
Adjuvant therapy for resected early-stage small-cell lung cancer: is now time to rethink about that?
Small-cell lung cancer (SCLC) has historically been considered a highly chemo-radiosensitive malignancy, rarely susceptible of surgical resection due to advanced stage presentation with bulky nodal disease and frequent systemic involvement. Actually, surgical series documented SCLC in just 2–3% of patients (1) and, although several randomized trials contributed to define the management of extensive and limited stage SCLC, very few data are available for the early stage, possibly resectable, disease. The National Comprehensive Cancer Network (NCCN) and European Society of Medical Oncology guidelines (2,3) recommend surgery followed by adjuvant chemotherapy and prophylactic cranial irradiation (PCI) as reasonable treatments for patients with T1-2N0M0 disease. Nevertheless, the evidence supporting the use of adjuvant chemotherapy comes from four, quite dated, phase II single arm studies (4-7) (Table 1), while the recommendation regarding PCI is exclusively based on data from trials evaluating the impact of chemotherapy and radiation in patients with not-resectable limited stage SCLC (13). For this reason, the management of patients with very limited stage SCLC (T1-2N0M0) is still far to be defined.
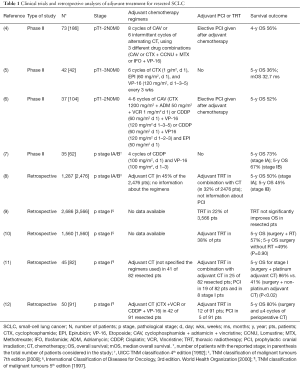
Full table
In the article by Yang et al. (14), a large retrospective analysis was performed using the National Cancer Data Base (NCDB) and including 1,574 patients with pathologic T1-2N0M0 SCLC who had undergone surgical resection in the predefined study period from 2003 to 2011. To minimize the possibility that the adjuvant treatment was used to treat recurrence rather than to prevent it, the investigators define adjuvant chemotherapy when administered within 5 months after surgery and adjuvant radiation when administered within 8 months after surgery. The time intervals reflect the condition in real world medical practice, in which patients often experience delays between surgery and initiation of adjuvant treatments.
Among 1,574 patients with resected T1-2N0M0 SCLC, 954 satisfied the investigator’s criteria, 354 patients underwent subsequent adjuvant chemotherapy, 190 patients underwent adjuvant chemo-radiation (87 received radiotherapy directed to the lung, 99 directed to the brain, 4 had unknown location of radiotherapy), 22 patients underwent radiation therapy alone [17 receiving thoracic radiotherapy (TRT) and 5 patients receiving PCI]. Treatment with adjuvant chemotherapy with or without radiation compared with no adjuvant therapy was associated with a significant increase in median overall survival (OS) of 24 months (66 vs. 42.1 months) and in 5-year OS of 12.3% (52.7% vs. 40.4%; P<0.01). Multivariate analysis showed that the use of adjuvant chemotherapy with or without radiation to the brain was significantly associated with improved survival compared with surgery alone, while there were no significant differences in 5-year survival between patients who received adjuvant chemotherapy with radiation to the lung or postoperative radiation only and patients who underwent only surgery.
The study by Yang et al. confirmed the survival benefit of adjuvant chemotherapy in resected pathologic T1-2N0M0 SCLC, with a 5-year OS rate of approximately 50%, that is comparable to the one reported in another retrospective analysis using the NCDB (8) and in four prospective trials, which documented a 5-year OS rate for resected early stage SCLC ranging from 36% to 70% (4-7). Furthermore, the investigators suggested a promising role of PCI after surgery and adjuvant chemotherapy, reporting a significant OS benefit of PCI in surgically resected SCLC. In contrast, they did not observed any significant survival benefit for adjuvant radiation alone and adjuvant chemotherapy with postoperative thoracic radiation compared to surgery alone. These last findings were remarkably consistent with the previous analyses of the SEER database (9,10).
