
Epidemiological characteristics of myxopapillary ependymoma and factors affecting overall survival: a SEER-based analysis
Highlight box
Key findings
• Age, marital status, surgery and radiotherapy are factors influencing the overall survival of myxopapillary ependymoma (MPE) patients. Surgery is still the main therapeutic choice, while radiotherapy plays a negative role in the management of MPE.
What is known and what is new?
• MPE is a rare and slow-growing tumor.
• Epidemiological characteristics and prognostic factors of MPE remain unclear.
What is the implication, and what should change now?
• Prospective research is required to verify and complement our findings for MPE control.
Introduction
Myxopapillary ependymoma (MPE), a rare and slow-growing tumor, frequently arises in the spinal cord’s cauda equina and filum terminale (1). According to the World Health Organization (WHO) classification (WHO grade I), it is considered as a low-grade endophytic adenoma (2). Epidemiological surveys have found that its incidence rate is 0.5–2.5 in 100,000 people per year (3). Although MPE has benign histopathology, it may become aggressive and may spread along the nerve axis to distant locations of the skull and spinal cord (4,5). Due to the rarity of MPE, there is a lack of clear treatment guidelines.
The current researches of MPE mainly focus on case reports. Vongsfak and others (6) presented a 13-year-old boy with pathologically examined MPE. To manage the disease following surgical removal, the patient underwent physiotherapy and entire spine irradiation. Ramkumar et al. (7) described a 69-year-old patient suffering from MPE of the fifth lumbar vertebra, and explored the role of magnetic resonance imaging (MRI) in the diagnosis of clinical cases presenting with MPE. The course of MPE progresses slowly, no specific symptoms appear in the early stage, surgical resection is still the preferred method for the treatment of MPE, and the impact of radiotherapy in the treatment of MPE is still debatable (8,9). So far, most MPE research has enrolled a limited number of patients, and MPE is usually studied as a subtype of spinal ependymoma. There is scarce research on the overall epidemiological characteristics of MPE and factors affecting survival which may benefit MPE prevention and treatment. Bates et al. (10) explored the epidemiology and prognostic factors of MPE with 773 individuals from the Surveillance, Epidemiology, and End Results (SEER) program between 2004 and 2012, and more recent investigation is required.
To address this research gap, this study intended to further explore the epidemiological characteristics and prognostic factors of MPE utilizing data from the SEER from 2004 to 2015, so as to optimize disease risk stratification, and provide a scientific and reasonable basis for selecting clinical treatments. We present this article in accordance with the STROBE reporting checklist (available at https://tcr.amegroups.com/article/view/10.21037/tcr-24-757/rc).
Methods
Study population
The SEER database of the National Cancer Institute gathers data on cancer diagnosis, therapy, and survival from around 30% of individuals in the United States, which is publicly available. In order to reflect the progress of research and oncology practices, cancer control methods are constantly evolving, from simply enumerating cancers based on organ sites in the population to including the surveillance of cancer occurrence through histopathology and molecular subtypes (11). Through the SEER-18 database, we inquired a total of 1,038 MPE patients registered from 2004 to 2015 in this retrospective cohort study. The earliest year was 2004 because it was the first year that benign brain tumor information had to be reported. All cases with the International Classification of Diseases (ICD-O-3) histology codes 9394/0 (MPE, benign), 9394/1 (MPE), and 9394/3 (MPE, malignant) were included in the current research. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). Since the data in this study are de-identified publicly available data, this retrospective cohort study was exempted from the institutional ethical committee by Jiangmen Central Hospital.
Data extraction
Age, gender, race, marital status, geographical location, tumor location, tumor histology, tumor size, surgical acceptance, gross total resection (GTR), radiotherapy acceptance, and other cancers were provided by the SEER database. Race was classified as whites, blacks and others. Marital status was divided into single/unmarried, divorced/separate, widowed and married. States of residence included California, Atlanta Georgia, New Jersey and Washington. Histological types were divided into malignant and non-malignant. The acceptance of surgery was classified into no surgery and surgery (local tumor destruction surgery, partial resection, subtotal resection, and GTR). The acceptance of radiotherapy was grouped into no radiotherapy (radiation therapy was declined by the patient or their guardian) and radiotherapy (patient received treatment with radioisotopes, beam radiation and radioactive implants). The outcomes were 5- and 10-year overall survival.
