
Elevated serum CD26 level is associated with metastasis and post-operation survival in pancreatic cancer patients
Introduction
CD26/dipeptidyl peptidase IV (DPPIV) has become a research focus in recent years, especially in cancer research. It is a 110-kD transmembrane glycoprotein distributed throughout almost all mammalian tissues and cells, including the epithelium cells of the intestine, liver, bile duct, kidney proximal tubules, pancreas, placenta, lung, thyroid gland, adrenal, and prostate gland (1-4). Soluble CD26 (sCD26) has also been detected in biological fluids, including serum, plasma, urine, synovial, semen, and cerebrospinal fluid (5).
The serum level of sCD26 in patients of a variety of diseases has been of research interest during the past decade, wherein sCD26 in cancer patients has attracted the most attention. Cordero and colleagues found a decreased sCD26 level in colorectal cancer patients, and they found that sCD26 can act as a prognostic marker of early CRC patients (6). Blanco-Prieto found that sCD26, together with calprotectin and EGF, can be applied to identify patients at high-risk for lung cancer (7). Molica and colleagues proved that sCD26 may provide a useful insight into the complex interrelationship of prognostic variables and predict clinical outcome of patients with early B-chronic lymphocytic leukemia. In addition, sCD26 has also been extensively studied in breast cancer (5), melanoma (8), and oral cancer (9). The altered concentration and its clinical significance of sCD26 in cancer patients have been well reviewed by Cordero et al. (10).
Pancreatic ductal adenocarcinoma (PDAC) is the fourth leading cause of cancer related death worldwide. The poor prognosis is mainly attributed to the malignant biological behavior of the tumor. Moreover, another important reason is due to the difficulty in early detection of this disease. Till now, the sCD26 in pancreatic cancer patients has rarely been studied. This study aimed to determine the sCD26 level in different types of pancreatic diseases, including pancreatitis, solid-pseudopapillary tumor (SPT) of the pancreas, pancreatic neuroendocrine neoplasms (pNENs), serous cystadenoma (SC), mucinous cystadenoma (MC), and PDAC. The correlation between sCD26 concentration and the clinicopathological characteristics or post-operation survival of patients with pancreatic cancer will also be assessed.
Methods
Patients
A total of 148 patients were enrolled in this study, including 92 patients with PDAC (age range, 28–82 years), 21 patients with pancreatitis (age range, 17–72 years), 17 patients with SPT (age range, 11–54 years), 9 patients with pNENs (age range, 29–63 years), 5 patients with SC (age range, 38–79 years), 4 patients with MC (age range, 47–81 years). Eighty-six healthy donors (46 men and 40 women; age range, 18–78 years) were also included. Type 2 diabetes mellitus patients were excluded from this study, and nobody received DPP4 inhibitors. All the pancreatic adenocarcinoma patients underwent surgical resection at Peking University Cancer Hospital between May 2010 and June 2012. Patients who had undergone preoperative (neoadjuvant) chemotherapy were excluded. Preoperative serum samples were collected from all the patients, and postoperative serum samples were collected from 49 of 92 PDAC patients, 10 of 17 SPT patients, 6 of 9 pNENs patients, 4 of 5 SC patients, 4 of 4 MC patients, while none of the pancreatitis patients. The median time elapsed between 1st and 2nd blood sampling was 8 (IQR =5) days. We were blind to the information of individual participants during the determination of sCD26 level. The protocol of this study was approved by the ethical committee of Peking University Cancer Hospital (ethical approval number: 2010031426), and written informed consent was obtained from each patient prior to initiation.
Preparation of samples
After collection by venipuncture, all blood samples were stored in serum storage tubes and centrifuged at 1,500 rpm for 15 min at 4 °C. And then the isolated serum was stored at −80 °C until use.
Determination of serum CD26 levels
The serum CD26 levels in all the patients and the healthy donors were determined using enzyme-linked immunosorbent assay (ELISA) kits (Human sCD26 Platinum ELISA kit, eBioscience, Austria) according to the manufacturer’s instructions. Mean sCD26 level was determined by calculating the mean value of duplicate measurements of each sample. Standard curve was developed by plotting the mean absorbance for each standard concentration (calculated in duplicate) on the ordinate against the human sCD26 concentration on the abscissa.