Although based on a large national database and clarifying interesting aspects about the management of resected SCLC, the analysis of Yang et al. could not avoid some limitations, mainly related to its retrospective nature. For what concerns adjuvant chemotherapy, important information are missing regarding the specific chemotherapy regimens administered in this population based cohort study. Nevertheless, considering that the study period was 2003–2011 and that the combination of platinum-etoposide became the standard of care for SCLC in the 1990’s, when this regimen demonstrated to be superior to cyclophosphamide-anthracycline-vincristine (CAV) in limited stage SCLC (15) and equivalent to CAV but with less toxicities in extensive stage SCLC (16), it is strongly plausible that platinum-etoposide was the preferred adjuvant chemotherapeutic regimen used in the analysis by Yang and colleagues. Furthermore, in 2005, the superiority of platinum-based chemotherapy compared to non-platinum regimens, was demonstrated also in the adjuvant setting, in a retrospective analysis including stage I SCLC who underwent surgical resection and postoperative adjuvant chemotherapy (11).
Although platinum-etoposide was probably the most common adjuvant regimen used in the analysis by Yang et al., the investigators did not address in their study the question regarding the use of cisplatin or carboplatin. In fact, despite the equivalence in terms of survival between cisplatin and carboplatin in advanced SCLC, as reported in the COCIS meta-analysis (17), this result should not be translated to the adjuvant setting, considering that only the 30% of patients included in COCIS meta-analysis had limited stage SCLC. Actually, the small percentage of limited stage SCLC in the COCIS meta-analysis and the absence of efficacy of carboplatin-based combination in adjuvant treatment for non-small-cell lung cancer (NSCLC) (18), might suggest a possible differential treatment effect between adjuvant carboplatin and cisplatin also for resected SCLC; however, no definitive conclusion regarding this issue can, ultimately, be drawn.
In the analysis of Yang et al., besides the specific chemotherapeutic agents used, also the number of administered cycles of chemotherapy cannot be ascertained. In this regard, in a retrospective review of early stage SCLC patients who underwent surgical resection, the administration of four or more cycles of adjuvant chemotherapy compared to one to three cycles was an independent factor to determine postoperative survival (12), suggesting that four or more cycles of adjuvant chemotherapy should be considered the standard treatment for resected SCLC.
Regarding PCI, its role is quite controversial in extensive stage SCLC, where a randomized trial reported a striking improvement in 1-year OS rate of 14% (27% vs. 13%, HR 0.68) (19) and a more recent meta-analysis outlined the absence of a significant survival benefit (HR 0.95, P=0.89) (20). For what concerns patients with limited stage SCLC and with complete remission after chemo-radiotherapy treatment, a large meta-analysis showed that PCI significantly improved the 3-years OS of 5.4% (15.3% vs. 20.7%, HR 0.84) (13). In the adjuvant setting, the analysis of Yang et al. represents the first study supporting PCI after surgery and adjuvant chemotherapy, showing at the multivariate analysis a significant reduction in the risk of death of 48% in favor of PCI (after adjuvant chemotherapy) compared to surgery alone (HR 0.52, P<0.01). Although the authors have performed a comparison of prognostic variables between the two groups, a further adjustment of the results with a propensity score analysis would have increase the value of the analysis, by minimizing the biases related to its retrospective nature.
In their retrospective analysis, the investigators did not find a significant survival benefit with adjuvant radiotherapy or adjuvant chemotherapy followed by thoracic radiation compared to surgery alone. This result seems to be in contrast with the benefit of 5.4% in 3-year OS observed by adding TRT to chemotherapy in limited stage SCLC (21), with the improvement of 10% in 2-year OS (13% vs. 3%, P=0.004) reported in patients with extensive stage SCLC included in the CREST trial (22) and with the significant reduction in the risk of death (HR 0.82, P=0.02) demonstrated in a meta-analysis of five trials comparing TRT vs. no TRT (20). The study of Yang and colleagues and the retrospective analyses of the SEER database (9,10), outlined that adjuvant TRT might have a less determinant role in controlling micrometastases compared to adjuvant chemotherapy and PCI. Furthermore, these results differ from a recently published retrospective analysis of the NCDB reporting a significant OS benefit for adjuvant chemo-radiotherapy compared to surgery alone (HR 0.41, P<0.0001) in resected SCLC (8). In the analysis of Yang and colleagues, the doses and the schedules of administration of TRT cannot be ascertained and it is possible that the lack of survival benefit from adjuvant TRT could depend on the use of inappropriate doses and/or old-fashioned schedules of radiotherapy. In fact, in limited stage SCLC, early concurrent TRT with a time of starting any therapy and end of radiotherapy (SER) ≤30 days (23) and accelerated TRT (24) were, respectively, associated with a significant 2-year OS benefit of 5% and 5-year OS benefit of 10% if compared to TRT with SER >30 days and to standard fractionation schemes.
