
Dephosphorylation-related signature predicts the prognosis of papillary renal cell carcinoma
Highlight box
Key findings
• We identified nine genes associated with dephosphorylation that were differentially expressed in papillary renal cell carcinoma (PRCC) tumor tissues, and constructed a prognostic model.
What is known and what is new?
• Up to now, mRNAs, lncRNAs and CENP-A, among others, have been used as independent prognostic factors in PRCC patients, but there is no research study on the role of dephosphorylation related genes in predicting the prognosis of PRCC.
• We conducted an in-depth analysis of the expression levels of genes related to PRCC dephosphorylation and found that these genes were differentially expressed in PRCC tumor tissues, thus constructing and validating a valid and reliable prognostic model that accurately predicts the prognosis of PRCC patients.
What is the implication, and what should change now?
• A prognostic model constructed based on the differential expression of nine dephosphorylation-related genes in PRCC tumor tissues can accurately predict the prognosis of PRCC patients. This study has great potential value for future research, and its clinical significance, utility, and accuracy need to be further investigated and validated.
Introduction
Kidney cancer is one of the most common malignancies worldwide with an increasing incidence rate (1). In 2020, there were approximately 431,288 new cases of kidney cancer worldwide, resulting in 179,368 deaths (2). The most common subtype of kidney cancer is renal cell carcinoma (RCC), including papillary RCC (PRCC), chromophobe RCC (ChRCC) and clear cell RCC (ccRCC) (3), of which PRCC being the second most prevalent subtype, accounting for 10 to 15% of all cases (4). Unfortunately, distant metastasis is found in approximately 30% of patients with RCC at initial diagnosis, resulting in a poor prognosis (5-year relative survival rate of 13.9%) (5,6). PRCC is the most heterogeneous type of RCC (7). ChRCC and ccRCC usually have a positive outcome (8), while, PRCC have a poor clinical prognosis in clinical studies. Therefore, identifying reliable predictors associated with PRCC prognosis will help in the treatment, diagnosis and prognostic assessment of this disease.
Post-translational modifications (PTM) is involved in a number of cellular activities (9), of which phosphorylation-dephosphorylation is the most common and critical one (10). Phosphorylation-dephosphorylation is catalyzed by kinases and phosphatases (11), respectively, which is a fully reversible process (12). This reversible process is essential for cell cycle control and leads to large changes in protein conformation, which can alter protein function and coordinate multiple functions such as cell metabolism, gene transcription and translation, signaling, growth, differentiation, and apoptosis (13,14). Phosphate-dephosphorylation acts as a molecular switch through which many enzymes and receptors are activated and inactivated. Aberrant phosphorylation is a major cause of alterations in many structural, functional and regulatory proteins in disease states, of which many studies in recent years have revealed a role in cancer manifestations (15-18). Dysregulation in the phosphorylation-dephosphorylation cascade of many signaling pathways is involved in the cell cycle such as tyrosine kinases, mitogen-activated protein (MAP) kinases, calmodulin-linked protein complexes, and cell cycle protein-dependent kinases has also been shown to be a form of various types of cancer (15).
Alterations in the phosphorylated proteome have been shown in numerous studies to affect gastrointestinal mesenchymal tumors (19), lung cancer (20,21), hematological malignancies (22,23), breast cancer (24), pancreatic cancer (25) and prostate cancer (26), and have led to the development of specific inhibitors that provide value for cancer therapy. In recent years, many phosphatases that catalyze dephosphorylation have been described as oncogenes and tumor suppressors (27-29), and more may be confirmed as oncogenes or tumor suppressors in the coming years. Therefore, we hypothesized that dephosphorylation-related genes may be valuable diagnostic and therapeutic indicators for PRCC patients. In this study, we conducted a thorough analysis of the expression levels of genes associated with PRCC dephosphorylation to construct and validate a feature set that predicts the prognosis of PRCC sufferers. We present this article in accordance with the TRIPOD reporting checklist (available at https://tcr.amegroups.com/article/view/10.21037/tcr-24-669/rc).
Methods
Data acquisition
All primitive data, including fragments per kilobase of exon model per million mapped fragments (FPKM) data and clinical information of PRCC patients were acquired from The Cancer Genome Atlas (TCGA) database. The messenger RNA (mRNA) expression data in FPKM format were converted into transcripts per kilobase of exon model per million mapped reads (TPM) format for subsequent analysis.
Selection of dephosphorylation-related genes
We selected 425 dephosphorylation related genes (GOBP_DEPHOSPHORYLATION.v.7.5.1) from the molecular signature database (MSigDB). Then 8 genes not included in TCGA were deleted. Finally, 417 dephosphorylation-related genes were obtained as candidate genes for subsequent analysis.
