
Percentage of fat in milk consumption and risk of six cancers: a Mendelian randomization study
Highlight box
Key findings
• Higher percentage fat in milk consumption was shown to have a causal effect on the increased risk of breast cancer.
What is known and what is new?
• There is a correlation between milk consumption and cancer risk, yet research on the causal link between fat percentage in milk and cancer risk is scarce. Elucidating whether fat percentage in milk is a primary factor influencing changes in cancer risk is of significant importance.
• Analyses at the genetic level, free from confounding factors, suggest that a higher fat content in milk has a causal relationship specifically with an increased risk of breast cancer, with no observed causal relationship with the risk of several other types of cancer associated with milk consumption.
What is the implication, and what should change now?
• Generally, the percentage of fat in milk is not a decisive factor in determining the impact of milk consumption on cancer risk. These findings may have significant implications for the development of cancer prevention strategies targeted at the European population.
Introduction
Background
Cancer remains a leading cause of mortality, imposing a substantial burden on patients, their families, and society (1,2). The escalating challenge of the rising cancer burden is multifaceted, attributable to factors such as the aging global population, unhealthy dietary habits, and sedentary lifestyles, among others (1). Modifiable factors associated with the reduction of cancer risk warrant attention. Diet, a prevalent modifiable factor, was shown in prior research to have influence on cancer risk (3). In the past few decades, a multitude of epidemiological studies have identified associations between specific dietary patterns or individual foods and the risk of cancer (3). For instance, milk, a common beverage with diverse health implications, has been shown to be correlated with cancer risk in previous studies (4,5).
Recent prospective epidemiological studies have identified commercial milk consumption as a significant risk factor for breast cancer (6,7). A meta-analysis encompassing 29 studies suggested that the intake of whole-fat milk and dairy products may be associated with a higher risk of ovarian cancer, whereas the consumption of low-fat milk may mitigate this risk (8). A meta-analysis incorporating five observational studies revealed a significant inverse correlation between dairy product consumption and the risk of endometrial cancer in women with a higher body mass index (BMI) (9). Milk consumption, as genetically proxied, has been found to be associated with a decreased risk of colorectal cancer (10). Meanwhile, milk consumption has been associated with an increase in prostate cancer risk (11). A meta-analysis showed an inverse association between milk consumption and bladder cancer risk (12). The influence of milk consumption on cancer risk has been a contentious issue, with existing literature demonstrating potential associations between dairy intake and specific malignancies. Milk is a complex biological fluid that contains a variety of components, including proteins, vitamins, minerals, and fats, and can be categorized into different types based on these compositional differences (6,13). For instance, the fat content can vary among different types of milk (14). A study based on the Danish population discovered that the intake of skimmed milk, semi-skimmed milk, and whole milk differentially impacts cancer mortality (15). Observational studies have suggested that variations in the fat content of milk may influence cancer risk (16). Diets high in fat content, coupled with the excessive accumulation of adipose tissue, are established risk factors for a myriad of pathologies, with cancer being a notable inclusion (9,17,18).
Rationale and knowledge gap
The presence of potential confounding factors inherent in observational research methodologies limits the available evidence, thereby preventing the establishment of a definitive causal link between the fat content in milk, body fat, and the risk of cancer.
Mendelian randomization (MR) offers a methodological approach leveraging genetic variants as instrumental variables (IVs) to discern causal associations between traits and disease phenotypes. Unaffected by environmental influences and advantageous in controlling for confounding factors and reverse causality, MR mimics a “naturally occurring randomized, double-blind trial” and serves as a supplement to randomized controlled trials (RCTs) (19,20).
Objective
Given the limitations of current evidence, this study aimed to examine the potential causal relationship between the percentage of fat in milk consumption and the risk of various cancers, specifically breast, ovarian, endometrial, colorectal, prostate, and bladder cancers, which are illnesses associated with milk consumption (or dairy products), using MR analysis. We present this manuscript in accordance with the STROBE-MR reporting checklist (available at https://tcr.amegroups.com/article/view/10.21037/tcr-24-802/rc).
Methods
Study design
Through univariable MR analysis, our study aimed to elucidate the causal relationships between the percentage of fat in milk consumption and the risk of breast, ovarian, endometrial, colorectal, prostate, and bladder cancers, utilizing summary statistics derived from genome-wide association studies (GWAS). To elucidate the potential impacts of adiposity on the etiology of these six cancers, we undertook an MR analysis to ascertain the causal links between body fat percentage and the risks associated with each cancer type.
MR analysis is a statistical approach that utilizes IVs to investigate causal relationships between exposures and outcomes. This analytical framework was grounded on three pivotal assumptions: firstly, IVs are expected to be associated with percentage of fat in milk consumption or body fat percentage; secondly, these genetic variants should demonstrate no correlation with potential confounding factors; and thirdly, their effects are hypothesized to influence the six abovementioned cancers solely through their impact on percentage of fat in milk consumption or body fat percentage.