Statistical analysis
Statistical analysis was performed using SPSS 16.0 (SPSS Inc., Chicago, IL, USA). All numeric data were tested for normal distribution using the one-sample Kolmogorov-Smirnov test. Results were presented as mean ± standard deviation (SD) values for variables that follow a normal distribution. The two-tailed Student’s t-test was performed for comparison. Simple bivariate Pearson test were used to study the correlation of sCD26 level before and after operation. The survival curves were estimated by Kaplan-Meier analysis, and P values were calculated by log rank test. The effect of different features on patient survival was evaluated by multivariate analysis with the Cox proportional hazards regression model. Diagnostic value of sCD26 was evaluated by receiver operating characteristic (ROC) curve. P˂0.05 was considered statistically significant.
Results
sCD26 level increased in preoperative pancreatic cancer patients while decreased after operation
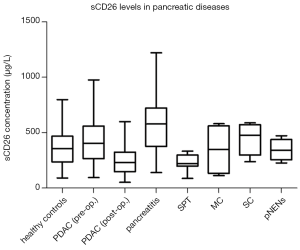
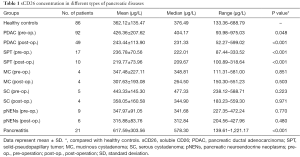
We measured sCD26 level in serum of 86 healthy donors and in preoperative serum of 92 pancreatic cancer patients. Patients in these two groups did not differ in age (P=0.574) and gender (P=0.684). The average sCD26 level in preoperative serum of pancreatic cancer patients was 426.36±207.62 µg/L, which was significantly higher than that in the healthy donors (361.30±154.64 µg/L, P=0.048). In addition, we also tested the postoperative sCD26 in 46 of the above 92 PDAC patients. Interestingly, a dramatic decrease was observed in postoperative sCD26 level, with a sCD26 level of 243.44±113.90 µg/L, which was significantly lower than healthy donors (P<0.001, Figure 1 and Table 1).
Full table
Variable sCD26 levels in other pancreatic diseases
In order to find out whether the changed sCD26 in pancreatic cancer was cancer specific, we also tested the sCD26 levels in other pancreatic diseases (Figure 1 and Table 1). We found variable preoperative sCD26 levels in different diseases, with the highest in pancreatitis (617.59±303.56 µg/L), and the lowest in SPT (236.78±70.56 µg/L). In pNENs patients, the mean sCD26 level was 347.97±91.05 µg/L, while in SC and MC, it was 443.33±145.30 µg/L and 347.48±227.11 µg/L, respectively. When compared with healthy donors and PDAC patients, patients with SPT (P<0.001, both) and pancreatitis (P<0.001, P=0.007, respectively) reached statistical significance.
In addition, we also tested the postoperative sCD26 levels in the above diseases except for pancreatitis, and results showed that after operation, the sCD26 levels decreased in almost all of them, which was consistent with PDAC (Table 1).
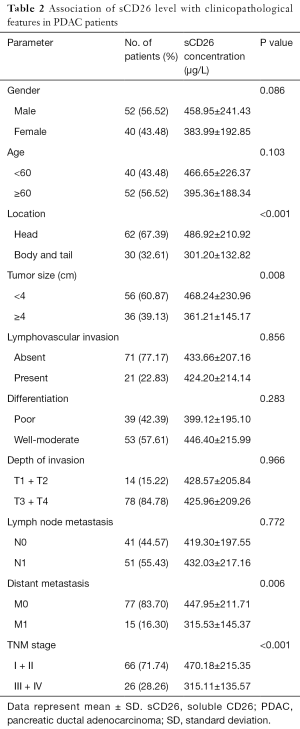
Increased sCD26 level was associated with favorable clinicopathological features of pancreatic cancer patients
Next, we evaluated the correlation of preoperative serum sCD26 level with the clinicopathological features of pancreatic cancer patients, and found that it was correlated with tumor location, tumor size, distant metastasis status and TNM stages. Specifically, sCD26 was associated with tumor location, that was, tumors located at the head of pancreas had a higher sCD26 level than those located at the body and tail of the pancreas (486.92 vs. 301.20 µg/L, P<0.001). sCD26 level was markedly lower in patients with a larger size (>4 cm) than those with a smaller size (≤4 cm) (361.21 vs. 468.24 µg/L, P=0.008).