Survival analysis and risk-prognosis model construction
Gene expression data and clinical data were used to construct an effective prognostic prediction model of PRCC patients. Lasso regression algorithm (LASSO) was used to eliminate the prediction genes with positive correlation and avoid over fitting. Subsequently, multivariate Cox regression was performed on genes associated with prognostic risk to construct the risk-prognosis model. The risk score was calculated by multiplying the multivariate Cox proportional hazards regression coefficients by their gene expression levels for each patient. The formula for risk score:
Where Coef represents coefficients, and X represents the gene expression level.
A total of 142 patients were assigned to the high-risk group and 143 to the low-risk group, based on the median risk score. The survival difference between the two risk groups was analyzed by Kaplan-Meier survival analysis and log-rank test.
Independent prognostic analysis was performed for risk scores and clinical parameters
To determine whether risk score is an independent prognostic factor, risk scores and traditional clinical variables (age, gender, stage, cancer status, TNM stage, new event) were included as likely independent prognostic factors. Univariate and multivariate Cox regression were used to assess the association of these factors with patient prognosis. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013).
Statistical analysis
Data were processed using the Perl programming language. Statistical analyses were performed with R software (version 4.0.3). P<0.05 was considered statistically significant.
Results
Construction of a signature model of dephosphorylation related genes associated with prognostic
The 425 dephosphorylation-related genes were selected from the MSigDB, and the 8 genes not included in TCGA were deleted to get 417 dephosphorylation related candidate genes (Figure 1). LASSO was employed to analyze these dephosphorylation-related genes associated with survival. As a result, we found 14 genes (ADORA1, CDC25C, CDCA2, CDKN3, CRY2, MYH6, PLPPR4, PPA2, PPP2R2B, PPP6R2, PTP4A1, PTPRQ, THNSL2, and TPTE2) significantly associated with prognosis in ccRCC patients and proceeded to the next analysis (Figure 2).


A prognostic nine-gene signature panel constructed in PRCC patients
Multivariate Cox regression analysis was performed for the 14 prognostic genes using the R software survival package, as shown in Table 1, 9 genes (ADORA1, CDKN3, CRY2, PLPPR4, PPA2, PPP2R2B, PPP6R2, PTP4A1, TPTE2) were ultimately identified to construct the gene signature panel. The following formula was used to calculate the risk score for each patient: Risk score = (0.3556 × ADORA1) + (0.6266 × CDKN3) + (−0.4708 × CRY2) + (0.5841 × PLPPR4) + (−0.9527 × PPA2) + (0.4254 × PPP2R2B) + (−0.5788 × PPP6R2) + (0.4000 × PTP4A1) + (1.6784 × TPTE2) (Table 1). ADORA1, CDKN3, PLPPR4, PPP2R2B, PTP4A1, TPTE2 were considered to be high-risk gene factors and, conversely, CRY2, PPA2, PPP6R2 were considered protective genes. Based on the median risk score, 142 of the 285 PRCC patients were classified into a high-risk group, while the remaining 143 patients were in the low-risk group.
Table 1
| mRNAs | Ensemble ID | Chromosome location | β (Cox) | HR (95% CI) | P |
|---|---|---|---|---|---|
| ADORA1 | ENSG00000163485 | Chr1: 203,090,654−203,167,405 | 0.3556 | 1.427 (1.113–1.829) | 0.005 |
| CDKN3 | ENSG00000100526 | Chr14: 54,396,849−54,420,218 | 0.6266 | 1.871 (1.275–2.746) | 0.001 |
| CRY2 | ENSG00000121671 | Chr11: 45,847,118−45,883,248 | −0.4708 | 0.625 (0.354–1.101) | 0.10 |
| PLPPR4 | ENSG00000117600 | Chr1: 99,264,292−99,309,590 | 0.5841 | 1.793 (1.001–3.212) | 0.049 |
| PPA2 | ENSG00000138777 | Chr4: 105,369,077−105,474,067 | −0.9527 | 0.386 (0.198–0.750) | 0.005 |
| PPP2R2B | ENSG00000156475 | Chr5: 146,580,742−147,084,784 | 0.4254 | 1.53 (0.916–2.555) | 0.10 |
| PPP6R2 | ENSG00000100239 | Chr22: 50,343,304−50,445,090 | −0.5788 | 0.561 (0.311–1.011) | 0.054 |
| PTP4A1 | ENSG00000112245 | Chr6: 63,521,746−63,583,588 | 0.4000 | 1.492 (0.967–2.301) | 0.07 |
| TPTE2 | ENSG00000132958 | Chr13: 19,422,876−19,561,625 | 1.6784 | 5.357 (1.434–20.010) | 0.01 |
mRNAs, messenger RNAs; HR, hazard ratio; CI, confidence interval.