Data sources
Through the Integrative Epidemiology Unit (IEU) database, we accessed exposure data on single nucleotide polymorphisms (SNPs) linked to percentage of fat in milk consumption (n=411,503) and body fat percentage (n=401,772) from the United Kingdom (UK) Biobank databases (21,22). IVs for the outcomes were sourced from the following GWAS: breast cancer (n=139,274), the relevant SNPs data were obtained from studies collaborating within the Breast Cancer Association Consortium (BCAC) and the Discovery, Biology and Risk of Inherited Variants in Breast Cancer (DRIVE) consortium (23); ovarian cancer (n=66,450), the associated SNPs data were obtained from the Ovarian Cancer Association Consortium (OCAC) and the Consortium of Investigators of Modifiers of BRCA1/2 (CIMBA) (24); endometrial cancer (n=121,885), the relevant SNPs data were extracted from studies of endometrial cancer within the European population, and additionally from studies identified through the Endometrial Cancer Association Consortium (ECAC), the Epidemiology of Endometrial Cancer Consortium (E2C2), and the UK Biobank (25); colorectal cancer (n=32,072), the relevant SNPs data were obtained from the Genetics and Epidemiology of Colorectal Cancer Consortium (GECCO), the Colorectal Cancer Transdisciplinary Study (CORECT), and the Colon Cancer Family Registry (CCFR) (26); prostate cancer (n=140,254), the relevant SNPs data were extracted from a large-scale GWAS meta-analysis of prostate cancer within the European population (27); bladder cancer (n=373,295), with the relevant SNPs data obtained from the UK Biobank. All participants were of European descent and had provided their written informed consent. Each study protocol was approved by the respective research Ethics Board or Institutional Review Board. No additional ethical approval was required as all data were obtained from publicly available sources. Details of the data utilized in this study can be found in Table S1. All data were ethically reviewed and approved prior to collection, with details provided in the articles or websites pertaining to the sources of the data. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013).
Genetic IVs selection
Initially, SNPs linked with percentage of fat in milk consumption or body fat percentage were identified based on the genome-wide significance threshold (P<5e−8) and designated as IVs to fulfill assumption 1. To mitigate potential bias resulting from linkage disequilibrium (LD), SNPs showing evidence of LD were systematically excluded from the analysis (r2=0.001, kb=10,000). Next, we utilized the LDlink application to identify SNPs potentially associated with six cancers and removed them to satisfy assumption 2 (28). The strength of instrumental SNPs was assessed by computing the F-statistic, with variables retaining a value of F>10 being retained. Ultimately, a set of SNPs representing exposure was identified as genetic instruments. Tables S2,S3 enumerate the SNPs selected to represent exposure, with discordant SNPs excluded. This process ensures that the allelic orientations of both exposure-SNPs and outcome-SNPs are aligned. Finally, SNPs associated with outcomes were excluded (P<5e−8), fulfilling assumption 3. The remaining SNPs were utilized for MR analysis. Table S4-S9 detail the SNPs employed as IVs in the current investigation for percentage of fat in milk consumption and six cancers.
Statistical analysis
The inverse-variance weighted (IVW) methodological framework was utilized for the primary MR analysis. Weighted mediation (WM) and MR-Egger methods were employed to conduct the supplementary MR analysis. Sensitivity analyses were instrumental in evaluating potential heterogeneity and genetic pleiotropy within our study. Heterogeneity was systematically assessed using the Q statistic, which was derived from both the MR-Egger and IVW methods. MR pleiotropy testing was conducted to examine potential pleiotropic effects. To assess the impact of individual IVs on the MR outcomes, we performed a leave-one-out sensitivity analysis. Comprehensive results of the MR analyses are detailed in Tables 1,2 and Figure 1. All MR analyses were conducted using R software (version 4.3.1; R Foundation for Statistical Computing, Vienna, Austria), adhering to standard practices in hypothesis testing, with statistical significance defined as a P value less than 0.05. We have utilized an artificial intelligence (AI) tool named KIMI for the refinement of our manuscript’s English, which can be accessed at the following URL: https://kimi.moonshot.cn/.