What’s more, in patients with (n=14) and without metastasis (n=78), sCD26 level was 315.53 and 447.95 µg/L, respectively. sCD26 level was markedly lower in patients with metastasis (P=0.006). Finally, sCD26 was higher in patients with earlier TNM stages than in later ones (470.18 vs. 315.11 µg/L, P<0.001). But in this group of patients, we did not find a significant correlation between preoperative serum sCD26 level and gender, age, differentiation, lymphovascular invasion, depth of invasion and lymph node metastasis of pancreatic cancer patients. Detailed results are shown in Table 2.
Full table
Increased sCD26 level was associated with a longer survival time in pancreatic cancer patients
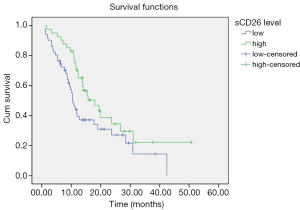
Follow-up data were available for all the 92 PDAC patients. Follow-up time was measured from the operation date to time of death or the last follow-up visit. The follow-up time of all the patients was from August 2010 to September 2015. During the follow-up period, 61 (66.30%) patients died of pancreatic cancer. Median follow-up duration was 11.85 months (range, 1.13–50.73 months). For survival analysis, we chose a cut-off level of 430 µg/L, which was around the mean concentration of preoperative sCD26 concentration, to define high and low sCD26 concentration groups (≤430 and >430 µg/L, respectively). Kaplan-Meier analysis and log-rank test showed that patients with lower sCD26 level had a poorer post-operation survival (median survival time 10.47 vs. 18.00 months, P=0.044) (Figure 2). Furthermore, to test whether sCD26 level was an independent prognostic factor for PDAC patients, we performed a multivariate survival analysis, in which those parameters associated with overall survival in univariate survival analysis were included. In multivariate analysis, lymph node metastasis (P=0.014), distant metastasis (P<0.001) were independent prognostic factors related with overall survival of pancreatic cancer patients, while preoperative sCD26 level was not (P=0.075). Detailed data are shown in Table 3.

Full table
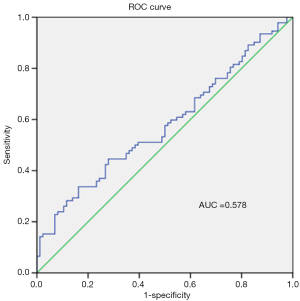
sCD26 was not a favourable diagnosis biomarker for PDAC patients
As sCD26 has been reported to be a potential diagnostic biomarker for certain diseases, we evaluated its diagnostic value in PDAC patients through ROC curve analysis. As shown in Figure 3, the area under curve (AUC) was only 0.578 (SE =0.043, 95% CI: 0.494–0.662), which was too low to be used as a diagnosis biomarker.
Discussion
As a multi-functional protein, CD26/DPPIV is involved in several biological and pathological processes, including activation of T lymphocytes; degradation of a variety of chemokines and peptides via DPPIV enzymatic activity, which mediates its function in immunological reaction and regulation and cancer progression (11-14). Soluble form of CD26 is important for the biological function of CD26. Previous studies have investigated the relationship between sCD26 level and different types of malignancies, including malignant hematological tumors and several types of solid tumors (6,15,16), but few examined the relationship between sCD26 level and pancreatic cancer. It has been well documented that CD26 is expressed by acinar cells, the islets, ductal cells, and the endothelium of venules or capillary bed of the pancreas (2,10,17,18). While in one study, sitagliptin, an approved CD26 inhibitor used for the treatment of type 2 diabetes, was proved to increase ductal cell turnover and to induce ductal cell metaplasia, both of which are significant risk factors for pancreatic ductal cancer (19). So it is very important to evaluate the sCD26 level and its clinical significance in malignant tumors patients (including PDAC) when CD26 inhibitors are listed as prescription drugs.