Evaluation and validation of prognostic models
Risk score distributions and scatter plots showed that patients with low-risk scores had better survival outcomes than the high-risk score group of ccRCC patients (Figure 3A,3B). The Kaplan-Meier analysis further confirmed the reliability of the prognosis model. The low-risk group had a significantly better prognosis than the high-risk group. The survival rate of low risk ccRCC patients was significantly higher than that of high risk ccRCC patients (Figure 3C). To assess the role of this signature in the diagnosis, we performed receiver operating characteristic (ROC) curve analysis. It showed that the area under curve (AUC) value of this model was 0.833, indicating that the model for these nine genes had good predictive power and high accuracy in predicting patient survival (Figure 3D). The heat map showed significant differences in the expression of nine genes between the two groups, with higher levels of expression of risk factors (ADORA1, CDKN3, PLPPR4, PPP2R2B, PTP4A1, TPTE2) in PRCC patients with high-risk scores, and protective factors (CRY2, PPA2, PPP6R2) expression levels were lower in PRCC patients with high-risk scores (Figure 4A,4B).


A Chi-squared test was used to compare the differences between clinical parameters in patients with different risk scores (Table 2), and the results showed that the type of American Joint Committee on Cancer (AJCC) stage, tumor (T) stage, node (N) stage, metastasis (M) stage, new events, cancer status were associated with their risk scores.
Table 2
| Variables | Total (n=285) | High risk (n=142) | Low risk (n=143) | P |
|---|---|---|---|---|
| Age (years) | 61.62±11.87 | 60.84±12.6 | 62.39±11.08 | 0.27 |
| Gender | ||||
| Female | 76 (27) | 47 (33) | 29 (20) | |
| Male | 209 (73) | 95 (67) | 114 (80) | |
| Stage | <0.001 | |||
| 1 | 177 (66) | 70 (52) | 107 (81) | |
| 2 | 25 (9) | 11 (8) | 14 (11) | |
| 3 | 50 (19) | 40 (30) | 10 (8) | |
| 4 | 15 (6) | 14 (10) | 1 (1) | |
| Cancer status | Cancer status | Cancer status | Cancer status | |
| Tumor free | 216 (85) | 95 (75) | 121 (95) | |
| With tumor | 38 (15) | 31 (25) | 7 (5) | |
| T | <0.001 | |||
| 1 | 197 (70) | 82 (59) | 115 (82) | |
| 2 | 36 (13) | 18 (13) | 18 (13) | |
| 3 | 46 (16) | 38 (27) | 8 (6) | |
| 4 | 2 (1) | 2 (1) | 0 (0) | |
| N | N | N | N | |
| 0 | 143 (85) | 63 (71) | 80 (100) | |
| 1 | 23 (14) | 23 (26) | 0 (0) | |
| 2 | 3 (2) | 3 (3) | 0 (0) | |
| M | M | M | M | |
| 0 | 203 (96) | 103 (93) | 100 (99) | |
| 1 | 9 (4) | 8 (7) | 1 (1) | |
| New event | New event | New event | New event | |
| No | 225 (79) | 100 (70) | 125 (87) | |
| Yes | 60 (21) | 42 (30) | 18 (13) | |
Data are presented as mean ± standard deviation or n (%).
The cBioPortal database was used to analyze the genetic alterations in PRCC for the selected 9 genes. There were 280 of 285 patients with mutation data. Only 10 of 280 cases had alterations in all query genes, including 4 (1.43%) cases of mutation, 4 (1.43%) cases of amplification, and 2 (0.71%) cases of homozygously deleted (HOMDEL) (Table 3). The heatmap of the alteration of these 9 genes showed that: the PPP6R2 gene had a change rate of 1.43%, including 2 cases of mutation, 1 case of amplification and 1 case of HOMDEL; the PTP4A1 gene had a change rate of 0.71%, including 1 case of HOMDEL and 1 case of amplification; the TPTE2 gene had a change rate of 0.71%, including 1 case of amplification and 1 case of mutation; the PLPPR4 gene had a change rate of 0.36%, including 1 case of mutation; the PPP2R2B gene had a change rate of 0.36%, including 1 case of amplification (Figure 5, Table 4).
Table 3
| Alteration | Number of cases | Frequency (%) |
|---|---|---|
| Mutation | 4 | 1.43 |
| Amplification | 4 | 1.43 |
| HOMDEL | 2 | 0.71 |
| Total | 10 | 3.57 |
HOMDEL, homozygously deleted.