Table 1
| Outcomes | No. of SNP | MR | Heterogeneity | Pleiotropy | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Method | Beta | SE | P value | Method | Q | P value | Intercept | SE | P value | ||||
| Breast cancer | 7 | IVW | 2.993 | 1.211 | 0.01 | MR-Egger | 10.067 | 0.07 | 0.004 | 0.018 | 0.82 | ||
| WM | 2.036 | 1.355 | 0.13 | IVW | 10.183 | 0.12 | |||||||
| MR-Egger | 1.572 | 6.067 | 0.81 | − | − | − | |||||||
| Ovarian cancer | 8 | IVW | −2.373 | 1.641 | 0.15 | MR-Egger | 2.516 | 0.87 | −0.006 | 0.022 | 0.79 | ||
| WM | −4.140 | 2.045 | 0.04 | IVW | 2.594 | 0.92 | |||||||
| MR-Egger | −0.594 | 6.570 | 0.93 | − | − | − | |||||||
| Endometrial cancer | 9 | IVW | −3.579 | 3.273 | 0.27 | MR-Egger | 25.712 | 0.001 | −0.010 | 0.041 | 0.81 | ||
| WM | −4.375 | 2.782 | 0.12 | IVW | 25.931 | 0.001 | |||||||
| MR-Egger | −0.498 | 13.092 | 0.97 | − | − | − | |||||||
| Colorectal cancer | 9 | IVW | −2.730 | 2.132 | 0.20 | MR-Egger | 8.286 | 0.31 | −0.015 | 0.026 | 0.59 | ||
| WM | −2.257 | 2.875 | 0.43 | IVW | 8.665 | 0.37 | |||||||
| MR-Egger | 1.828 | 8.361 | 0.83 | − | − | − | |||||||
| Prostate cancer | 9 | IVW | 1.708 | 1.548 | 0.27 | MR-Egger | 14.928 | 0.04 | −0.026 | 0.017 | 0.16 | ||
| WM | 1.573 | 1.346 | 0.24 | IVW | 20.195 | 0.01 | |||||||
| MR-Egger | 9.812 | 5.350 | 0.11 | − | − | − | |||||||
| Bladder cancer | 9 | IVW | −0.011 | 0.018 | 0.55 | MR-Egger | 8.937 | 0.26 | 1.9e−04 | 2.2e−04 | 0.41 | ||
| WM | −0.003 | 0.021 | 0.90 | IVW | 9.924 | 0.27 | |||||||
| MR-Egger | −0.069 | 0.068 | 0.35 | − | − | − | |||||||
SNP, single nucleotide polymorphism; MR, Mendelian randomization; SE, standard error; IVW, inverse-variance weighted; WM, weighted median.
Table 2
| Outcomes | No. of SNP | MR | Heterogeneity | Pleiotropy | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Method | Beta | SE | P value | Method | Q | P value | Intercept | SE | P value | ||||
| Breast cancer | 285 | IVW | −0.025 | 0.045 | 0.58 | MR-Egger | 553.159 | 1.1e−19 | 0.001 | 0.002 | 0.52 | ||
| WM | −0.046 | 0.052 | 0.38 | IVW | 553.960 | 1.3e−19 | |||||||
| MR-Egger | −0.127 | 0.165 | 0.44 | − | − | − | |||||||
| Ovarian cancer | 291 | IVW | 0.225 | 0.073 | 0.002 | MR-Egger | 365.741 | 0.001 | 4.4e−05 | 0.003 | 0.99 | ||
| WM | 0.225 | 0.104 | 0.03 | IVW | 365.741 | 0.002 | |||||||
| MR-Egger | 0.222 | 0.229 | 0.33 | − | − | − | |||||||
| Endometrial cancer | 307 | IVW | 0.669 | 0.088 | 3.0e−14 | MR-Egger | 452.744 | 7.7e−08 | −0.001 | 0.004 | 0.84 | ||
| WM | 0.637 | 0.120 | 1.1e−07 | IVW | 452.808 | 9.3e−08 | |||||||
| MR-Egger | 0.725 | 0.281 | 0.01 | − | − | − | |||||||
| Colorectal cancer | 300 | IVW | 0.344 | 0.094 | 2.5e−04 | MR-Egger | 407.648 | 2.4e−05 | −1.3e−04 | 0.004 | 0.98 | ||
| WM | 0.281 | 0.125 | 0.03 | IVW | 407.650 | 2.9e−05 | |||||||
| MR-Egger | 0.353 | 0.296 | 0.23 | − | − | − | |||||||
| Prostate cancer | 306 | IVW | −0.104 | 0.519 | 0.046 | MR-Egger | 555.523 | 5.8e−17 | −4.1e−04 | 0.002 | 0.86 | ||
| WM | −0.219 | 0.066 | 0.001 | IVW | 555.582 | 7.8e−17 | |||||||
| MR-Egger | −0.076 | 0.164 | 0.65 | − | − | − | |||||||
| Bladder cancer | 301 | IVW | −2.7e−05 | 6.7e−04 | 0.97 | MR-Egger | 330.303 | 0.10 | 2.0e−06 | 2.9e−05 | 0.95 | ||
| WM | 7.2e−05 | 9.8e−04 | 0.94 | IVW | 330.308 | 0.11 | |||||||
| MR-Egger | −1.6e−04 | 0.002 | 0.94 | − | − | − | |||||||
SNP, single nucleotide polymorphism; MR, Mendelian randomization; SE, standard error; IVW, inverse-variance weighted; WM, weighted median.
