0%) and 139 (45.6%) patients with elevated serum CEA and CA15-3 levels, respectively. The median serum CEA and CA15-3 values in patients with elevated tumor marker levels were 16.9 (range, 5.1–3,515.0) ng/mL and 85.1 (range, 25.6–3,000.0) U/mL, respectively. The initial treatments after a diagnosis of metastatic breast cancer are shown in Table 2.
Full table
Full table
Sites of distant metastases
A total of 489 sites of distant metastases were identified in patients diagnosed with metastatic disease. One hundred and seventy-six (57.7%) patients had single region metastases and 129 (42.3%) patients had multiple region metastases. The common sites of distant metastases were as follows: bone (31.3%), lung/mediastina (23.7%), abdomen/pelvis (21.7%), axillary and/or neck lymph nodes (7.2%), brain (6.7%), pleura (6.3%), and other distant soft tissue (3.0%; Table 1).
Patient clinicopathologic characteristics according to serum CEA and CA15-3 levels
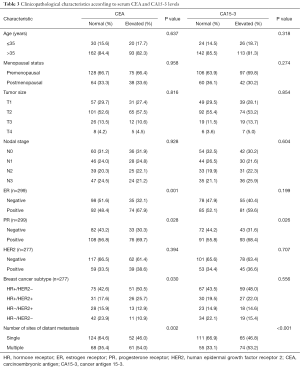
Table 3 shows patient clinicopathologic characteristics according to serum CEA and CA15-3 levels. When classified according to normal or elevated serum tumor marker levels, serum CEA levels were less frequently elevated in patients with ER negative disease (P=0.001), PR negative disease (P=0.028) and HR−/HER2− subtype (P=0.030), while elevated serum CA15-3 levels were less commonly observed in PR negative disease (P=0.026). In addition, elevated serum CEA (P=0.002) and CA15-3 (P<0.001) levels were significantly correlated with the number of metastatic organs.
Full table
Association of serum tumor markers levels and the sites of distant metastases
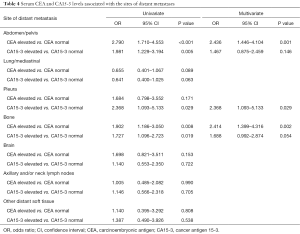
Table 4 shows the metastatic characteristics according to serum CEA and CA15-3 levels. Univariate analysis showed that elevated serum CEA levels were more frequently associated with abdomen/pelvis and bone metastases in patients. Furthermore, elevated serum CA15-3 levels were frequently observed in abdomen/pelvis, pleura, and bone metastases.
Full table
In multivariate logistic regression analysis, abdomen/pelvis metastases [odds ratio (OR) 2.436; 95% confidence interval (CI), 1.446–4.104, P=0.001] and bone metastases (OR 2.414; 95% CI, 1.399–4.316, P=0.002) showed strong correlations with elevated serum CEA levels. Elevated serum CA15-3 levels were significantly correlated with pleura metastases (OR 2.368; 95% CI, 1.093–5.133, P=0.029). Abnormal serum CA15-3 levels were also a marginally predictive factor for bone metastases (OR 1.688; 95% CI, 0.992–2.874, P=0.054). Elevation of CA15-3 levels were not associated with abdomen/pelvis metastases (P=0.146) in multivariate analysis. Elevation of CEA and CA15-3 level was not significant association with the other sites of distant recurrence including lung/mediastina, axillary and/or neck lymph nodes, and other distant soft tissue.
Discussion
In this study, we explored the relationship between serum CEA and CA15-3 levels and the risk of site-specific metastases in metastatic breast cancer. Our results showed that patients with elevated serum CA15-3 levels were more likely to have abdomen/pelvis and bone metastases, while patients with elevated serum CA15-3 levels were more prone to pleura metastases.
Serum CEA and CA15-3 levels were elevated in 37.0% and 45.6% of patients in this study, respectively, which is similar to results in other studies (CEA: 36.0–50.7%; CA15-3: 36.4–55.6%) (21,22). As a special type of breast cancer classification, the prognosis of triple negative breast cancer (TNBC) is significantly worse than other BCS; however, our study found that the probability of elevated serum CEA levels in TNBC was significantly lower than the HR positive subtype (10.9% vs. 25.7–50.5%), which was similar to results from our previous research regarding preoperative tumor markers of breast cancer (10). In addition, Yerushalmi et al. found that tumor markers were less frequently elevated in TNBC (CEA: 31.3%; CA15-3: 68.4%) than luminal subtypes (CEA: 59.6–65.0%; CA15-3: 83.4–86.8%; P<0.001) (23). Kos et al. also showed that elevated tumor marker levels were less frequently observed in TNBC patients as compared to luminal groups (22). Therefore, monitoring of tumor marker levels in HR positive groups may be beneficial in determining early distant recurrence in breast cancer, while TNBC may have little value during follow up for timely detection of distant relapse.
