
Atezolizumab in lung cancer—appreciating the differences
Once felt to be a non-immunogenic tumor, non-small cell lung cancer (NSCLC) has become an example of the transformative potential of immunotherapy in oncology. Nivolumab and pembrolizumab, two monoclonal antibodies that target PD-1, have become firmly established as standard second-line therapy for patients with advanced NSCLC. Nivolumab was compared to second-line docetaxel in squamous NSCLC [Checkmate 017 (1)] and non-squamous NSCLC [Checkmate 057 (2)] and in both histologic subtypes, demonstrated an improvement in overall survival. Similarly, pembrolizumab was compared to second line docetaxel in the KEYNOTE-010 study (3) and also showed a significant improvement in overall survival. What differs between nivolumab and pembrolizumab is the role of PD-L1 expression as a biomarker. Nivolumab is currently approved independent of PD-L1 expression while use of pembrolizumab is limited to tumors expressing PD-L1. While the improvement in median survival with both agents was significant, the more impressive feature of these agents is the durability of response. Longer follow-up of patients with advanced NSCLC who received salvage nivolumab reveals a survival rate of approximately 20% at 3 years (4). This durable benefit has forced us to re-examine our approach to metastatic lung cancer and has appropriately raised our expectations for the future.
There are now several PD-L1 inhibitors in development. Antibodies specific for the PD-L1 ligand disrupt its interaction with the PD-1 receptor in a manner similar to the established PD-1 inhibitors. PD-L1 inhibitors differ in that they preserve the interaction between PD-1 and PD-L2, which may promote immune homeostasis and potentially mediate toxicity. PD-L1 inhibitors can also target the interaction between PD-L1 and B7.1, or CD80, receptors. Blocking B7.1 receptor activation may mediate T-cell activation and contribute to a therapeutic immune response. For now, any potential benefit of a PD-L1 inhibitor over a PD-1 inhibitor remains theoretical and with no direct comparisons available (or expected), most would consider PD-L1 inhibitors to be somewhat interchangeable with PD-1 inhibitors. Recently, Fehrenbacher et al. (5) reported results from POPLAR, exploring atezolizumab, an antibody targeting PD-L1, in NSCLC and indeed, outcomes were comparable to those seen with PD-1 inhibitors in this population. As this field rapidly moves forward, it will be important to acknowledge both the similarities and differences between the available agents and to identify the important gaps in our understanding.
In the POPLAR study, 287 patients with advanced NSCLC were randomized to atezolizumab (1,200 mg) or docetaxel (75 mg/m2) given every 3 weeks in the second- or third-line setting. All patients provided tumor tissue for central PD-L1 testing but PD-L1 expression was not mandated for study entry. The primary endpoint of the study was overall survival in the intention-to-treat population and in subgroups based on PD-L1 expression, defined using immunohistochemistry with the Ventana SP142 assay. In this assay, a percentage of tumor cells with PD-L1 staining is reported and a grade is assigned: TC3 describes tumors with ≥50% of cells staining positive, TC2 refers to tumors with 5–49% of cells positive, TC1 reflects tumors with 1–4% of cells positive and TC0 is reserved for tumors with no PD-L1 expression. While the antibody, platform, and cutoffs are different from those used for nivolumab and pembrolizumab, the general approach to tumor PD-L1 status is the same. However, the atezolizumab program also examines PD-L1 expression in tumor infiltrating immune cells and provides a score for the tumor microenvironment. IC3 is defined as ≥10% of infiltrating immune cells with PD-L1 positivity, IC2 is 5–9%, IC1 is 1–4% and IC0 is no PD-L1 staining. Samples are considered PD-L1 positive based on expression on tumor cells, immune cells, or both.
As seen with PD-1 inhibitors, atezolizumab was better tolerated than docetaxel, with fewer treatment related grade 3+ adverse events (11% vs. 39%) and fewer patients discontinuing therapy due to adverse event (8% vs. 22%). Atezolizumab improved median overall survival as compared to docetaxel (12.6 vs. 9.7 months, P=0.04). The duration of response was impressive, echoing the experience with PD-1 inhibitors, with atezolizumab offering a median duration of 14.3 months and docetaxel 7.2 months. Overall survival benefit was greater for those with PD-L1 positive tumors, using either a TC or IC designation, with no appreciable difference in survival between the two arms for the TC0/IC0 population. Interestingly, there was very little overlap between the TC3 and IC3 population. The study also analyzed a T-effector and interferon-gamma immune gene signature associated with T-cell activation. Analysis was performed using the Fluidigm real-time PCR platform and the gene signature included CD8A, GZMA, GZMB, IFN-γ, EOMES, CXCL9, CXCL10, and TBX21. Tumors were scored as above or below the median for expression and patients with tumors scoring above the median fared better with atezolizumab. There was also an association between the gene signature and PD-L1 expression on immune cells, though not on tumor cells.
