
Intracranial efficacy of crizotinib versus chemotherapy in PROFILE 1014: shining a light on central nervous system endpoints in clinical trials
PROFILE 1014
The appropriate management of non-small cell lung cancer (NSCLC) that has spread to the central nervous system (CNS) is becoming an increasingly important clinical issue and nowhere is this more obvious than in the battleground of developing tyrosine kinase inhibitors (TKI) for the treatment of anaplastic lymphoma kinase (ALK)-positive disease. In the 1st line PROFILE 1014 study, which compared crizotinib with platinum-pemetrexed chemotherapy in patients with ALK positive disease, 23% of patients had baseline CNS disease, with estimates of the lifetime incidence of CNS disease in ALK positive NSCLC approaching 50% (1,2). In contrast, baseline estimates of CNS disease in potentially operable NSCLC (not otherwise specified) have been quoted at 7%, and in 1st line trials for advanced epidermal growth factor receptor (EGFR) mutant NSCLC at 12–14% (3,4). Consequently, although small studies looking at the pattern of metastatic spread at diagnosis of stage IV disease have not identified the CNS as a site of spread that is significantly different between dominant oncogene-addicted subtypes of NSCLC, including ALK, there may still be some inherent tropism of ALK positive disease for the CNS (5). In addition, the successful development of therapies to control the extra-CNS disease in ALK positive NSCLC for months and sometimes years is likely to exaggerate the impact of even a small propensity for CNS spread through its cumulative manifestation over time.
Recognizing the distinct risks of CNS metastases in ALK positive NSCLC, PROFILE 1014 represents the first completed phase III clinical trial to prospectively measure the efficacy of an ALK inhibitor in the CNS as one of its defined endpoints (1). Patients with baseline CNS metastases were permitted to enter the trial provided that their CNS disease was treated and neurologically stable for ≥2 weeks with no ongoing corticosteroid requirement. The main CNS efficacy endpoint in PROFILE 1014 was intracranial time-to-progression (IC-TTP) which was defined as the time from randomization to objective worsening of existing intracranial lesions or the development of new intracranial lesions. Patients with a baseline history of CNS disease had repeat CNS imaging every 6 weeks and if they had no history of CNS disease, every 12 weeks.
In the intention-to-treat (ITT) population (343 patients), crizotinib demonstrated a non-significant trend towards an improved IC-TTP compared to up to 6 cycles of platinum-pemetrexed chemotherapy (with no pemetrexed continuation maintenance option): median not reached vs. 17.8 months, HR =0.60; 95% confidence intervals (CI): 0.34 to 1.05; P=0.068. In the subgroup with baseline treated and stable brain metastases (tBM; 79 patients), a similar non-significant trend was noted with median IC-TTP 15.7 vs. 12.5 months for crizotinib and chemotherapy, respectively (HR =0.45; 95% CI: 0.19 to 1.07; P=0.063). In the subgroup without baseline CNS disease (263 patients), again a non-significant trend was noted with the median IC-TTP not reached in either treatment group (HR =0.69; 95% CI: 0.33 to 1.45; P=0.323).
Therefore, with regard to the protocol defined IC-TTP endpoint, no significant difference between the interventions can be claimed, although there were consistent non-significant trends in favor of the crizotinib in all groups analyzed. Importantly, much of the dataset remains immature and therefore whether any of these trending differences will become significant later remains to be seen.
To generate CNS efficacy data which would mature sooner than IC-TTP, the study investigators performed a post-hoc analysis of the intracranial disease control rate (IC-DCR) at 12 and 24 weeks, which is the major new data contained in this publication, separate from the previously published main trial report (6). The IC-DCR was defined as the percentage of patients with confirmed complete response, partial response, or stable disease in the tBM subgroup at the defined time points. At 12 weeks, the IC-DCR was 85% (95% CI: 70% to 94%) and 45% (95% CI: 29% to 62%) for crizotinib and chemotherapy, respectively (P<0.001). At 24 weeks, the IC-DCR was 56% (95% CI: 40% to 72%) and 25% (95% CI: 13% to 41%) for crizotinib and chemotherapy, respectively (P=0.006).