Statistical analysis
All statistical tests were two-sided, and P<0.05 was deemed to be statistically significant. Univariate and multivariate analyses were carried out with SAS 9.4 software (SAS Institute Inc., Cary, NC, USA). Restricted cubic splines and Kaplan-Meier (KM) curves were drawn using SAS 9.4 and R 4.20 software (R foundation for Statistical Computing, Vienna, Austria).
The Shapiro-Wilk test was employed to examine normality. Normally distributed measurement data were described as mean ± standard deviation (mean ± SD), and the independent sample t-test was applied for intergroup comparison. Non-normal data were described by median and quartile [M (Q1, Q3)], and comparison of two groups was conducted by the Mann-Whitney U rank sum test. Enumeration data were reported by the number of cases and composition ratio [n (%)], and comparison between groups was subject to the Chi-squared test or Fisher’s exact test.
According to the purpose of the study, the incidence of MPE and MPE incidence stratified by age, gender and race from 2004 to 2015 were initially shown, and then 5- and 10-year overall survival were analyzed. Univariate Cox regression was performed for the variables studied. Afterwards, the variables of which the P values in univariate Cox regression were less than 0.05 and which may affect overall survival were incorporated into multivariate Cox regression analysis. Finally, subgroup analysis based on histological types was performed. Due to the small number of malignant MPE samples, only non-malignant MPE was studied here.
Results
Participants and baseline characteristics
A total of 1,038 MPE cases from 2004 to 2015 were obtained from the SEER-18 database. After 12 cases with missing survival time were excluded, 1,026 subjects were finally enrolled in this study (Figure 1). These subjects had a median age at diagnosis of 41 [27, 54] years old. There were 437 (42.59%) women and 589 (57.41%) men. Fifty (4.96%) patients were blacks, 903 (89.49%) were whites, and 56 (5.55%) belonged to other races. As regards marital status, 366 (37.77%) were single/unmarried, 66 (6.81%) were divorced/separate, 22 (2.27%) were widowed, and 515 (53.15%) were married. Tumors were located in the spinal cord in 934 patients (91.03%) and the cauda equina in 60 patients (5.85%), 14 (1.36%) patients had intracranial tumors, and tumors were located in other sites in 18 (1.75%) patients. Twenty-six (2.53%) patients suffered from malignant MPE, and 1,000 (97.47%) had non-malignant MPE. Surgery was performed in 965 (94.05%) patients. Moreover, 265 (25.83%) underwent GTR, and 761 (74.17%) did not underwent GTR. And 169 (16.47%) cases received radiotherapy. There were 981 (95.61%) survivors, and 45 (4.39%) were dead. The median follow-up time was 63 months; 5- and 10-year survival rate was 96.20% (94.85%, 97.55%) and 92.60% (90.05%, 95.15%), respectively. Table 1 summarizes patient basic characteristics.