Table 4
| mRNAs | No alterations | Genetic alteration | Altered/profiled (%) | ||
|---|---|---|---|---|---|
| Mutation | Amplification | HOMDEL | |||
| PPP6R2 | 276 | 2 | 1 | 1 | 1.43 |
| PTP4A1 | 278 | 0 | 1 | 1 | 0.71 |
| TPTE2 | 278 | 1 | 1 | 0 | 0.71 |
| PLPPR4 | 279 | 1 | 0 | 0 | 0.36 |
| PPP2R2B | 279 | 0 | 1 | 0 | 0.36 |
| ADORA1 | 280 | 0 | 0 | 0 | 0.00 |
| CDKN3 | 280 | 0 | 0 | 0 | 0.00 |
| CRY2 | 280 | 0 | 0 | 0 | 0.00 |
| PPA2 | 280 | 0 | 0 | 0 | 0.00 |
PRCC, papillary renal cell carcinoma; mRNAs, messenger RNAs; HOMDEL, homozygously deleted.
Independent prognostic value of the 9-gene signature
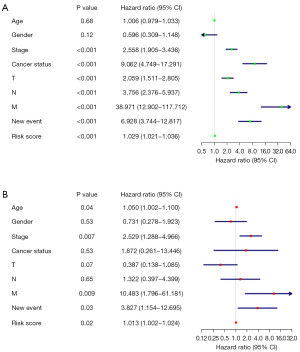
We integrated risk scores and clinical parameters including gender, stage, age, cancer status, T stage, N stage, M stage and new event, using univariate Cox regression analysis and multivariate Cox regression analysis to evaluate whether the 9-gene signature could be an independent prognostic predictor for the outcome of PRCC patients’ survival. Univariate Cox regression analysis showed that the risk score of the 9-gene signature was an independent prognostic indicator significantly associated with patient survival [hazard ratio (HR) =1.029, 95% confidence interval (CI): 1.021–1.036, P<0.001, Figure 6A]. After confounders adjustment, the results of multivariate Cox regression analysis showed that risk score remained as an independent indicator of prognosis (HR =1.013, 95% CI: 1.002–1.024, P=0.02, Figure 6B). As well, it was found that age (P=0.04), stage (P=0.007), new event (P=0.03) and M stage (P=0.009) were also independent factors of prognosis.
Discussion
RCC is a common malignance of the genitourinary system (30), accounting for about 2–3% of adult malignancies (31). Researches have shown that the mortality rate of RCC remains steadily increasing despite early diagnosis and aggressive intervention (32). PRCC is the second most common subtype of RCC, in which most patients diagnosed with PRCC are already in advanced stages (33). Although some patients can receive early surgical treatment, however, the recurrence or metastasis rate of surgically resected patients is as high as 40% with poor prognosis (34). PRCC remains a clinical challenge due to its unclear oncogenic mechanism, high histological specificity and absence of evidence for early diagnosis and treatment. Most studies on RCC in the past have focused on ccRCC, with relatively little work on PRCC (35-38). There were only a few researches focused on mRNAs or long non-coding RNAs (lncRNAs) to predict survival outcomes. For example, Yin et al. identified a five ferroptosis-related genes signature that could be used to predict the prognosis of PRCC patients (39). Gao et al. found that five mRNAs were an independent prognostic factor in PRCC patients (40). Lan et al. used 7 lncRNAs to construct a prognostic model for PRCC (6). Li et al. revealed that centromere protein-A (CENP-A) may be a biomarker for predicting therapeutic outcome and prognosis in PRCC cases (41). However, up to now, there is no research study on the role of dephosphorylation related genes in predicting the prognosis of PRCC.
PTM are involved in a number of pathological and physiological processes. Phosphorylation modifications are one of the most important covalent modifications in living organisms, and the dephosphorylation regulates all life activities including evolution, cell proliferation, signal transduction, differentiation, neural activity, apoptosis, tumorigenesis and muscle contraction. Mutation of kinases or phosphatases causes aberrant phosphorylation that leads to abnormalities in correlated signaling pathways which are strongly associated with cancer development. In recent years, catalyzed dephosphorylated phosphatases have been shown to not only be tumor suppressors but also to function as oncogenes, and more phosphatases may be confirmed as tumor suppressors or oncogenes (27,42). In this study, we established the first prognostic model based on dephosphorylation associated genes in PRCC patients. We identified nine genes associated with survival in PRCC patients, namely ADORA1, CDKN3, CRY2, PLPPR4, PPA2, PPP2R2B, PPP6R2, PTP4A1, and TPTE2. Risk score formulae were constructed by Cox regression model. According to the risk score, the patients were divided into low-risk and high-risk groups, and the results showed that low-risk patients had significantly longer survival time.