Finally, it is possible that, in Yang’s analysis, a selection bias contributed to the much higher survival reported in patients treated with both adjuvant chemotherapy and PCI and to the absence of survival benefit for patients treated with only adjuvant thoracic radiation. In fact, although the authors tried to minimize this bias including the Charles/Deyo Comorbidity Condition (CDCC) score as a covariate in the multivariable model, it is still possible that patients with best performance status received both adjuvant chemotherapy and PCI after surgery, while patients who received only adjuvant TRT had more comorbidities or poorer clinical conditions.
The observations made by Yang and colleagues, supporting the use of a multimodal adjuvant treatment for early stage SCLC including surgery, adjuvant chemotherapy and PCI, are extremely helpful for medical oncologist to manage patients with resected T1-2N0M0 SCLC. Nevertheless, the 5-year survival rate of stage I SCLC still ranges between 40% and 60% (25); for this reason, the development of more powerful and modern adjuvant therapeutic tools and radiation techniques, both integrated in a multimodal treatment strategy, will represent one of the toughest challenge for the future research in this field of thoracic oncology.
Acknowledgments
Funding: This work was supported by a specific grant of the Italian Association for Cancer Research (AIRC, My First AIRC Grant n 14282) and by a Young Investigational Award of the International Association for the Study of Lung Cancer (IASLC).
Footnote
Provenance and Peer Review: This article was commissioned and reviewed by the Section Editor Ke-Jun Liu (Department of Medical Oncology, Affiliated Dongguan People’s Hospital of Southern Medical University, Dongguan, China).
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2016.09.09). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Vallières E, Shepherd FA, Crowley J, et al. The IASLC Lung Cancer Staging Project: proposals regarding the relevance of TNM in the pathologic staging of small cell lung cancer in the forthcoming (seventh) edition of the TNM classification for lung cancer. J Thorac Oncol 2009;4:1049-59. [Crossref] [PubMed]
- National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology: Small Cell Lung Cancer (version 2.2015).
- Früh M, De Ruysscher D, Popat S, et al. Small-cell lung cancer (SCLC): ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2013;24:vi99-105. [Crossref] [PubMed]
- Karrer K, Ulsperger E. Surgery for cure followed by chemotherapy in small cell carcinoma of the lung. For the ISC-Lung Cancer Study Group. Acta Oncol 1995;34:899-906. [Crossref] [PubMed]
- Macchiarini P, Hardin M, Basolo F, et al. Surgery plus adjuvant chemotherapy for T1-3N0M0 small-cell lung cancer. Rationale for current approach. Am J Clin Oncol 1991;14:218-24. [Crossref] [PubMed]
- Rea F, Callegaro D, Favaretto A, et al. Long term results of surgery and chemotherapy in small cell lung cancer. Eur J Cardiothorac Surg 1998;14:398-402. [Crossref] [PubMed]
- Tsuchiya R, Suzuki K, Ichinose Y, et al. Phase II trial of postoperative adjuvant cisplatin and etoposide in patients with completely resected stage I-IIIa small cell lung cancer: the Japan Clinical Oncology Lung Cancer Study Group Trial (JCOG9101). J Thorac Cardiovasc Surg 2005;129:977-83. [Crossref] [PubMed]
- Combs SE, Hancock JG, Boffa DJ, et al. Bolstering the case for lobectomy in stages I, II, and IIIA small-cell lung cancer using the National Cancer Data Base. J Thorac Oncol 2015;10:316-23. [Crossref] [PubMed]
- Weksler B, Nason KS, Shende M, et al. Surgical resection should be considered for stage I and II small cell carcinoma of the lung. Ann Thorac Surg 2012;94:889-93. [Crossref] [PubMed]