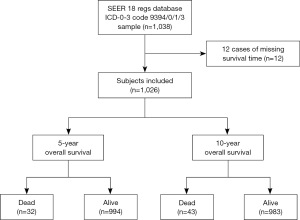
Table 1
| Variables | Values |
|---|---|
| Age (years), median (Q1, Q3) | 41.00 (27.00, 54.00) |
| Gender, n (%) | |
| Female | 437 (42.59) |
| Male | 589 (57.41) |
| Race, n (%) | |
| Black | 50 (4.96) |
| White | 903 (89.49) |
| Other | 56 (5.55) |
| Marital status, n (%) | |
| Single/unmarried | 366 (37.77) |
| Divorced/separated | 66 (6.81) |
| Widowed | 22 (2.27) |
| Married | 515 (53.15) |
| State, n (%) | |
| California | 374 (36.45) |
| Atlanta Georgia | 105 (10.23) |
| New Jersey | 108 (10.53) |
| Washington | 97 (9.45) |
| Other | 342 (33.33) |
| Rural/urban, n (%) | |
| Rural | 108 (10.53) |
| Urban | 918 (89.47) |
| Location, n (%) | |
| Spinal cord | 934 (91.03) |
| Intracranial | 14 (1.36) |
| Horsetail | 60 (5.85) |
| Other | 18 (1.75) |
| Histology, n (%) | |
| Malignant | 26 (2.53) |
| Non-malignant | 1,000 (97.47) |
| Size, n (%) | |
| <3 cm | 329 (55.29) |
| ≥3 cm | 266 (44.71) |
| Surgery, n (%) | |
| No | 61 (5.95) |
| Yes | 965 (94.05) |
| GTR, n (%) | |
| No | 761 (74.17) |
| Yes | 265 (25.83) |
| Radiation, n (%) | |
| No | 857 (83.53) |
| Yes | 169 (16.47) |
| Other cancers, n (%) | |
| No | 1,002 (97.66) |
| Yes | 24 (2.34) |
| Vital status, n (%) | |
| Live | 981 (95.61) |
| Dead | 45 (4.39) |
| Survival months, median (Q1, Q3) | 63.00 (29.00, 105.00) |
GTR, gross total resection.
Epidemiology
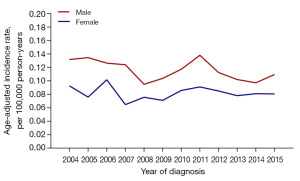
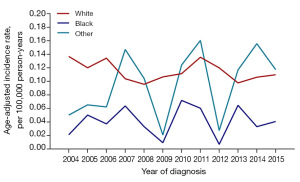
No significant difference was obtained between the incidence of total MPE and the incidence of non-malignant MPE in any year from 2004 to 2015, and no incidence trend of total MPE and non-malignant MPE was shown (Figure 2). According to Figure 3, the incidence of MPE remained low in pediatric populations. Two peaks appeared in the incidence of MPE, with one in people aged 30–34 years and the other in the middle-aged people of 45–49 years old. In 2005, 2007, and 2011, the MPE incidence of male was higher than that of female (P<0.001). No statistically significant difference existed in the incidence between genders in other years, and there was no trend of MPE incidence (Figure 4). Comparable MPE incidences were found between whites and blacks in 2007, 2010, and 2013, and no trend of MPE incidence was demonstrated. In other years, the MPE incidence of whites was greater, as compared with that of blacks (P<0.001) (Figure 5).
Univariate analysis
Univariate Cox analysis evaluated the influence magnitude of different factors on the overall survival of MPE patients (Table 2). For 5-year overall survival, the risk of death in older patients was greater than that in younger patients [older vs. younger: hazard ratio (HR) =1.055, 95% confidence interval (CI): 1.033–1.078, P<0.001]; in contrast to single/unmarried patients, divorced/separated patients exhibited an elevated risk of death (divorced/separated vs. single/unmarried: HR =4.953, 95% CI: 1.664–14.740, P=0.004); the risk of death was higher in patients not receiving surgery versus those undergoing surgery (surgery vs. no surgery: HR =0.249, 95% CI: 0.102–0.605, P=0.002); in contrast to patients who did not undergo radiotherapy, patients receiving radiotherapy had an elevated risk of death (radiotherapy vs. no radiotherapy: HR =3.102, 95% CI: 1.516–6.346, P=0.002). For 10-year overall survival, older age (HR =1.068, 95% CI: 1.047–1.089, P<0.001), divorced/separated and widowed states (divorced/separated vs. single/unmarried: HR =5.808, 95% CI: 2.180–15.477, P<0.001; widowed vs. single/unmarried: HR =7.904, 95% CI: 2.379–26.263), no surgery (surgery vs. no surgery: HR =0.251, 95% CI: 0.116–0.541, P<0.001), and radiotherapy (radiotherapy vs. no radiotherapy: HR =2.547, 95% CI: 1.345–4.820, P=0.004) were significantly negative prognostic factors. Gender, race, tumor location, geographical location, histology, tumor size, GTR, and other cancers did not show significant relationships with the risk of death.