Among the nine genes associated with PRCC prognosis, some have been reported to be expressed in cancer or other diseases, but none have been examined in PRCC. For example, ADORA1 mRNA was highly expressed in hepatocellular carcinoma (43), papillary thyroid carcinoma (44), melanoma, ovarian cancer (45) and nasopharyngeal carcinoma, and it boosts cancer cell growth and inhibits cell apoptosis. While metformin (46) and omega 3 unsaturated fatty acids (47) exert anti-tumor effects by upregulating ADORA1 to induce apoptosis in colorectal and gastric cancer cells, respectively. The expression of PPA2 was lower in ccRCC tissues than normal renal tissues significantly, and its downregulation was associated with poor prognosis of ccRCC. It was shown that PPA2 is an independent prognostic factor for ccRCC patients. The result of Gene Set Enrichment Analysis (GSEA) indicated that the low expression of PPA2 may be associated with epithelial-mesenchymal transition in ccRCC (48). PPP2R2B is a regulatory subunit of protein phosphatase 2A. Downregulation of PPP2R2B levels plays an important role in both development and progression of breast cancer, and findings suggest that PPP2R2B is closely associated with immunosuppressive genes (49). It improves the anti-tumor function of T lymphocytes and inhibits immune evasion, which is associated with poor clinical outcomes and resistance to human epidermal growth factor receptor 2 (HER2) targeted therapy (50). TPTE2 encodes a homolog of PTEN tumor suppressor protein, which is involved in carcinogenesis during the transformation of cirrhosis to hepatocellular carcinoma (51). PTP4A1, an isoform of regenerative liver phosphatase (protein tyrosine phosphatase), is highly expressed in most cancers (metastatic colorectal cancer, cervical cancer, non-small cell lung cancer) and plays a pathogenic role in cancer metastasis and progression (52). Cui et al. constructed a panel of four tumor-associated antigens (containing anti-PTP4A1) to help distinguish gastric cancer patients from healthy patients (53). CRY2 is a biological clock protein involved in the cell cycle. Fang et al. observed that colorectal cancer patients with CRY2 overexpression had lower survival rates and were resistant to chemotherapy. Knockdown of CRY2 increases the sensitivity of colorectal cancer cells to oxaliplatin (54). Mao et al. identified that the decreased CRY2 expression in breast cancer tissues was associated with higher tumor grade and shorter overall survival time. Research shows that CRY2 can be used as a biomarker for breast cancer prognosis, progression and susceptibility (55). Liu et al. found that CRY2 acts as a key role in the clinical prognosis of RCC and is closely associated with apoptosis, cell cycle and immune cell regulation (56). Yu et al. showed that CRY2 may be an anti-oncogene in osteosarcoma and inhibits the migration and proliferation of osteosarcoma cells (57). Cen et al. discovered that the circSDHC/miR-127-3p/CDKN3/E2F1 axis has a major role in RCC progression (58). CDKN3 could be an independent prognostic factor for cervical cancer, ovarian cancer, nasopharyngeal cancer, lung adenocarcinoma, and colorectal cancer. The results of these studies verified the reliability of the gene signatures. None of the above 7 genes have been reported in any PRCC studies. The remaining 2 genes (PLPPR4, PPP6R2) have not been reported to be prognostic in any relevant tumor studies. All of these genes will provide new insights for future studies to explore new molecular targets and biomarkers for PRCC.
Conclusions
In summary, we identified nine genes (ADORA1, CDKN3, CRY2, PLPPR4, PPA2, PPP2R2B, PPP6R2, PTP4A1, TPTE2) associated with dephosphorylation that were differentially expressed in PRCC tumor tissues, and constructed a prognostic model. Following validation, it was shown to be a valid and reliable prognostic indicator that could predict the prognosis of PRCC patients accurately. This study has a lot of potential value for future studies, and further research and validation are needed to be performed for its clinical significance, practicality and accuracy.
Acknowledgments
The authors thank Longyang Jiang for his help with data analysis.
Funding: None.