The POPLAR study is an important addition to the immunotherapy experience in NSCLC. In achieving responses and outcomes comparable to PD-1 inhibitors, it confirms PD-L1 is a viable therapeutic target in NSCLC. More importantly, it helps expand our search for a viable predictive marker for checkpoint inhibitors in NSCLC. PD-L1 expression remains the best available biomarker but it is neither sensitive nor specific enough. Immunohistochemistry introduces many challenges including identification of the optimal clone and platform, standardizing tissue processing, addressing tissue heterogeneity, and preventing subjective analysis. Though the PD-L1 scoring system used in POPLAR has been reproducible, a more objective test would be welcome. The gene signature explored in POPLAR may be a step in the right direction. What may eventually prove more significant is the identification of a subset of patients with PD-L1 expression in immune cells but not on tumor cells. This subset of patients also seems to derive benefit from immunotherapy. Ostensibly, these are patients who would be classified as PD-L1 negative on other assays. The median duration of response with PD-1 and PD-L1 inhibitors in NSCLC often approaches or exceeds the median overall survival, suggesting that the true benefit is limited to a relatively small subset of patients. Optimal delivery of these agents is dependent on prospective identification of this subset. PD-L1 expression will have to serve that function for now but its use alone is neither sensitive nor specific enough. Examining PD-L1 expression in immune cells is a positive step that may not only lead to a more robust biomarker, but may also provide insight into overcoming primary resistance. It is worth noting that prior studies with nivolumab did not reveal a predictive role for PD-L1 expression on immune cells. This is one of many inconsistent results seen with PD-L1 expression across studies (Table 1). These varied results may reflect differences in the tests used, as it is readily apparent that these tests are not equal. Biomarker development for immunotherapy has been hindered by a complete lack of harmonization between these PD-L1 assays with different antibodies and platforms moving forward in parallel. A critical assessment of these tests is desperately overdue and we cannot expect to move the field forward without some effort to standardize this biomarker. One test may ultimately emerge as the best predictor of benefit for all PD-1 or PD-L1 inhibitors. Alternatively, if the assays are identifying different patient populations, they may be complementary and a panel may be more useful than a single assay. Efforts are underway now by the International Association for the Study of Lung Cancer to compare these different assays and will be invaluable. However, with trials moving to completion quickly, the science will always be catching up.
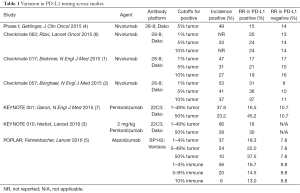
Full table
Nivolumab, pembrolizumab, and now atezolizumab have all provided better outcomes than docetaxel in the salvage setting. For all three, response rates in an unselected population have been modest at best but no worse than standard therapy. As immunotherapy moves to the first-line setting, the stakes are higher and we must improve our ability to recognize the patients that will benefit and those that would be better served with standard chemotherapy. With a critical paradigm shift in NSCLC management already underway, optimal biomarker development will require rational prospective study design and true collaboration between all involved parties.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned and reviewed by the Section Editor Long Jiang (Second Affiliated Hospital, Institute of Respiratory Diseases, Zhejiang University School of Medicine, Hangzhou, China).
Conflicts of Interest: SV Liu has served as a consultant for Genentech/Roche and Pfizer. Dr. Giaccone has no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Brahmer J, Reckamp KL, Baas P, et al. Nivolumab versus Docetaxel in Advanced Squamous-Cell Non-Small-Cell Lung Cancer. N Engl J Med 2015;373:123-35. [PubMed]
- Borghaei H, Paz-Ares L, Horn L, et al. Nivolumab versus Docetaxel in Advanced Nonsquamous Non-Small-Cell Lung Cancer. N Engl J Med 2015;373:1627-39. [PubMed]
- Herbst RS, Baas P, Kim DW, et al. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet 2016;387:1540-50. [PubMed]
- Gettinger SN, Horn L, Gandhi L, et al. Overall Survival and Long-Term Safety of Nivolumab (Anti-Programmed Death 1 Antibody, BMS-936558, ONO-4538) in Patients With Previously Treated Advanced Non-Small-Cell Lung Cancer. J Clin Oncol 2015;33:2004-12. [PubMed]
- Fehrenbacher L, Spira A, Ballinger M, et al. Atezolizumab versus docetaxel for patients with previously treated non-small-cell lung cancer (POPLAR): a multicentre, open-label, phase 2 randomised controlled trial. Lancet 2016;387:1837-46. [PubMed]
- Rizvi NA, Mazières J, Planchard D, et al. Activity and safety of nivolumab, an anti-PD-1 immune checkpoint inhibitor, for patients with advanced, refractory squamous non-small-cell lung cancer (CheckMate 063): a phase 2, single-arm trial. Lancet Oncol 2015;16:257-65. [PubMed]
- Garon EB, Rizvi NA, Hui R, et al. Pembrolizumab for the treatment of non-small-cell lung cancer. N Engl J Med 2015;372:2018-28. [PubMed]