Table 2
| Variables | 5-year overall survival | 10-year overall survival | |||
|---|---|---|---|---|---|
| HR (95% CI) | P | HR (95% CI) | P | ||
| Age | 1.055 (1.033–1.078) | <0.001 | 1.068 (1.047–1.089) | <0.001 | |
| Gender | |||||
| Female | Reference | ||||
| Male | 1.052 (0.520–2.131) | 0.89 | 0.988 (0.539–1.811) | 0.97 | |
| Race | |||||
| Black | Reference | Reference | |||
| White | 0.467 (0.142–1.541) | 0.21 | 0.639 (0.197–2.070) | 0.46 | |
| Other | 0.589 (0.098–3.522) | 0.56 | 0.590 (0.099–3.533) | 0.56 | |
| Marital status | |||||
| Single/unmarried | Reference | Reference | |||
| Divorced/separated | 4.953 (1.664–14.740) | 0.004 | 5.808 (2.180–15.477) | <0.001 | |
| Widowed | 4.366 (0.907–21.017) | 0.07 | 7.904 (2.379–26.263) | <0.001 | |
| Married | 1.690 (0.701–4.075) | 0.24 | 2.047 (0.915–4.576) | 0.08 | |
| State | |||||
| California | Reference | Reference | |||
| Atlanta Georgia | 1.238 (0.399–3.838) | 0.71 | 1.493 (0.619–3.602) | 0.37 | |
| New Jersey | 0.290 (0.038–2.227) | 0.23 | 0.396 (0.091–1.712) | 0.22 | |
| Washington | 0.924 (0.261–3.276) | 0.90 | 0.618 (0.181–2.109) | 0.44 | |
| Other | 1.083 (0.487–2.411) | 0.85 | 0.893 (0.440–1.812) | 0.76 | |
| Rural/urban | |||||
| Rural | Reference | ||||
| Urban | 1.195 (0.364–3.921) | 0.77 | 1.598 (0.494–5.165) | 0.44 | |
| Location | |||||
| Spinal cord | Reference | Reference | |||
| Intracranial | 2.678 (0.364–19.691) | 0.33 | 1.940 (0.266–14.132) | 0.51 | |
| Horsetail | 1.611 (0.490–5.299) | 0.43 | 1.104 (0.341–3.573) | 0.87 | |
| Other | – | 0.99 | – | 0.99 | |
| Histology | |||||
| Malignant | 1.227 (0.168–8.991) | 0.84 | 0.963 (0.133–6.996) | 0.97 | |
| Non-malignant | Reference | Reference | |||
| Size | |||||
| <3 cm | Reference | Reference | |||
| ≥3 cm | 0.695 (0.233–2.072) | 0.51 | 0.556 (0.211–1.462) | 0.23 | |
| Surgery | |||||
| No | Reference | Reference | |||
| Yes | 0.249 (0.102–0.605) | 0.002 | 0.251 (0.116–0.541) | <0.001 | |
| GTR | |||||
| No | Reference | Reference | |||
| Yes | 0.633 (0.273–1.470) | 0.29 | 0.509 (0.247–1.050) | 0.07 | |
| Radiation | |||||
| No | Reference | Reference | |||
| Yes | 3.102 (1.516–6.346) | 0.002 | 2.547 (1.345–4.820) | 0.004 | |
| Other cancers | |||||
| No | Reference | Reference | |||
| Yes | 1.431 (0.195–10.484) | 0.72 | 1.141 (0.157–8.294) | 0.90 | |
HR, hazard ratio; CI, confidence interval; GTR, gross total resection.
In order to more rigorously evaluate the relationship between age and survival, a Cox model with restricted cubic splines was created, which produced an inflection point of the risk function at age 41 years (Figure S1). The use of restricted cubic splines has been widely described as an effective strategy for analyzing the relationship between age and survival outcomes. For 5- or 10-year overall survival, the risk of death in patients older than 41 years was increased, therefore age was the main factor influencing the survival of MPE patients. Additionally, as shown in Figures S2,S3, performing surgery was related to the improvement of overall survival (P<0.001), and the application of radiotherapy was correlated with reduced overall survival (P<0.001).