Footnote
Reporting Checklist: The authors have completed the TRIPOD reporting checklist. Available at https://tcr.amegroups.com/article/view/10.21037/tcr-24-669/rc
Peer Review File: Available at https://tcr.amegroups.com/article/view/10.21037/tcr-24-669/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tcr.amegroups.com/article/view/10.21037/tcr-24-669/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Siegel RL, Miller KD, Fuchs HE, et al. Cancer statistics, 2022. CA Cancer J Clin 2022;72:7-33. [Crossref] [PubMed]
- Bray F, Laversanne M, Sung H, et al. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2024;74:229-63. [Crossref] [PubMed]
- Moch H, Cubilla AL, Humphrey PA, et al. The 2016 WHO Classification of Tumours of the Urinary System and Male Genital Organs-Part A: Renal, Penile, and Testicular Tumours. Eur Urol 2016;70:93-105. [Crossref] [PubMed]
- Akhtar M, Al-Bozom IA, Al Hussain T. Papillary Renal Cell Carcinoma (PRCC): An Update. Adv Anat Pathol 2019;26:124-32. [Crossref] [PubMed]
- Wang YL, Zhang YY. cg04448376, cg24387542, cg08548498, and cg14621323 as a Novel Signature to Predict Prognosis in Kidney Renal Papillary Cell Carcinoma. Biomed Res Int 2020;2020:4854390. [Crossref] [PubMed]
- Lan H, Zeng J, Chen G, et al. Survival prediction of kidney renal papillary cell carcinoma by comprehensive LncRNA characterization. Oncotarget 2017;8:110811-29. [Crossref] [PubMed]
- Wang Q, Zhang Y, Zhang B, et al. Single-cell chromatin accessibility landscape in kidney identifies additional cell-of-origin in heterogenous papillary renal cell carcinoma. Nat Commun 2022;13:31. [Crossref] [PubMed]
- Makhov P, Joshi S, Ghatalia P, et al. Resistance to Systemic Therapies in Clear Cell Renal Cell Carcinoma: Mechanisms and Management Strategies. Mol Cancer Ther 2018;17:1355-64. [Crossref] [PubMed]
- Roskoski R Jr. Targeting ERK1/2 protein-serine/threonine kinases in human cancers. Pharmacol Res 2019;142:151-68. [Crossref] [PubMed]
- Gelens L, Saurin AT. Exploring the Function of Dynamic Phosphorylation-Dephosphorylation Cycles. Dev Cell 2018;44:659-63. [Crossref] [PubMed]
- Fontanillo M, Köhn M. Phosphatases: Their Roles in Cancer and Their Chemical Modulators. Adv Exp Med Biol 2016;917:209-40. [Crossref] [PubMed]
- Ardito F, Giuliani M, Perrone D, et al. The crucial role of protein phosphorylation in cell signaling and its use as targeted therapy Int J Mol Med 2017;40:271-80. (Review). [Crossref] [PubMed]
- Wang J, Wang F, Wang N, et al. Diagnostic and Prognostic Value of Protein Post-translational Modifications in Hepatocellular Carcinoma. J Clin Transl Hepatol 2023;11:1192-200. [Crossref] [PubMed]
- Singh V, Ram M, Kumar R, et al. Phosphorylation: Implications in Cancer. Protein J 2017;36:1-6. [Crossref] [PubMed]
- Hanahan D, Weinberg RA. The hallmarks of cancer. Cell 2000;100:57-70. [Crossref] [PubMed]
- Roberts PJ, Der CJ. Targeting the Raf-MEK-ERK mitogen-activated protein kinase cascade for the treatment of cancer. Oncogene 2007;26:3291-310. [Crossref] [PubMed]
- Gorini G, Harris RA, Mayfield RD. Proteomic approaches and identification of novel therapeutic targets for alcoholism. Neuropsychopharmacology 2014;39:104-30. [Crossref] [PubMed]
- Appella E, Anderson CW. Post-translational modifications and activation of p53 by genotoxic stresses. Eur J Biochem 2001;268:2764-72. [Crossref] [PubMed]
- Javidi-Sharifi N, Traer E, Martinez J, et al. Crosstalk between KIT and FGFR3 Promotes Gastrointestinal Stromal Tumor Cell Growth and Drug Resistance. Cancer Res 2015;75:880-91. [Crossref] [PubMed]
- Pathak AK, Husain N, Shukla S, et al. Impact of glutathione S transferases P1 (Ile105Val) variants on the risk of GSTp, phosphorylated c-Jun kinase, and P53 phenotypic expression and their implications on overall survival outcomes in non-small cell lung cancer patients treated with chemotherapy. Mutat Res 2022;824:111775. [Crossref] [PubMed]