Studies of preoperative CEA and CA15-3 levels have shown that elevated serum tumor marker levels represent an increase in tumor burden, such as advanced tumor size and nodal stage (10,11,24,25). In this study, we also found that patients with multiple organ metastases were more prone to abnormal serum tumor marker levels, which suggests a relationship between elevated tumor marker levels and tumor burden in breast cancer with distant metastases.
ESO-ESMO guidelines recommend observing serum CEA and CA15-3 levels to monitor the therapeutic response in advanced breast cancer (13,14). However, the correlation between serum tumor marker levels and the risk of site-specific metastases has not yet been well established. Yerushalmi et al. found that CEA (P=0.17) and CA15-3 (P=0.2) levels did not correlate with the distant metastatic sites in metastatic breast cancer patients (23). Lee et al. found that elevated CEA was significantly correlated with liver metastases (P=0.002), and CA15-3 levels had a significant correlation with bone (P=0.021), liver (P=0.013), and multiple region metastases (P<0.001) (21). Caglar and colleagues also found that the mean CEA and CA15-3 levels in patients with bone metastases were significantly elevated compared to patients without bone metastases (P<0.001) (26). Furthermore, CEA and CA15-3 may be used as candidate biomarkers in diagnosing different causes of malignant pleural effusion (27). In a study of lung cancer, Lee et al. also found that bone metastases (P<0.001) and brain metastases (P=0.005) showed a significant correlation with elevated serum CEA levels; furthermore, CEA levels ≥100 ng/mL were correlated with abdominal/pelvic metastases (P<0.001) (20). Our results found that patients with elevated serum CA15-3 levels were more prone to have abdomen/pelvis (P=0.001) and bone metastases (P=0.002), while CA15-3 levels were also potentially correlated with pleura (P=0.029) and bone metastases (P=0.054). Therefore, CEA and CA15-3 levels may serve as good biomarkers to assess the risk of site-specific metastases, especially the liver, bone and pleura metastases. The correlation between tumor marker levels and site of metastases requires further investigation, which may be of great value for the targeted therapy of breast cancer with organ-specific metastases.
CA15-3 is a member of the Mucin-1 (MUC-1) family (28). Previous studies have found that MUC-1 can be immunogenic and could be a suitable target for breast cancer immunotherapy (29,30). It was also found that targeting the MUC1-C oncoprotein inhibits the self-renewal capacity of breast cancer cells (31). Overexpress of CEA promotes the adhesion and metastatic processes in cancer cells (32). The oncofetal antigens include CEA, which may serve as a target for the active immune response against cancer. Our findings strongly support the feasibility of using an inhibitor targeting CEA, which may result in a delay of site-specific metastatic processes and prolonged survival in breast cancer (33,34). New cancer vaccines targeting both CEA and MUC-1 have also been developed (35-37). Our results provide a greater understanding for future investigative anti-CEA and CA15-3 targeting and intensive systemic assessment in advanced breast patients with site-specific metastases.
The American Society of Clinical Oncology does not recommend monitoring CEA and CA15-3 levels for routine surveillance of patients with breast cancer after primary therapy (38). However, the European Group on Tumor Markers has suggested routine measurement of tumor markers such as CEA and CA15-3 in patients with breast cancer since 2005 (39). In our previous studies, we have confirmed that preoperative serum CEA and CA15-3 levels can not only serve as prognostic factors in breast cancer patients (10), but they also have a potential impact on axillary treatment considerations (19). In this study, we further found that serum CEA and CA15-3 levels in metastatic breast cancer patients can predict the risk of site-specific metastases. Based on our results, we suggest that serum CEA and CA15-3 levels have potential clinical value in postoperative survival prediction and follow up monitoring of breast cancer patients.
There are several limitations in our study. First, retrospective studies have an inherent problem of selection bias. Secondly, CEA and CA15-3 levels may also be elevated in other benign conditions (40). In addition, the majority of patients with metastatic disease were diagnosed by clinical and imaging approaches and diagnosis was not confirmed by pathological examination. Furthermore, the molecular mechanisms between elevated serum tumor marker levels and the risk of site-specific metastatic affinity remain unclear.
Conclusions
In conclusion, elevated serum CEA and CA15-3 levels may cause an increased risk of site-specific metastase in metastatic breast cancer. Further experimental studies investigating the specific role of serum CEA and CA15-3 levels in the organ-specific metastatic cascade are required.