In previous studies, sCD26 level was found to be decreased in most types of cancers. Javidroozi and colleagues found that in 139 healthy volunteers and 561 cancer patients (with various malignancies), plasma sCD26 level was significantly lower in cancer patients compared with healthy subjects (4.38 vs. 5.65 µg/mL, P<0.001) (20), and this was supported by other studies, such as in lung cancer (7), melanoma (8), colorectal cancer (6). But in pancreatic and hepatic cancer patients, things seem to be different. In Javidroozi’s study, liver/pancreas cancers (n=8, together) had significantly higher sCD26 (5.99±3.05 µg/mL) than healthy donors (20). And in our study, the average serum CD26 level was higher in 92 PDAC patients than healthy donors. In addition, we also found that higher sCD26 levels were detected in patients with smaller tumor size, without distant metastasis, earlier TNM stages and a longer survival time. This was in accordance with Javidroozi’s study, that a higher DPPIV was associated with better survival in a cohort of 346 cancers patients (20). It seems that sCD26 might play a protective role in pancreatic cancer and other cancers, and we presume that the underlying mechanisms may be attributed to its regulatory effect on the immunity status of the human body. Through binding with the extracellular matrix proteins CD45 and ADA, sCD26 may enhance T cell activation, which is a very crucial process of immunoregulation (21,22). sCD26 can also promote T cell proliferation independent of ADA binding or even its enzymatic activity (23,24). A recent study in colon cancer has given us new clues. Cutler and colleagues (25) found that conventional chemotherapeutic agents could attenuate CXCL12-mediated migration of colon cancer cells by selecting for CXCR4-negative cells and increasing peptidase CD26, because increased sCD26 can degrade CXCL12 through its DPPIV activity, leading to the blockade of CXCL12-CXCR4 axis, which plays an important role in a variety of cancers, including pancreatic cancer (26,27).
Cordero et al. reported that DPPIV enzymatic activity was high in patients with hepatic cancer, hepatitis, osteoporosis, cholestasis and other liver diseases (10). Based on this, the hepatobiliary system was the first to be suggested as the origin of sCD26 in the human body (28), which is still an open question. Our results provided evidence that the pancreas organ may also be among physiologic sources of sCD26, which has been assumed before but without bold evidence. First, unlike in other cancers, the sCD26 level is increased in pancreatic cancer and pancreatitis patients, which is similar with hepatic diseases. In addition, we found that after operation, the sCD26 level was prominently decreased in almost all the patients who suffered a tumor, malignant or non-malignant, in some even lower than the healthy donors. Immune cells, especially activated T lymphocytes, stimulated B and natural killer (NK) cells, and the hepatobiliary system were traditionally thought to be the origins of sCD26. Now in this study, we provided new possibilities.
As the sCD26 level always changed in response to diseases including but not limited to cancers (8,29), we also tested in this study the sCD26 levels in other pancreatic diseases, including pancreatitis, SPT, pNENs, SC, MC, with variable results in different groups. Specially, we noted that sCD26 level strikingly increased in pancreatitis patients. As pancreatitis patients always suffer a systemic inflammatory response, we presume that the increase of sCD26 level in these patients might be attributed to its established role in T-cell immune responses.
As sCD26 has been suggested as a diagnostic marker in different cancer types, such as gastric cancer (30), colorectal cancer (6), and lung cancer (7), we also performed the ROC analysis to evaluate the diagnostic value of sCD26 for PDAC patients. While unfortunately, the result was unsatisfactory. This might be caused by relatively small sample size, so in future study, we will enlarge the sample size so as to further assess the potential diagnosis value of sCD26 in PDAC patients.
There are some limitations of this study. First, the sample size was not large enough, which might bring statistical bias into the results. Second, as it has been reported that serum sCD26 levels are associated with state of patient nourishment (31), in this cohort, poor-nourished patients after pancreas surgery might be included in this cohort, so the post-operative results might not be accurate enough. Still we think this may not affect final results markedly, because it is indicated that in patients with eating disorders, the DPPIV activity was consequently increased (31). This suggests that although we detected a significantly decreased sCD26 concentration after operation, the actual effect of the pancreas operations on the sCD26 level might be even more potent. Blood collected after a longer interval from the operation will provide more accuracy of the tested results. Lastly, as CD26 molecule contains DPPIV enzyme activity and almost 90–95% of DPPIV activity are associated with serum sCD26, it is better to measure the serum level of DPPIV enzyme activity, so as to provide more evidence to confirm the results.