Results
MR results of relationship between percentage of fat in milk consumption and cancers
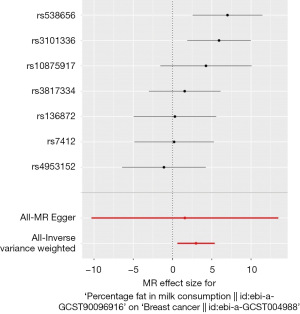
Using MR analysis, observed a causal association between percentage of fat in milk consumption and breast cancer (refer to Table 1), under the IVW method (β=2.993, P=0.01). Figure 1 displays a forest plot that delineates the findings from the MR analysis, which explores the potential influence of the fat content in milk consumption on the risk of breast cancer. Supplementary figures, including a scatter plot (Figure S1), a funnel plot (Figure S2), and a leave-one-out analysis plot (Figure S3), are also presented to offer a visual representation of the data. In the sensitivity analysis, no indication of heterogeneity was observed (MR-Egger, Q=10.067, P=0.07), and there was no evidence to suggest horizontal pleiotropy (intercept =0.004, P=0.82).
We found no potential causal association between percentage fat in milk consumption on risk of ovarian cancer (IVW, β=−2.373, P=0.15), endometrial cancer (IVW, β=−3.579, P=0.27), colorectal cancer (IVW, β=−2.730, P=0.20), prostate cancer (IVW, β=1.708, P=0.27), and bladder cancer (IVW, β=−0.011, P=0.55) (Table 1). Additionally, the MR analysis revealed no evidence of heterogeneity in the associations between the percentage of fat in milk consumption and the risks of ovarian, colorectal, and bladder cancers. The results of the MR analysis between the percentage of fat in milk consumption and the risk of endometrial and prostate cancers may exhibit heterogeneity. The analysis results presented above provide no evidence of horizontal pleiotropy. Due to the absence of evidence for causal relationships between percentage of fat in milk consumption and the five cancers, and given that the sensitivity analysis results indicated that the findings from the MR analysis were robust, we had elected to omit the forest plots, scatter plots, leave-one-out analysis plots, and funnel plots that pertain to these results.
MR results of relationship between body fat percentage and cancers
Using MR analysis, we discerned causal relationships between body fat percentage and ovarian, endometrial, colorectal, and prostate cancers (Table 2). The causal association between body fat percentage and ovarian cancer was evident in the results derived from both the IVW and the WM analytical approaches (IVW, β=0.225, P=0.002; WM, β=0.225, P=0.03), with a certain degree of heterogeneity observed among the results (MR-Egger, Q=365.741, P=0.001). The results of the scatter plot and funnel plot can be found in Figures S4,S5.
The results derived from the IVW, WM, and MR-Egger analytical methods collectively demonstrated a clear causal association between body fat percentage and the incidence of endometrial cancer (IVW, β=0.669, P<0.001; WM, β=0.637, P<0.001; MR-Egger, β=0.725, P=0.01), with a certain degree of heterogeneity observed among the results (MR-Egger, Q=452.744, P<0.001). The results of the scatter plot and funnel plot are displayed in Figures S6,S7.
The causal link between body fat percentage and the risk of colorectal cancer was apparent in the findings obtained through both the IVW and WM analytical approaches (IVW, β=0.344, P<0.001; WM, β=0.281, P=0.03), with a certain degree of heterogeneity observed among the results (MR-Egger, Q=407.648, P<0.001). The results of the scatter plot and funnel plot are shown in Figures S8,S9.
The presence of a causal association between body fat percentage and prostate cancer was discernible from the results generated by the IVW analytical method (β=−0.104, P=0.046), with a certain degree of heterogeneity observed among the results (MR-Egger, Q=555.523, P<0.001). The results of the scatter plot and funnel plot can be found in Figures S10,S11.
No potential causal association was identified between body fat percentage and the risk of breast cancer in our analysis (IVW, β=−0.025, P=0.58), or between body fat percentage and the risk of bladder cancer (IVW, β=−2.7e−05, P=0.97) (Table 2). The results of the MR analysis between the body fat percentage and breast and prostate cancers risk exhibited heterogeneity. Due to the absence of evidence for causal relationships between body fat percentage and breast and bladder cancers, and given that the sensitivity analysis results indicated that the findings from the MR analysis were robust, we had elected to omit the forest plots, scatter plots, leave-one-out analysis plots, and funnel plots that pertain to these results. The analysis results detailed above do not yield evidence supporting the presence of horizontal pleiotropy.