Overall antitumor activity demonstrated similar statistically significant improvements in progression free survival (PFS; the primary endpoint of the study) with crizotinib over chemotherapy, as in the main IIT analysis, regardless of the presence or absence of baseline CNS disease. In the tBM group, median PFS was 9 vs. 4 months for crizotinib and chemotherapy, respectively (HR =0.40; 95% CI: 0.23–0.69, P≤0.001). In the BM absent group, median PFS was 11.1 vs. 7.2 months for crizotinib and chemotherapy, respectively (HR =0.51; 95% CI: 0.38–0.69, P≤0.001). Similarly, the objective response rate (ORR) was significantly higher with crizotinib than with chemotherapy, as in the main IIT analysis, regardless of the presence or absence of baseline CNS disease.
Discussion on the CNS activity of crizotinib in PROFILE 1014
Previously it has been reported that 46–72% of ALK positive NSCLC patients receiving treatment with crizotinib first progress within the brain and that this is the only site of progression in over 80% of these cases (7,8). From a single case, in which matched blood and cerebrospinal fluid (CSF) crizotinib levels were assessed <0.3% of the levels present in the blood were seen in the CSF, suggesting a plausible pharmacokinetic explanation for the disconnect in activity seen between the body and the brain with this drug (9). Consistent with this, when CNS activity was assessed retrospectively within the Pfizer trials database, among those with untreated measurable disease in the brain at the start of crizotinib therapy, the CNS objective response rate was only 18% (compared to 53% systemically); the median duration of these CNS responses was nearly half that of the systemic response data (26.4 vs. 47.9 weeks, respectively) and the median time to progression was 7 months intracranially, compared to 12.7 months systemically (8).
With multiple prior retrospective reports commenting on the limited activity of crizotinib in the CNS, does the prospective data from PROFILE 1014 now make the case stronger for using crizotinib as first line therapy in patients with CNS disease at baseline?
Well, the answer is both ‘yes’ and ‘no.’ In addition, understanding why there isn’t a simple answer to this question starts to shine a revealing light on just how we are learning to better design and interpret efficacy endpoints relating to CNS metastases within modern cancer clinical trials.
At the most superficial level, it is simple enough to argue that the protocol defined endpoint of a statistically significant improvement in IC-TTP was not met and the IC-DCRs at 12 and 24 weeks represent post-hoc assessments based on relatively few events and are therefore of more questionable validity. For example, among the tBM group, only 21 of 79 patients (27%), across both arms had experienced a CNS progression event at the time of analysis. Among the BM absent group, only 30 of 263 patients (11%) across both arms had experienced a CNS progression event. In addition, as CNS lesions were previously treated and did not have to be of a given size, these were not assessed as RECIST target lesions. Instead, intracranial progression as it related to both IC-TTP and IC-DCR was only defined as either the development of new lesions or ‘worsening’ of disease. In the absence of specific size or percentile change criteria, the term ‘worsening’ was therefore open to subjective variations in interpretation. The consistent general anti-cancer benefit of crizotinib over chemotherapy in terms of both PFS and ORR in the tBM and absent BM subgroups (just as in the overall ITT population) also cannot be interpreted as clearly showing CNS benefit, as the events driving these endpoints (progression/non-progression and non-response/response) will have been overwhelmingly dominated by extra-CNS events. When progression did occur, the CNS was still the sole site of progression in a higher proportion of crizotinib than chemotherapy treated cases, in both the tBM (38% vs. 23%, respectively) and absent BM (19% vs. 6%, respectively) subgroups suggesting that the CNS remains a prominent Achilles heel for crizotinib. That said, it should be recognized that progression occurred on average at a significantly later date with crizotinib than with chemotherapy. So while PROFILE 1014 may not have conclusively proven that crizotinib is better than chemotherapy within the CNS, it also hasn’t shown that it is any worse and it is clearly better when considering efficacy within the patient as a whole, reliably solidifying crizotinib’s case as the initial treatment choice in advanced ALK positive NSCLC compared to chemotherapy.