In summary, in this study, we have found an increased preoperative sCD26 level in PDAC patients than healthy donors, and the preoperative sCD26 level was associated with favorable clinicopathological features and a longer survival time of the patients. sCD26 levels in other pancreatic diseases, including pancreatitis, SPT, pNENs, SC, MC were also determined. Although we did not initially intend to explore the origin of sCD26, we did provide possible evidence that the pancreas may be listed as one of the physiological sources of sCD26.
Acknowledgments
Funding: This study was supported by International Science & Technology Cooperation Program of China (approval No. 2013DFG32720); the Capital Health Research and Development of Special (approval No. 2016-2-2151); National Natural Science Foundation of China (approval No. 81272765, 61571437, 61372028, and 81441071); Capital Characteristic Clinical Application Research (approval No. Z161100000516065).
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2016.08.38). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The protocol of this study was approved by the ethical committee of Peking University Cancer Hospital (ethical approval number: 2010031426), and written informed consent was obtained from each patient prior to initiation.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Heike M, Möbius U, Knuth A, et al. Tissue distribution of the T cell activation antigen Ta1. Serological, immunohistochemical and biochemical investigations. Clin Exp Immunol 1988;74:431-4. [PubMed]
- Hartel S, Gossrau R, Hanski C, et al. Dipeptidyl peptidase (DPP) IV in rat organs. Comparison of immunohistochemistry and activity histochemistry. Histochemistry 1988;89:151-61. [Crossref] [PubMed]
- Fleischer B. CD26: a surface protease involved in T-cell activation. Immunol Today 1994;15:180-4. [Crossref] [PubMed]
- Mizutani S, Sumi S, Narita O, et al. Purification and properties of human placental dipeptidyl peptidase IV. Nihon Sanka Fujinka Gakkai Zasshi 1985;37:769-75. [PubMed]
- Erić-Nikolić A, Matić IZ, Dorđević M, et al. Serum DPPIV activity and CD26 expression on lymphocytes in patients with benign or malignant breast tumors. Immunobiology 2011;216:942-6. [Crossref] [PubMed]
- Cordero OJ, Ayude D, Nogueira M, et al. Preoperative serum CD26 levels: diagnostic efficiency and predictive value for colorectal cancer. Br J Cancer 2000;83:1139-46. [Crossref] [PubMed]
- Blanco-Prieto S, Vázquez-Iglesias L, Rodríguez-Girondo M, et al. Serum calprotectin, CD26 and EGF to establish a panel for the diagnosis of lung cancer. PLoS One 2015;10:e0127318 [Crossref] [PubMed]
- Matić IZ, Ðorđić M, Grozdanić N, et al. Serum activity of DPPIV and its expression on lymphocytes in patients with melanoma and in people with vitiligo. BMC Immunol 2012;13:48. [Crossref] [PubMed]
- Urade M, Komatsu M, Yamaoka M, et al. Serum dipeptidyl peptidase activities as a possible marker of oral cancer. Cancer 1989;64:1274-80. [Crossref] [PubMed]
- Cordero OJ, Salgado FJ, Nogueira M. On the origin of serum CD26 and its altered concentration in cancer patients. Cancer Immunol Immunother 2009;58:1723-47. [Crossref] [PubMed]
- Oravecz T, Pall M, Roderiquez G, et al. Regulation of the receptor specificity and function of the chemokine RANTES (regulated on activation, normal T cell expressed and secreted) by dipeptidyl peptidase IV (CD26)-mediated cleavage. J Exp Med 1997;186:1865-72. [Crossref] [PubMed]
- Thompson MA, Ohnuma K, Abe M, et al. CD26/dipeptidyl peptidase IV as a novel therapeutic target for cancer and immune disorders. Mini Rev Med Chem 2007;7:253-73. [Crossref] [PubMed]