Multivariate analysis
Age, gender, marital status, histological type, surgery, radiotherapy, and other cancers were adjusted in a multivariate Cox regression model (Table 3). Regarding 5-year overall survival, the older the patients, the higher the risk of death (older vs. younger: HR =1.068, 95% CI: 1.041–1.096, P<0.001); the risk of death was increased in widowed patients versus single/unmarried patients (widowed vs. single/unmarried: HR =3.199, 95% CI: 1.215–8.421, P=0.02); patients undergoing surgery showed a lower risk of death than those who did not have surgery (HR =0.277, 95% CI: 0.110–0.699, P=0.007); in contrast to patients without radiotherapy, the risk of death for patients receiving radiotherapy was raised (HR =4.645, 95% CI: 2.179–9.902, P<0.001). The results of 10-year overall survival presented that older age (HR =1.081, 95% CI: 1.056–1.107, P<0.001), widowed (widowed vs. single/unmarried: HR =3.257, 95% CI: 1.410–7.523, P=0.006), no surgery (surgery vs. no surgery: HR =0.281, 95% CI: 0.125–0.631, P=0.002), and radiotherapy (radiotherapy vs. no radiotherapy: HR =4.095, 95% CI: 2.088–8.031, P<0.001) were in significant associations with a greater risk of death for MPE patients.
Table 3
| Variables | 5-year overall survival | 10-year overall survival | |||
|---|---|---|---|---|---|
| HR (95% CI) | P | HR (95% CI) | P | ||
| Age | 1.068 (1.041–1.096) | <0.001 | 1.081 (1.056–1.107) | <0.001 | |
| Gender | |||||
| Female | 0.779 (0.364–1.669) | 0.52 | 0.716 (0.370–1.107) | 0.32 | |
| Male | Reference | Reference | |||
| Marital status | |||||
| Single/unmarried | Reference | Reference | |||
| Divorced/separated | 1.557 (0.603–4.02) | 0.36 | 1.589 (0.675–3.741) | 0.29 | |
| Widowed | 3.199 (1.215–8.421) | 0.02 | 3.257 (1.410–7.523) | 0.006 | |
| Married | 0.810 (0.153–4.289) | 0.80 | 1.068 (0.310–3.682) | 0.92 | |
| Histology | |||||
| Malignant | 1.030 (0.125–8.508) | 0.98 | 0.933 (0.120–7.259) | 0.95 | |
| Non-malignant | Reference | Reference | |||
| Surgery | |||||
| No | Reference | Reference | |||
| Yes | 0.277 (0.110–0.699) | 0.007 | 0.281 (0.125–0.631) | 0.002 | |
| Radiation | |||||
| No | Reference | Reference | |||
| Yes | 4.645 (2.179–9.902) | <0.001 | 4.095 (2.088–8.031) | <0.001 | |
| Other cancers | |||||
| No | Reference | Reference | |||
| Yes | 0.819 (0.097–6.888) | 0.85 | 0.490 (0.060–4.012) | 0.51 | |
HR, hazard ratio; CI, confidence interval.
Multivariate analysis for non-malignant MPE patients
Through controlling for age, gender, marital status, surgery, radiotherapy, and other cancers in multivariate Cox regression analysis, for both 5- and 10-year overall survival, it was revealed that older age (older vs. younger: 5-year overall survival: HR =1.067, 95% CI: 1.039–1.096, P<0.001; 10-year overall survival: HR =1.081, 95% CI: 1.055–1.107, P<0.001), widowed (widowed vs. single/unmarried: 5-year overall survival: HR =2.995, 95% CI: 1.066–8.191, P=0.04; 10-year overall survival: HR =3.058, 95% CI: 1.282–7.296, P=0.01), no surgery (surgery vs. no surgery: 5-year overall survival: HR =0.281, 95% CI: 0.111–0.712, P=0.007; 10-year overall survival: HR =0.283, 95% CI: 0.126–0.635, P=0.002), and radiotherapy (radiotherapy vs. no radiotherapy: 5-year overall survival: HR =5.049, 95% CI: 2.348–10.856, P<0.001; 10-year overall survival: HR =4.355, 95% CI: 2.211–8.578, P<0.001) were significantly independent adverse factors for overall survival (Table 4).