Discussion
Key findings
In the present study, we employed MR analysis to elucidate the causal effects of genetically elevated levels of fat percentage in milk consumption on the risk of breast cancer. Furthermore, our investigation identified a causal link between an increased body fat percentage, as determined genetically, and the risks associated with ovarian, endometrial, and colorectal cancers. Additionally, we observed a protective effect of the same against prostate cancer.
Strengths and limitations
This study has several attributes and strengths, notably its substantial sample size in the application of SNPs as genetic IVs. Particularly noteworthy is the employment of MR, facilitating the evaluation of causal associations between exposure and outcome. Compared to traditional observational studies and basic experimental studies, MR methodology offers a robust approach that effectively mitigates biases stemming from issues such as reverse causation and confounding variables.
However, several limitations of this study warrant acknowledgment. First, there is heterogeneity in the MR analysis results concerning the causal relationships between body fat percentage and risk of ovarian, endometrial, colorectal, and prostate cancers. However, the scatter plots and funnel plots indicated robustness in the MR analysis results, with the observed heterogeneity having minimal impact on the outcomes. Second, although our objective was to utilize novel, broad, and demographically representative datasets for the two-sample MR analysis, there exists a potential overlap between a subset of the exposure and outcome data. Specifically, the data pertaining to both exposure and bladder cancer were extracted from the UK Biobank database. Nonetheless, sensitivity analyses confirmed the robustness of our findings. Third, the absence of a comprehensive medical record database within our study limits the validation of both the presence and severity of the symptoms under investigation, as well as the verification of various disease types. Consequently, our analysis could only infer generalized causal links among disease categories, rather than delineate specific causal pathways within disease subtypes. Fourth, the scope of our study was confined to European populations, which restricts the generalizability of our findings to this demographic.
Comparison with similar studies
Although diet may play a significant role in the etiology of cancers, our study did not detect a correlation between the percentage of fat in milk consumption and the risk of developing colorectal, ovarian, and endometrial cancers. Nonetheless, in an effort to more thoroughly understand the influence of body fat percentage on tumorigenesis, we have pursued further investigation. This line of inquiry is supported by a body of existing research that has already begun to elucidate the ties between personal adiposity levels and the initiation of cancer. The International Agency for Research on Cancer (IARC) Working Group utilizes BMI, defined as the weight in kilograms divided by the square of the height in meters, as a robust indicator for assessing overall adiposity (29). It is widely accepted that colorectal, ovarian, and endometrial cancers are associated with “excess body fat” with sufficient evidence (29). Additionally, research utilizing whole-body fat mass (WBFM) and whole-body fat-free mass as metrics has revealed significant associations between these anthropometric indicators and an elevated genetic susceptibility to colorectal cancer risk in male cases (30). Our research has paralleled findings within the scientific community by establishing a causal link between elevated adiposity, as denoted by a high body fat percentage, and an increased risk of developing colorectal, ovarian, and endometrial cancers.
Multiple epidemiological studies have robustly demonstrated a positive correlation between milk consumption and the risk of developing prostate cancer (31,32). A comprehensive systematic review, inclusive of studies published up to May 2020, has focused on examining the relationship between the fat content in milk and the risk of prostate cancer (16). However, the current evidence base regarding the association between prostate cancer risk and the consumption of milk of varying fat content—categorized as skim, low-fat, and whole—is limited. It remains unclear whether the elevated risk of prostate cancer is indeed linked to the dietary fat content present in milk (16). Our research findings indicated no correlation between the percentage of fat in milk consumption and the risk of prostate cancer, leading us to hypothesize that other non-fat components within milk may be associated with prostate cancer risk. This notion is supported by other studies. Elevated levels of estrogen within the milk supply have been correlated with the industrialization processes inherent to modern dairy production (33,34). An emerging consensus within the scientific literature indicates that estrogen may exert an influential role in the pathogenesis of prostate cancer (35,36). Focusing on the non-fat components of milk to further assess their impact on the risk of prostate cancer development may be beneficial (16).
A comprehensive analysis has indicated a negative correlation between WBFM, measured by bioelectrical impedance, and the genetic predisposition to prostate cancer (30). Our findings are in concordance with existing literature, illustrating an inverse correlation between body fat percentage and the risk of developing prostate cancer. However, a clinical trial has found that weight management interventions can improve body composition, biomarkers of prostate cancer, and quality of life (37). An increase in a body shape index is significantly correlated with a heightened risk of prostate cancer, particularly in individuals who are classified as underweight/normal weight or obese (38). Visceral adipose tissue has been identified as a risk factor for the recurrence of prostate cancer (39). Therefore, we contend that even if a higher body fat percentage appears to have a modest protective effect against prostate cancer, the pursuit of an excessively high body fat percentage should not be encouraged. The relationship between tumorigenesis and both BMI and body fat remains a subject of debate.