- Ohtsuki T, Tsuda H, Morimoto C. Good or evil: CD26 and HIV infection. J Dermatol Sci 2000;22:152-60. [Crossref] [PubMed]
- Arscott WT, LaBauve AE, May V, et al. Suppression of neuroblastoma growth by dipeptidyl peptidase IV: relevance of chemokine regulation and caspase activation. Oncogene 2009;28:479-91. [Crossref] [PubMed]
- Molica S, Digiesi G, Mirabelli R, et al. Serum level of CD26 predicts time to first treatment in early B-chronic lymphocytic leukemia. Eur J Haematol 2009;83:208-14. [Crossref] [PubMed]
- de la Haba-Rodríguez J, Macho A, Calzado MA, et al. Soluble dipeptidyl peptidase IV (CD-26) in serum of patients with colorectal carcinoma. Neoplasma 2002;49:307-11. [PubMed]
- McCaughan GW, Wickson JE, Creswick PF, et al. Identification of the bile canalicular cell surface molecule GP110 as the ectopeptidase dipeptidyl peptidase IV: an analysis by tissue distribution, purification and N-terminal amino acid sequence. Hepatology 1990;11:534-44. [Crossref] [PubMed]
- Abbott CA, Baker E, Sutherland GR, et al. Genomic organization, exact localization, and tissue expression of the human CD26 (dipeptidyl peptidase IV) gene. Immunogenetics 1994;40:331-8. [Crossref] [PubMed]
- Matveyenko AV, Dry S, Cox HI, et al. Beneficial endocrine but adverse exocrine effects of sitagliptin in the human islet amyloid polypeptide transgenic rat model of type 2 diabetes: interactions with metformin. Diabetes 2009;58:1604-15. [Crossref] [PubMed]
- Javidroozi M, Zucker S, Chen WT. Plasma seprase and DPP4 levels as markers of disease and prognosis in cancer. Dis Markers 2012;32:309-20. [Crossref] [PubMed]
- Ludwig K, Fan H, Dobers J, et al. 3D structure of the CD26-ADA complex obtained by cryo-EM and single particle analysis. Biochem Biophys Res Commun 2004;313:223-9. [Crossref] [PubMed]
- Busek P, Malík R, Sedo A. Dipeptidyl peptidase IV activity and/or structure homologues (DASH) and their substrates in cancer. Int J Biochem Cell Biol 2004;36:408-21. [Crossref] [PubMed]
- Röhrborn D, Wronkowitz N, Eckel J. DPP4 in Diabetes. Front Immunol 2015;6:386. [Crossref] [PubMed]
- Yu DM, Slaitini L, Gysbers V, et al. Soluble CD26 / dipeptidyl peptidase IV enhances human lymphocyte proliferation in vitro independent of dipeptidyl peptidase enzyme activity and adenosine deaminase binding. Scand J Immunol 2011;73:102-11. [Crossref] [PubMed]
- Cutler MJ, Lowthers EL, Richard CL, et al. Chemotherapeutic agents attenuate CXCL12-mediated migration of colon cancer cells by selecting for CXCR4-negative cells and increasing peptidase CD26. BMC Cancer 2015;15:882. [Crossref] [PubMed]
- Dupont VN, Gentien D, Oberkampf M, et al. A gene expression signature associated with metastatic cells in effusions of breast carcinoma patients. Int J Cancer 2007;121:1036-46. [Crossref] [PubMed]
- Ying X, Jing L, Ma S, et al. GSK3β mediates pancreatic cancer cell invasion in vitro via the CXCR4/MMP-2 Pathway. Cancer Cell Int 2015;15:70. [Crossref] [PubMed]
- Hino M, Nagatsu T, Kakumu S, et al. Glycylprolyl beta-naphthylamidase activity in human serum. Clin Chim Acta 1975;62:5-11. [Crossref] [PubMed]
- Mahmoudi M, Hedayat M, Aghamohammadi A, et al. Soluble CD26 and CD30 levels in patients with common variable immunodeficiency. J Investig Allergol Clin Immunol 2013;23:120-4. [PubMed]
- Boccardi V, Marano L, Rossetti RR, et al. Serum CD26 levels in patients with gastric cancer: a novel potential diagnostic marker. BMC Cancer 2015;15:703. [Crossref] [PubMed]
- Hildebrandt M, Rose M, Mönnikes H, et al. Eating disorders: a role for dipeptidyl peptidase IV in nutritional control. Nutrition 2001;17:451-4. [Crossref] [PubMed]