Table 4
| Variables | 5-year overall survival | 10-year overall survival | |||
|---|---|---|---|---|---|
| HR (95% CI) | P | HR (95% CI) | P | ||
| Age | 1.067 (1.039–1.096) | <0.001 | 1.081 (1.055–1.107) | <0.001 | |
| Gender | |||||
| Female | 0.768 (0.354–1.669) | 0.51 | 0.706 (0.361–1.379) | 0.31 | |
| Male | Reference | Reference | |||
| Marital status | |||||
| Single/unmarried | Reference | Reference | |||
| Divorced/separated | 1.518 (0.584–3.942) | 0.39 | 1.566 (0.663–3.699) | 0.31 | |
| Widowed | 2.955 (1.066–8.191) | 0.04 | 3.058 (1.282–7.296) | 0.01 | |
| Married | 0.806 (0.146–4.440) | 0.80 | 1.086 (0.309–3.817) | 0.90 | |
| Surgery | |||||
| No | Reference | Reference | |||
| Yes | 0.281 (0.111–0.712) | 0.007 | 0.283 (0.126–0.635) | 0.002 | |
| Radiation | |||||
| No | Reference | Reference | |||
| Yes | 5.049 (2.348–10.856) | <0.001 | 4.355 (2.211–8.578) | <0.001 | |
| Associated with other cancers | |||||
| No | Reference | Reference | |||
| Yes | 1.090 (0.127–9.337) | 0.94 | 0.576 (0.069–4.826) | 0.61 | |
HR, hazard ratio; CI, confidence interval; MPE, myxopapillary ependymoma.
Discussion
It is believed that the datasets acquired from the National Cancer Institute can provide the most comprehensive and representative data on cancer diagnosis, treatment and survival. To the best of our knowledge, this is the largest study with 1,026 MPE patients. Our analysis emphasized an overall social demographic change and influencing factors of survival in MPE patients from 2004 to 2015. More specifically, it found that the incidence of MPE and non-malignant MPE remained largely steady from 2004 to 2015. There were two peaks in MPE incidence: one was at 30–34 years old and the other was at 45–49 years old. In 2005, 2007, and 2011, the incidence of male was higher than that of female. Compared with blacks, whites had a higher incidence in years other than 2007, 2010, and 2013. Multivariate analysis revealed that age, marital status, surgery, and radiotherapy were factors affecting both 5- and 10-year overall survival of MPE patients.
Age has potential impacts on overall survival among patients with MPE. This may be attributed to the gradual emergence of related clinical signs, or it may be caused by the aggressive behavior of the tumor (12,13). Due to this finding, preoperative or postoperative screening for metastatic disease is performed for cerebrospinal fluid cytology and complete central nervous system (CNS) imaging is recommended as a routine protocol for patients with MPE (14). Wang et al. (15) uncovered that marital status was independent prognostic factors for overall survival in primary intramedullary ependymoma. This was true for intracranial grade II/III ependymoma in adults (16). Further, we discovered that widowed MPE patients had a greater risk of death than single/unmarried ones.
Surgery is the standard treatment for MPE. It provides tissues for histological diagnosis, re-establishes cerebrospinal fluid flow, and allows tumor reduction or complete removal. For adults and children older than 3 years of age, 5-year PFS can reach 50–60% (17,18). Batich and others confirmed that after diagnosis of MPE, surgery should be performed as soon as possible. The less the tumor compresses and erodes the spinal cord, the less damage is brought to the neurological function (19). According to a case report that neurological function is preserved and restored in 79% of patients after MPE surgery (20). The overall resection rate of tumors depends on their location. It can reach 100% in the roof of the fourth ventricle, 86% in the spinal cord, and 54% in the cauda equina (21). Although not supported by large-sample data, many studies have shown that patients with total MPE resection have better survival and prognosis (19-21). The present study revealed that the acceptance of surgery was an important independent predictor of overall survival. It has been illustrated that since surgical complications are of concern, the extent of the operation is an essential issue (19,22).