Acknowledgments
Funding: This work was supported by grants from the National Natural Science Foundation of China (No. 81402527, 81302529), the Sci-Tech Office of Guangdong Province (No. 2013B021800157, 2013B021800458) and the Natural Science Foundation of Fujian Province (No. 2016J01635).
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2016.08.39). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the ethics committee of the First Affiliated Hospital of Xiamen University and SYSUCC (approval number of institutional review board, 2013B021800157) and individual consent for this retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin 2016;66:7-30. [Crossref] [PubMed]
- Zheng R, Zeng H, Zhang S, et al. National estimates of cancer prevalence in China, 2011. Cancer Lett 2016;370:33-8. [Crossref] [PubMed]
- DeSantis CE, Fedewa SA, Goding Sauer A, et al. Breast cancer statistics, 2015: Convergence of incidence rates between black and white women. CA Cancer J Clin 2016;66:31-42. [Crossref] [PubMed]
- Miller E, Lee HJ, Lulla A, et al. Current treatment of early breast cancer: adjuvant and neoadjuvant therapy. F1000Res 2014;3:198. [PubMed]
- Eckhardt BL, Francis PA, Parker BS, et al. Strategies for the discovery and development of therapies for metastatic breast cancer. Nat Rev Drug Discov 2012;11:479-97. [Crossref] [PubMed]
- Kennecke H, Yerushalmi R, Woods R, et al. Metastatic behavior of breast cancer subtypes. J Clin Oncol 2010;28:3271-7. [Crossref] [PubMed]
- Redig AJ, McAllister SS. Breast cancer as a systemic disease: a view of metastasis. J Intern Med 2013;274:113-26. [Crossref] [PubMed]
- Hou MF, Chen YL, Tseng TF, et al. Evaluation of serum CA27.29, CA15-3 and CEA in patients with breast cancer. Kaohsiung J Med Sci 1999;15:520-8. [PubMed]
- Clinton SR, Beason KL, Bryant S, et al. A comparative study of four serological tumor markers for the detection of breast cancer. Biomed Sci Instrum 2003;39:408-14. [PubMed]
- Wu SG, He ZY, Zhou J, et al. Serum levels of CEA and CA15-3 in different molecular subtypes and prognostic value in Chinese breast cancer. Breast 2014;23:88-93. [Crossref] [PubMed]
- Shao Y, Sun X, He Y, et al. Elevated Levels of Serum Tumor Markers CEA and CA15-3 Are Prognostic Parameters for Different Molecular Subtypes of Breast Cancer. PLoS One 2015;10:e0133830 [Crossref] [PubMed]
- Park BW, Oh JW, Kim JH, et al. Preoperative CA 15-3 and CEA serum levels as predictor for breast cancer outcomes. Ann Oncol 2008;19:675-81. [Crossref] [PubMed]
- Cardoso F, Costa A, Norton L, et al. ESO-ESMO 2nd international consensus guidelines for advanced breast cancer (ABC2). Breast 2014;23:489-502. [Crossref] [PubMed]
- Cardoso F, Costa A, Norton L, et al. ESO-ESMO 2nd international consensus guidelines for advanced breast cancer (ABC2)†. Ann Oncol 2014;25:1871-88. [Crossref] [PubMed]
- Coates AS, Winer EP, Goldhirsch A, et al. Tailoring therapies--improving the management of early breast cancer: St Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2015. Ann Oncol 2015;26:1533-46. [Crossref] [PubMed]
- van 't Veer LJ, Dai H, van de Vijver MJ, et al. Gene expression profiling predicts clinical outcome of breast cancer. Nature 2002;415:530-6. [Crossref] [PubMed]
- Sorlie T, Tibshirani R, Parker J, et al. Repeated observation of breast tumor subtypes in independent gene expression data sets. Proc Natl Acad Sci U S A 2003;100:8418-23. [Crossref] [PubMed]
- Tang P, Skinner KA, Hicks DG. Molecular classification of breast carcinomas by immunohistochemical analysis: are we ready? Diagn Mol Pathol 2009;18:125-32. [Crossref] [PubMed]
- Wu SG, He ZY, Ren HY, et al. Use of CEA and CA15-3 to Predict Axillary Lymph Node Metastasis in Patients with Breast Cancer. J Cancer 2016;7:37-41. [Crossref] [PubMed]
- Lee DS, Kim SJ, Kang JH, et al. Serum Carcinoembryonic Antigen Levels and the Risk of Whole-body Metastatic Potential in Advanced Non-small Cell Lung Cancer. J Cancer 2014;5:663-9. [Crossref] [PubMed]