A comprehensive meta-analysis of observational studies has indicated a positive association between higher consumption of whole-fat milk and an elevated risk of bladder cancer (40). However, a previous MR study did not identify a correlation between milk consumption and the risk of bladder cancer (10). Consistent with this MR study, our research has also failed to observe an association between the percentage of fat in milk consumption and bladder cancer. In an effort to further investigate the relationship between body fat percentage and bladder cancer, our study also yielded no significant association between the two variables. The IARC has stated that, given the limited and often incongruous data currently available, a definitive link between BMI (indicator of overall body fat) and the incidence of bladder cancer has yet to be established (29).
Explanations of findings
Recent studies have provided support for an association between milk consumption and the risk of breast cancer (7,41). An MR study suggests that milk consumption is not associated with breast cancer (10). Discrepancies among various studies have generated controversy over the impact of milk consumption on breast cancer risk. Although some research suggests a potential association, the body of evidence is not uniform. Milk includes proteins, fats, and vitamins (6). Recent research in the realms of basic science and clinical studies has shed light on the health effects of milk polar lipids, suggesting their potential to positively modulate dysfunctional lipid metabolism, gut dysbiosis, and inflammation (14). However, both animal experimentation and ecological studies in human populations imply that an increased intake of dietary fat may be associated with a higher incidence of the disease (42). A study has observed that mice fed a diet high in olive oil exhibited fewer and smaller surface nodules in breast cancer compared to those on a high dairy fat diet (17). Our findings are congruent with these studies, indicating a correlation between a high percentage of fat in milk consumption and an elevated risk of breast cancer.
Interestingly, an MR study suggests that milk consumption is positively correlated with overweight and obesity (5). There is a recognized association between body fat and the risk of developing breast cancer, with the majority of studies indicating a positive correlation between obesity and increased breast cancer risk (43-45). However, an MR study has suggested that general obesity may be inversely associated with the risk of breast cancer (18), whereas another MR study found no significant link between visceral fat and the risk of breast cancer (46). However, our research has determined that the body fat percentage does not correlate with the risk of developing breast cancer. This may indicate that the mechanism by which percentage of fat in milk consumption influences breast cancer risk does not involve the impact on body fat percentage. Milk, a complex biological fluid (6), contains a multitude of components for which associations with the etiological mechanisms of breast cancer require further in-depth research.
Implications and actions needed
Given these constraints, future research endeavors should concentrate on elucidating the mechanisms underlying the causal associations within disease subtype classifications, taking into account genetic and other contributing factors across diverse populations. Subsequent inquiries should aim to broaden their scope to include a thorough evaluation of how both the lipid and non-lipid components of milk may influence the risk stratification of neoplastic development.
Conclusions
Our study results indicate that a higher percentage fat in milk consumption is associated with an increased risk of breast cancer. The percentage of body fat demonstrated a positive correlation with the risk factors associated with ovarian, endometrial, and colorectal cancers, contrasting with an inverse correlation observed in the context of prostate cancer risk. The fat percentage in milk was not the decisive factor in determining the impact of milk consumption on the risk of cancer in general. These insights are instrumental for informing the development of targeted cancer prevention strategies within the European population.
Acknowledgments
We extend our heartfelt appreciation to Dr. Jie Liu of the Department of Vascular and Endovascular Surgery at the Chinese PLA General Hospital for his exceptional contributions to our work. His expertise was instrumental in several critical aspects of our research, including the consultation on study design, statistical analysis, and the provision of insightful feedback on the manuscript.