Previous research found that those initially with radiotherapy and patients who received subtotal resection (STR) had a greater rate of tumor development and recurrence (23). However, it is believed that radiotherapy has no auxiliary effects as the effects are uncertain at present, and there is a risk of spinal cord injury. Moreover, after radiotherapy, normal tissue structure will be damaged, and subsequent surgery will become difficult if the tumor recurs (24). Vitanovics et al. (25) reviewed MPE, remarking that MPE is low-grade neoplasm with favorable prognosis in the absence of adjuvant radiotherapy. Similarly, Jahanbakhshi et al. (26) and Strojnik et al. (27) both opposed radiotherapy following subtotal resection. Likewise, our findings showed that radiation therapy may be associated with a reduction of 5- and 10-year overall survival. The use of radiotherapy as additional treatment after surgical resection should be carefully considered.
There were several strengths and limitations in this study. The study enrolled 1,026 MPE cases from 2004 to 2015 from the SEER database. This latest research had a large time span and covered a wide range of ages, making it easy to analyze the epidemiological characteristics of MPE. The limitations are acknowledged as below. The SEER database focuses on the American population, indicating that the findings may not apply to other populations. Besides, some important covariates were not measured, such as radiation dosage, the extent of surgical resection, type of comorbidities and MPE progression, so this remaining confusion may underestimate our results.
Conclusions
Age, gender and race were associated with MPE incidence. Age, marital status, surgery, and radiotherapy were all influential factors for 5- and 10-year overall survival of patients with MPE. More researches on the epidemiology and prognostic factors of MPE are warranted to verify and complement our findings.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://tcr.amegroups.com/article/view/10.21037/tcr-24-757/rc
Peer Review File: Available at https://tcr.amegroups.com/article/view/10.21037/tcr-24-757/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tcr.amegroups.com/article/view/10.21037/tcr-24-757/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). Since the data in this study are de-identified publicly available data, this retrospective cohort study was exempted from the institutional ethical committee by Jiangmen Central Hospital.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Limaiem F, Das JM. Myxopapillary Ependymoma. 2023. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing.
- Louis DN, Perry A, Wesseling P, et al. The 2021 WHO Classification of Tumors of the Central Nervous System: a summary. Neuro Oncol 2021;23:1231-51. [Crossref] [PubMed]
- Bertrand KC, Klimo P. Recent Advancements in Ependymoma: Challenges and Therapeutic Opportunities. Pediatr Neurosurg 2023;58:307-12. [Crossref] [PubMed]
- Khan NR, VanLandingham M, O'Brien T, et al. Primary Seeding of Myxopapillary Ependymoma: Different Disease in Adult Population? Case Report and Review of Literature. World Neurosurg 2017;99:812.e21-6. [Crossref] [PubMed]
- Liu T, Yang C, Deng X, et al. Clinical characteristics and surgical outcomes of spinal myxopapillary ependymomas. Neurosurg Rev 2020;43:1351-6. [Crossref] [PubMed]
- Vongsfak J, Jetjumnong C, Cullen J. Image report: Extensive disseminated thoracolumbosacral myxopapillary ependymoma. Surg Neurol Int 2020;11:297. [Crossref] [PubMed]
- Ramkumar S, Wanniang CA, Wahlang AR, et al. Subcutaneous Sacro Coccygeal Myxopapillary Ependymoma: A Case Report and a Comprehensive Review of the Literature Reappraising Its Current Diagnostic Approach and Management. Cureus 2021;13:e14931. [Crossref] [PubMed]