- Lee JS, Park S, Park JM, et al. Elevated levels of serum tumor markers CA 15-3 and CEA are prognostic factors for diagnosis of metastatic breast cancers. Breast Cancer Res Treat 2013;141:477-84. [Crossref] [PubMed]
- Kos T, Aksoy S, Sendur MA, et al. Variations in tumor marker levels in metastatic breast cancer patients according to tumor subtypes. J BUON 2013;18:608-13. [PubMed]
- Yerushalmi R, Tyldesley S, Kennecke H, et al. Tumor markers in metastatic breast cancer subtypes: frequency of elevation and correlation with outcome. Ann Oncol 2012;23:338-45. [Crossref] [PubMed]
- Molina R, Auge JM, Farrus B, et al. Prospective evaluation of carcinoembryonic antigen (CEA) and carbohydrate antigen 15.3 (CA 15.3) in patients with primary locoregional breast cancer. Clin Chem 2010;56:1148-57. [Crossref] [PubMed]
- Lee JS, Park S, Park JM, et al. Elevated levels of preoperative CA 15-3 and CEA serum levels have independently poor prognostic significance in breast cancer. Ann Oncol 2013;24:1225-31. [Crossref] [PubMed]
- Caglar M, Kupik O, Karabulut E, et al. Detection of bone metastases in breast cancer patients in the PET/CT era: Do we still need the bone scan? Rev Esp Med Nucl Imagen Mol 2016;35:3-11. [Crossref] [PubMed]
- Wang XF, Wu YH, Wang MS, et al. CEA, AFP, CA125, CA153 and CA199 in malignant pleural effusions predict the cause. Asian Pac J Cancer Prev 2014;15:363-8. [Crossref] [PubMed]
- Duffy MJ, Evoy D, McDermott EW. CA 15-3: uses and limitation as a biomarker for breast cancer. Clin Chim Acta 2010;411:1869-74. [Crossref] [PubMed]
- Milani A, Sangiolo D, Aglietta M, et al. Recent advances in the development of breast cancer vaccines. Breast Cancer (Dove Med Press) 2014;6:159-68. [PubMed]
- Rivalland G, Loveland B, Mitchell P. Update on Mucin-1 immunotherapy in cancer: a clinical perspective. Expert Opin Biol Ther 2015;15:1773-87. [Crossref] [PubMed]
- Alam M, Rajabi H, Ahmad R, et al. Targeting the MUC1-C oncoprotein inhibits self-renewal capacity of breast cancer cells. Oncotarget 2014;5:2622-34. [Crossref] [PubMed]
- Benchimol S, Fuks A, Jothy S, et al. Carcinoembryonic antigen, a human tumor marker, functions as an intercellular adhesion molecule. Cell 1989;57:327-34. [Crossref] [PubMed]
- Blumenthal RD, Hansen HJ, Goldenberg DM. Inhibition of adhesion, invasion, and metastasis by antibodies targeting CEACAM6 (NCA-90) and CEACAM5 (Carcinoembryonic Antigen). Cancer Res 2005;65:8809-17. [Crossref] [PubMed]
- Turriziani M, Fantini M, Benvenuto M, et al. Carcinoembryonic antigen (CEA)-based cancer vaccines: recent patents and antitumor effects from experimental models to clinical trials. Recent Pat Anticancer Drug Discov 2012;7:265-96. [Crossref] [PubMed]
- Madan RA, Arlen PM, Gulley JL. PANVAC-VF: poxviral-based vaccine therapy targeting CEA and MUC1 in carcinoma. Expert Opin Biol Ther 2007;7:543-54. [Crossref] [PubMed]
- Mohebtash M, Tsang KY, Madan RA, et al. A pilot study of MUC-1/CEA/TRICOM poxviral-based vaccine in patients with metastatic breast and ovarian cancer. Clin Cancer Res 2011;17:7164-73. [Crossref] [PubMed]
- Gulley JL, Arlen PM, Tsang KY, et al. Pilot study of vaccination with recombinant CEA-MUC-1-TRICOM poxviral-based vaccines in patients with metastatic carcinoma. Clin Cancer Res 2008;14:3060-9. [Crossref] [PubMed]
- Khatcheressian JL, Hurley P, Bantug E, et al. Breast cancer follow-up and management after primary treatment: American Society of Clinical Oncology clinical practice guideline update. J Clin Oncol 2013;31:961-5. [Crossref] [PubMed]
- Molina R, Barak V, van Dalen A, et al. Tumor markers in breast cancer- European Group on Tumor Markers recommendations. Tumour Biol 2005;26:281-93. [Crossref] [PubMed]
- Cheung KL, Graves CR, Robertson JF. Tumour marker measurements in the diagnosis and monitoring of breast cancer. Cancer Treat Rev 2000;26:91-102. [Crossref] [PubMed]