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE-MR reporting checklist. Available at https://tcr.amegroups.com/article/view/10.21037/tcr-24-802/rc
Peer Review File: Available at https://tcr.amegroups.com/article/view/10.21037/tcr-24-802/prf
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at https://tcr.amegroups.com/article/view/10.21037/tcr-24-802/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- The Lancet. Our commitment to help accelerate progress against cancer. Lancet 2022;399:769. [Crossref] [PubMed]
- Schlander M, van Harten W, Retèl VP, et al. The socioeconomic impact of cancer on patients and their relatives: Organisation of European Cancer Institutes task force consensus recommendations on conceptual framework, taxonomy, and research directions. Lancet Oncol 2024;25:e152-63. [Crossref] [PubMed]
- Key TJ, Bradbury KE, Perez-Cornago A, et al. Diet, nutrition, and cancer risk: what do we know and what is the way forward? BMJ 2020;368:m511. [Crossref] [PubMed]
- Lumsden AL, Mulugeta A, Hyppönen E. Milk consumption and risk of twelve cancers: A large-scale observational and Mendelian randomisation study. Clin Nutr 2023;42:1-8. [Crossref] [PubMed]
- Yuan S, Sun J, Lu Y, et al. Health effects of milk consumption: phenome-wide Mendelian randomization study. BMC Med 2022;20:455. [Crossref] [PubMed]
- Melnik BC, John SM, Carrera-Bastos P, et al. The Role of Cow's Milk Consumption in Breast Cancer Initiation and Progression. Curr Nutr Rep 2023;12:122-40. [Crossref] [PubMed]
- Fraser GE, Jaceldo-Siegl K, Orlich M, et al. Dairy, soy, and risk of breast cancer: those confounded milks. Int J Epidemiol 2020;49:1526-37. [Crossref] [PubMed]
- Liao MQ, Gao XP, Yu XX, et al. Effects of dairy products, calcium and vitamin D on ovarian cancer risk: a meta-analysis of twenty-nine epidemiological studies. Br J Nutr 2020;124:1001-12. [Crossref] [PubMed]
- Li X, Zhao J, Li P, et al. Dairy Products Intake and Endometrial Cancer Risk: A Meta-Analysis of Observational Studies. Nutrients 2017;10:25. [Crossref] [PubMed]
- Larsson SC, Mason AM, Kar S, et al. Genetically proxied milk consumption and risk of colorectal, bladder, breast, and prostate cancer: a two-sample Mendelian randomization study. BMC Med 2020;18:370. [Crossref] [PubMed]
- López-Plaza B, Bermejo LM, Santurino C, et al. Milk and Dairy Product Consumption and Prostate Cancer Risk and Mortality: An Overview of Systematic Reviews and Meta-analyses. Adv Nutr 2019;10:S212-23. [Crossref] [PubMed]
- Wu J, Yu Y, Huang L, et al. Dairy Product Consumption and Bladder Cancer Risk: A Meta-Analysis. Nutr Cancer 2020;72:377-85. [Crossref] [PubMed]
- Xiao J, Ma J, Khan MZ, et al. Unlocking the potential of milk whey protein components in colorectal cancer prevention and therapy. Crit Rev Food Sci Nutr 2024;64:12961-98. [Crossref] [PubMed]
- Anto L, Warykas SW, Torres-Gonzalez M, et al. Milk Polar Lipids: Underappreciated Lipids with Emerging Health Benefits. Nutrients 2020;12:1001. [Crossref] [PubMed]
- Bergholdt HKM, Nordestgaard BG, Varbo A, et al. Lactase persistence, milk intake, and mortality in the Danish general population: a Mendelian randomization study. Eur J Epidemiol 2018;33:171-81. [Crossref] [PubMed]
- Sargsyan A, Dubasi HB. Milk Consumption and Prostate Cancer: A Systematic Review. World J Mens Health 2021;39:419-28. [Crossref] [PubMed]
- Velazquez FN, Viscardi V, Montemage J, et al. A Milk-Fat Based Diet Increases Metastasis in the MMTV-PyMT Mouse Model of Breast Cancer. Nutrients 2021;13:2431. [Crossref] [PubMed]
- Freuer D, Linseisen J, O'Mara TA, et al. Body Fat Distribution and Risk of Breast, Endometrial, and Ovarian Cancer: A Two-Sample Mendelian Randomization Study. Cancers (Basel) 2021;13:5053. [Crossref] [PubMed]
- Lawlor DA, Harbord RM, Sterne JA, et al. Mendelian randomization: using genes as instruments for making causal inferences in epidemiology. Stat Med 2008;27:1133-63. [Crossref] [PubMed]
- Verduijn M, Siegerink B, Jager KJ, et al. Mendelian randomization: use of genetics to enable causal inference in observational studies. Nephrol Dial Transplant 2010;25:1394-8. [Crossref] [PubMed]
- Pirastu N, McDonnell C, Grzeszkowiak EJ, et al. Using genetic variation to disentangle the complex relationship between food intake and health outcomes. PLoS Genet 2022;18:e1010162. [Crossref] [PubMed]