- Chai YH, Jung S, Lee JK, et al. Ependymomas: Prognostic Factors and Outcome Analysis in a Retrospective Series of 33 Patients. Brain Tumor Res Treat 2017;5:70-6. [Crossref] [PubMed]
- Merchant TE, Bendel AE, Sabin ND, et al. Conformal Radiation Therapy for Pediatric Ependymoma, Chemotherapy for Incompletely Resected Ependymoma, and Observation for Completely Resected, Supratentorial Ependymoma. J Clin Oncol 2019;37:974-83. [Crossref] [PubMed]
- Bates JE, Choi G, Milano MT. Myxopapillary ependymoma: a SEER analysis of epidemiology and outcomes. J Neurooncol 2016;129:251-8. [Crossref] [PubMed]
- Duggan MA, Anderson WF, Altekruse S, et al. The Surveillance, Epidemiology, and End Results (SEER) Program and Pathology: Toward Strengthening the Critical Relationship. Am J Surg Pathol 2016;40:e94-e102. [Crossref] [PubMed]
- Lee JC, Sharifai N, Dahiya S, et al. Clinicopathologic features of anaplastic myxopapillary ependymomas. Brain Pathol 2019;29:75-84. [Crossref] [PubMed]
- Omerhodžić I, Pojskić M, Rotim K, et al. MYXOPAPILLARY EPENDYMOMA OF THE SPINAL CORD IN ADULTS: A REPORT OF PERSONAL SERIES AND REVIEW OF LITERATURE. Acta Clin Croat 2020;59:329-37. [PubMed]
- Mastorakos P, Pomeraniec IJ, Shah S, et al. Mobile Myxopapillary Ependymoma with Associated Filum Terminale Cyst. World Neurosurg 2020;139:337-42. [Crossref] [PubMed]
- Wang C, Yuan X, Zuo J. Individualized Prediction of Overall Survival for Primary Intramedullary Spinal Cord Grade II/III Ependymoma. World Neurosurg 2020;143:e149-56. [Crossref] [PubMed]
- Deng X, Zhang X, Yang L, et al. Personalizing age-specific survival prediction and risk stratification in intracranial grade II/III ependymoma. Cancer Med 2020;9:615-25. [Crossref] [PubMed]
- Schiavello E, Biassoni V, Antonelli M, et al. Pediatric extraspinal sacrococcygeal ependymoma (ESE): an Italian AIEOP experience of six cases and literature review. Childs Nerv Syst 2018;34:1291-8. [Crossref] [PubMed]
- Borges LF. Spinal intramedullary ependymoma: surgical approaches and outcome. J Neurosurg Sci 2018;62:51-62. [PubMed]
- Batich KA, Riedel RF, Kirkpatrick JP, et al. Recurrent Extradural Myxopapillary Ependymoma With Oligometastatic Spread. Front Oncol 2019;9:1322. [Crossref] [PubMed]
- Lien BV, Brown NJ, Himstead AS, et al. Surgical management of a rare myxopapillary ependymoma of the gluteal region: A case report. Surg Neurol Int 2021;12:130. [Crossref] [PubMed]
- Aftahy AK, Barz M, Krauss P, et al. Intraventricular neuroepithelial tumors: surgical outcome, technical considerations and review of literature. BMC Cancer 2020;20:1060. [Crossref] [PubMed]
- Mewada TB, Bishnoi IH, Singh H, et al. Occipital Intraparenchymal Myxopapillary Ependymoma: Case Report and Literature Review. Asian J Neurosurg 2017;12:731-4. [Crossref] [PubMed]
- Rudà R, Reifenberger G, Frappaz D, et al. EANO guidelines for the diagnosis and treatment of ependymal tumors. Neuro Oncol 2018;20:445-56. [Crossref] [PubMed]
- Fonseca L, Cicuendez M, Martínez-Ricarte F, et al. A rare case of an intramedullary metastasis of a myxopapillary ependymoma. Surg Neurol Int 2019;10:83. [Crossref] [PubMed]
- Vitanovics D, Áfra D, Nagy G, et al. Symptomatic subependymomas of the ventricles. Review of twenty consecutive cases. Ideggyogy Sz 2014;67:415-9. [PubMed]
- Jahanbakhshi A, Najafi M, Jafari F, et al. Adjunctive treatment of myxopapillary ependymoma. Oncol Rev 2021;15:518. [Crossref] [PubMed]
- Strojnik T, Bujas T, Velnar T. Invasive myxopapillary ependymoma of the lumbar spine: A case report. World J Clin Cases 2019;7:1142-8. [Crossref] [PubMed]