- Bonanno L, Dal Maso A, Pavan A, et al. 51P Liver kinase B1 (LKB1) and phosphorylated AMP kinase (AMPK) expression in small cell lung cancer (SCLC): Association with prognosis and tumour immune microenvironment (TIME) features. J Thorac Oncol 2021;16:S723-4. [Crossref]
- Zhu N, Xiao H, Wang LM, et al. Mutations in tyrosine kinase and tyrosine phosphatase and their relevance to the target therapy in hematologic malignancies. Future Oncol 2015;11:659-73. [Crossref] [PubMed]
- Kraus J, Kraus M, Liu N, et al. The novel β2-selective proteasome inhibitor LU-102 decreases phosphorylation of I kappa B and induces highly synergistic cytotoxicity in combination with ibrutinib in multiple myeloma cells. Cancer Chemother Pharmacol 2015;76:383-96. [Crossref] [PubMed]
- Jagarlamudi KK, Hansson LO, Eriksson S. Breast and prostate cancer patients differ significantly in their serum Thymidine kinase 1 (TK1) specific activities compared with those hematological malignancies and blood donors: implications of using serum TK1 as a biomarker. BMC Cancer 2015;15:66. [Crossref] [PubMed]
- Paladino D, Yue P, Furuya H, et al. A novel nuclear Src and p300 signaling axis controls migratory and invasive behavior in pancreatic cancer. Oncotarget 2016;7:7253-67. [Crossref] [PubMed]
- Mehraein-Ghomi F, Church DR, Schreiber CL, et al. Inhibitor of p52 NF-κB subunit and androgen receptor (AR) interaction reduces growth of human prostate cancer cells by abrogating nuclear translocation of p52 and phosphorylated AR(ser81). Genes Cancer 2015;6:428-44. [Crossref] [PubMed]
- Sacco F, Perfetto L, Castagnoli L, et al. The human phosphatase interactome: An intricate family portrait. FEBS Lett 2012;586:2732-9. [Crossref] [PubMed]
- Labbé DP, Hardy S, Tremblay ML. Protein tyrosine phosphatases in cancer: friends and foes! Prog Mol Biol Transl Sci 2012;106:253-306. [Crossref] [PubMed]
- Laczmanska I, Skiba P, Karpinski P, et al. Customized Array Comparative Genomic Hybridization Analysis of 25 Phosphatase-encoding Genes in Colorectal Cancer Tissues. Cancer Genomics Proteomics 2017;14:69-74. [Crossref] [PubMed]
- Su X, Hou NN, Yang LJ, et al. The first competing risk survival nomogram in patients with papillary renal cell carcinoma. Sci Rep 2021;11:11835. [Crossref] [PubMed]
- Cardenas LM, Sigurdson S, Wallis CJD, et al. Advances in the management of renal cell carcinoma. CMAJ 2024;196:E235-40. [Crossref] [PubMed]
- Habeeb M, Arsey S, You H W, et al. Targeted nanomedicine modulating intercellular communications to arrest renal cell carcinoma progression. J Drug Deliv Sci Technol 2024;99:105983. [Crossref]
- Zhang G, Yu Z, Fu S, et al. Correction to: ERCC6L that is up-regulated in high grade of renal cell carcinoma enhances cell viability in vitro and promotes tumor growth in vivo potentially through modulating MAPK signalling pathway. Cancer Gene Ther 2022;29:1296. [Crossref] [PubMed]
- Yan H, Wei X, Wu A, et al. Nomograms for predicting overall and cancer-specific survival in patients with papillary renal cell carcinoma: a population-based study using SEER database. Transl Androl Urol 2020;9:1146-58. [Crossref] [PubMed]
- Luo L, Zhou H, Su H. Identification of 4-genes model in papillary renal cell tumor microenvironment based on comprehensive analysis. BMC Cancer 2021;21:553. [Crossref] [PubMed]
- Qian K, Li W, Ren S, et al. HDAC8 Enhances the Function of HIF-2α by Deacetylating ETS1 to Decrease the Sensitivity of TKIs in ccRCC. Adv Sci (Weinh) 2024;11:e2401142. [Crossref] [PubMed]
- Chen H, Chen Q, Chen J, et al. Deciphering the Effects of the PYCR Family on Cell Function, Prognostic Value, Immune Infiltration in ccRCC and Pan-Cancer. Int J Mol Sci 2024;25:8096. [Crossref] [PubMed]
- Xu H, Li Y. ASO Author Reflections: Accurate and User-Friendly Models for Predicting Oncologic Outcomes Following Partial Nephrectomy in Patients with cT1-ccRCC. Ann Surg Oncol 2024;31:5862-3. [Crossref] [PubMed]