- Mbatchou J, Barnard L, Backman J, et al. Computationally efficient whole-genome regression for quantitative and binary traits. Nat Genet 2021;53:1097-103. [Crossref] [PubMed]
- Michailidou K, Lindström S, Dennis J, et al. Association analysis identifies 65 new breast cancer risk loci. Nature 2017;551:92-4. [Crossref] [PubMed]
- Phelan CM, Kuchenbaecker KB, Tyrer JP, et al. Identification of 12 new susceptibility loci for different histotypes of epithelial ovarian cancer. Nat Genet 2017;49:680-91. [Crossref] [PubMed]
- O'Mara TA, Glubb DM, Amant F, et al. Identification of nine new susceptibility loci for endometrial cancer. Nat Commun 2018;9:3166. [Crossref] [PubMed]
- Huyghe JR, Bien SA, Harrison TA, et al. Discovery of common and rare genetic risk variants for colorectal cancer. Nat Genet 2019;51:76-87. [Crossref] [PubMed]
- Schumacher FR, Al Olama AA, Berndt SI, et al. Association analyses of more than 140,000 men identify 63 new prostate cancer susceptibility loci. Nat Genet 2018;50:928-36. [Crossref] [PubMed]
- Machiela MJ, Chanock SJ. LDlink: a web-based application for exploring population-specific haplotype structure and linking correlated alleles of possible functional variants. Bioinformatics 2015;31:3555-7. [Crossref] [PubMed]
- Lauby-Secretan B, Scoccianti C, Loomis D, et al. Body Fatness and Cancer--Viewpoint of the IARC Working Group. N Engl J Med 2016;375:794-8. [Crossref] [PubMed]
- Harris BHL, Di Giovannantonio M, Zhang P, et al. New role of fat-free mass in cancer risk linked with genetic predisposition. Sci Rep 2024;14:7270. [Crossref] [PubMed]
- Aune D, Navarro Rosenblatt DA, Chan DS, et al. Dairy products, calcium, and prostate cancer risk: a systematic review and meta-analysis of cohort studies. Am J Clin Nutr 2015;101:87-117. [Crossref] [PubMed]
- Ganmaa D, Li XM, Wang J, et al. Incidence and mortality of testicular and prostatic cancers in relation to world dietary practices. Int J Cancer 2002;98:262-7. [Crossref] [PubMed]
- Haimov-Kochman R, Shore LS, Laufer N. The milk we drink, food for thought. Fertil Steril 2016;106:1310-1. [Crossref] [PubMed]
- Maruyama K, Oshima T, Ohyama K. Exposure to exogenous estrogen through intake of commercial milk produced from pregnant cows. Pediatr Int 2010;52:33-8. [Crossref] [PubMed]
- Qin LQ, Wang PY, Kaneko T, et al. Estrogen: one of the risk factors in milk for prostate cancer. Med Hypotheses 2004;62:133-42. [Crossref] [PubMed]
- Bonkhoff H, Berges R. The evolving role of oestrogens and their receptors in the development and progression of prostate cancer. Eur Urol 2009;55:533-42. [Crossref] [PubMed]
- Bechtel MD, Michel C, Srinivasan P, et al. Impact of Weight Management on Obesity-Driven Biomarkers of Prostate Cancer Progression. J Urol 2024;211:552-62. [Crossref] [PubMed]
- Liu X, Shi H, Shi Y, et al. Association between a body shape index and prostate cancer: a cross-sectional study of NHANES 2001-2018. Int Urol Nephrol 2024;56:1869-77. [Crossref] [PubMed]
- Greco F, Piccolo CL, D'Andrea V, et al. Fat Matters: Exploring Cancer Risk through the Lens of Computed Tomography and Visceral Adiposity. J Clin Med 2024;13:453. [Crossref] [PubMed]
- Bermejo LM, López-Plaza B, Santurino C, et al. Milk and Dairy Product Consumption and Bladder Cancer Risk: A Systematic Review and Meta-Analysis of Observational Studies. Adv Nutr 2019;10:S224-38. [Crossref] [PubMed]
- Thorning TK, Raben A, Tholstrup T, et al. Milk and dairy products: good or bad for human health? An assessment of the totality of scientific evidence. Food Nutr Res 2016;60:32527. [Crossref] [PubMed]
- Boyd NF, Martin LJ, Noffel M, et al. A meta-analysis of studies of dietary fat and breast cancer risk. Br J Cancer 1993;68:627-36. [Crossref] [PubMed]
- Poltronieri TS, Pérsico RS, Viana LV. Body adipose tissue depots and treatment outcomes for women with breast cancer: A systematic review. Clin Nutr 2024;43:1033-42. [Crossref] [PubMed]
- Islami F, Marlow EC, Thomson B, et al. Proportion and number of cancer cases and deaths attributable to potentially modifiable risk factors in the United States, 2019. CA Cancer J Clin 2024;74:405-32. [Crossref] [PubMed]
- Kakkat S, Suman P, Turbat-Herrera EA, et al. Exploring the multifaceted role of obesity in breast cancer progression. Front Cell Dev Biol 2024;12:1408844. [Crossref] [PubMed]
- Lu Y, Tang H, Huang P, et al. Assessment of causal effects of visceral adipose tissue on risk of cancers: a Mendelian randomization study. Int J Epidemiol 2022;51:1204-18. [Crossref] [PubMed]