- Yu JB, Decker RH, Detterbeck FC, et al. Surveillance epidemiology and end results evaluation of the role of surgery for stage I small cell lung cancer. J Thorac Oncol 2010;5:215-9. [Crossref] [PubMed]
- Brock MV, Hooker CM, Syphard JE, et al. Surgical resection of limited disease small cell lung cancer in the new era of platinum chemotherapy: Its time has come. J Thorac Cardiovasc Surg 2005;129:64-72. [Crossref] [PubMed]
- Inoue M, Miyoshi S, Yasumitsu T, et al. Surgical results for small cell lung cancer based on the new TNM staging system. Thoracic Surgery Study Group of Osaka University, Osaka, Japan. Ann Thorac Surg 2000;70:1615-9. [Crossref] [PubMed]
- Aupérin A, Arriagada R, Pignon JP, et al. Prophylactic cranial irradiation for patients with small-cell lung cancer in complete remission. Prophylactic Cranial Irradiation Overview Collaborative Group. N Engl J Med 1999;341:476-84. [Crossref] [PubMed]
- Yang CF, Chan DY, Speicher PJ, et al. Role of Adjuvant Therapy in a Population-Based Cohort of Patients With Early-Stage Small-Cell Lung Cancer. J Clin Oncol 2016;34:1057-64. [Crossref] [PubMed]
- Sundstrøm S, Bremnes RM, Kaasa S, et al. Cisplatin and etoposide regimen is superior to cyclophosphamide, epirubicin, and vincristine regimen in small-cell lung cancer: results from a randomized phase III trial with 5 years' follow-up. J Clin Oncol 2002;20:4665-72. [Crossref] [PubMed]
- Roth BJ, Johnson DH, Einhorn LH, et al. Randomized study of cyclophosphamide, doxorubicin, and vincristine versus etoposide and cisplatin versus alternation of these two regimens in extensive small-cell lung cancer: a phase III trial of the Southeastern Cancer Study Group. J Clin Oncol 1992;10:282-91. [PubMed]
- Rossi A, Di Maio M, Chiodini P, et al. Carboplatin- or cisplatin-based chemotherapy in first-line treatment of small-cell lung cancer: the COCIS meta-analysis of individual patient data. J Clin Oncol 2012;30:1692-8. [Crossref] [PubMed]
- Pisters KM, Evans WK, Azzoli CG, et al. Cancer Care Ontario and American Society of Clinical Oncology adjuvant chemotherapy and adjuvant radiation therapy for stages I-IIIA resectable non small-cell lung cancer guideline. J Clin Oncol 2007;25:5506-18. [Crossref] [PubMed]
- Slotman B, Faivre-Finn C, Kramer G, et al. Prophylactic cranial irradiation in extensive small-cell lung cancer. N Engl J Med 2007;357:664-72. [Crossref] [PubMed]
- Soon YY, Koh WY, Leong CN, et al. Prophylactic cranial irradiation (PCI) and consolidation thoracic radiotherapy (TRT) for extensive stage small cell lung cancer (ES-SCLC): A systematic review and meta-analysis. J Clin Oncol 2015;33:abstr 7568.
- Pignon JP, Arriagada R, Ihde DC, et al. A meta-analysis of thoracic radiotherapy for small-cell lung cancer. N Engl J Med 1992;327:1618-24. [Crossref] [PubMed]
- Slotman BJ, van Tinteren H, Praag JO, et al. Use of thoracic radiotherapy for extensive stage small-cell lung cancer: a phase 3 randomised controlled trial. Lancet 2015;385:36-42. [Crossref] [PubMed]
- De Ruysscher D, Pijls-Johannesma M, Bentzen SM, et al. Time between the first day of chemotherapy and the last day of chest radiation is the most important predictor of survival in limited-disease small-cell lung cancer. J Clin Oncol 2006;24:1057-63. [Crossref] [PubMed]
- Turrisi AT 3rd, Kim K, Blum R, et al. Twice-daily compared with once-daily thoracic radiotherapy in limited small-cell lung cancer treated concurrently with cisplatin and etoposide. N Engl J Med 1999;340:265-71. [Crossref] [PubMed]
- Schneider BJ, Saxena A, Downey RJ. Surgery for early-stage small cell lung cancer. J Natl Compr Canc Netw 2011;9:1132-9. [PubMed]