- Yin H, Lin M, Liang S, et al. Ferroptosis-related gene signature predicts prognosis in kidney renal papillary cell carcinoma. Front Oncol 2022;12:988867. [Crossref] [PubMed]
- Gao Z, Zhang D, Duan Y, et al. A five-gene signature predicts overall survival of patients with papillary renal cell carcinoma. PLoS One 2019;14:e0211491. [Crossref] [PubMed]
- Li J, Li Q, Yuan Y, et al. High CENPA expression in papillary renal cell carcinoma tissues is associated with poor prognosis. BMC Urol 2022;22:157. [Crossref] [PubMed]
- Yang Y, Huang B, Liu J, et al. Heparanase-induced proliferation and inhibition of apoptosis are associated with the phosphatase and tensin homologue deleted on chromosome 10/focal adhesion kinase signaling pathway in multiple myeloma. Mater Express 2021;11:634-46. [Crossref]
- Ni S, Wei Q, Yang L. ADORA1 Promotes Hepatocellular Carcinoma Progression via PI3K/AKT Pathway. Onco Targets Ther 2020;13:12409-19. [Crossref] [PubMed]
- Lin X, Wang ZY, Xue G, et al. ADORA1 is a diagnostic-related biomarker and correlated with immune infiltrates in papillary thyroid carcinoma. J Cancer 2021;12:3997-4010. [Crossref] [PubMed]
- Takeiwa T, Mitobe Y, Ikeda K, et al. Long Intergenic Noncoding RNA OIN1 Promotes Ovarian Cancer Growth by Modulating Apoptosis-Related Gene Expression. Int J Mol Sci 2021;22:11242. [Crossref] [PubMed]
- Lan B, Zhang J, Zhang P, et al. Metformin suppresses CRC growth by inducing apoptosis via ADORA1. Front Biosci (Landmark Ed) 2017;22:248-57. [Crossref] [PubMed]
- Sheng H, Chen X, Liu B, et al. Omega-3 Polyunsaturated Fatty Acids Enhance Cisplatin Efficacy in Gastric Cancer Cells by Inducing Apoptosis via ADORA1. Anticancer Agents Med Chem 2016;16:1085-92. [Crossref] [PubMed]
- Zhu W, Jiang H, Xie S, et al. Downregulation of PPA2 expression correlates with poor prognosis of kidney renal clear cell carcinoma. PeerJ 2021;9:e12086. [Crossref] [PubMed]
- Li Z, Li Y, Wang X, et al. PPP2R2B downregulation is associated with immune evasion and predicts poor clinical outcomes in triple-negative breast cancer. Cancer Cell Int 2021;21:13. [Crossref] [PubMed]
- Bao Y, Oguz G, Lee WC, et al. EZH2-mediated PP2A inactivation confers resistance to HER2-targeted breast cancer therapy. Nat Commun 2020;11:5878. [Crossref] [PubMed]
- Lusche DF, Buchele EC, Russell KB, et al. Overexpressing TPTE2 (TPIP), a homolog of the human tumor suppressor gene PTEN, rescues the abnormal phenotype of the PTEN(-/-) mutant. Oncotarget 2018;9:21100-21. [Crossref] [PubMed]
- Hardy S, Kostantin E, Hatzihristidis T, et al. Physiological and oncogenic roles of the PRL phosphatases. FEBS J 2018;285:3886-908. [Crossref] [PubMed]
- Cui C, Duan Y, Qiu C, et al. Identification of Novel Autoantibodies Based on the Human Proteomic Chips and Evaluation of Their Performance in the Detection of Gastric Cancer. Front Oncol 2021;11:637871. [Crossref] [PubMed]
- Fang L, Yang Z, Zhou J, et al. Circadian Clock Gene CRY2 Degradation Is Involved in Chemoresistance of Colorectal Cancer. Mol Cancer Ther 2015;14:1476-87. [Crossref] [PubMed]
- Mao Y, Fu A, Hoffman AE, et al. The circadian gene CRY2 is associated with breast cancer aggressiveness possibly via epigenomic modifications. Tumour Biol 2015;36:3533-9. [Crossref] [PubMed]
- Liu S, Cheng Y, Wang S, et al. Circadian Clock Genes Modulate Immune, Cell Cycle and Apoptosis in the Diagnosis and Prognosis of Pan-Renal Cell Carcinoma. Front Mol Biosci 2021;8:747629. [Crossref] [PubMed]
- Yu Y, Li Y, Zhou L, et al. Cryptochrome 2 (CRY2) Suppresses Proliferation and Migration and Regulates Clock Gene Network in Osteosarcoma Cells. Med Sci Monit 2018;24:3856-62. [Crossref] [PubMed]
- Cen J, Liang Y, Huang Y, et al. Circular RNA circSDHC serves as a sponge for miR-127-3p to promote the proliferation and metastasis of renal cell carcinoma via the CDKN3/E2F1 axis. Mol Cancer 2021;20:19. [Crossref] [PubMed]