However, before we come to the conclusion that we don’t have to pay any particular attention to the CNS when we start patients on crizotinib, we should recall the specific details of this study. Patients with CNS disease were only permitted to be enrolled when that disease was treated and stable. Consequently, any conclusions from this study regarding the ‘efficacy’ of using crizotinib for those with established CNS metastases can only be applied to those with CNS disease that has been treated before the drug is commenced. More importantly though, we also have to consider whether this requirement for a priori CNS treatment could, in fact, have influenced the endpoints being assessed within the study.
Among the tBM subgroup, while the significant improvement in IC-DCR at both 12 weeks and 24 weeks for crizotinib over chemotherapy could reflect a true benefit from ALK inhibition in the brain, it could also have been confounded by other differences between the two arms. One of the major variables not presented (besides the number of CNS deposits present in the patients) was the exact nature of the CNS treatments used. While stereotactic surgery or radiosurgery (SRS) should reduce the potential of an individual CNS locus to later progress, the risk of progression at other sites within the brain remains unchanged. In contrast, whole brain radiotherapy (WBRT) may have more of a general protective effect across the entire brain parenchyma and/or cerebral leptomeninges, in addition to any impact on the overall permeability of the blood-brain barrier to subsequent systemic drug exposure (9). In the absence of detailed information on the type of prior treatment, in order to ascribe the IC-DCR benefit to the differences in drug intervention alone, we are left to assume that the rate of WBRT (and its potential broader CNS benefit) was equally distributed between the crizotinib and chemotherapy arms. Yet, given that there were only 39 and 40 cases in the crizotinib and chemotherapy arms of the tBM subgroup, respectively, significant imbalances in the rate of WBRT, when it was not a planned stratification factor, could certainly have occurred.
Admittedly, the consistent numerical improvements in IC-TTP present in both the tBM and the absent BM subgroups suggest that differences in CNS efficacy are unlikely to solely be due to imbalances in prior radiation (given that the absent BM subgroup would not have received any prior therapy). However, as all of these IC-TTP improvements remain non-significant to date, any use of these data to support such an argument would have to be qualified by noting that any of these IC-TTP trends could also have occurred by chance.
So where does this leave us from the trials perspective?
A recent survey of 413 open trials assessing systemic drug therapy for adult patients with advanced NSCLC within the clinicaltrials.gov database revealed that 14 and 19% of trials excluded patients with any history of CNS parenchymal or leptomeningeal metastatic disease, respectively (10). Furthermore, 19% of trials contained no explicit mention of CNS disease in their available inclusion/exclusion criteria. Consequently, given the increasing clinical concern about the CNS as a relevant battleground in the treatment of advanced NSCLC, PROFILE 1014 should be applauded for specifically permitting patients with CNS disease entry into the trial in the first place. In addition, it should be applauded for making prospectively defined CNS efficacy (IC-TTP) a prominent secondary endpoint, heralding a move away from some of the problems commonly associated with retrospective analyses of CNS data (9). To further address the issues associated with optimizing clinical trial designs for assessing CNS activity in metastatic disease, the Response Assessment in Neuro-Oncology (RANO) group, an independent, international, collaborative effort, has now begun to publish a series of guidelines on this topic (11,12). Clearly, one area in need of greater attention is the issue of accurately documenting and assessing the potential impact of prior CNS therapy on CNS related endpoints in subsequent drug trials. As in PROFILE 1014, in the clinicaltrials.gov analysis, 41% of trials permitted CNS disease only after prior CNS-directed treatment, which, at best, may limit the interpretation of CNS drug activity due to an overall stabilizing/protecting effect on the CNS and, at worst, if the specific modality (i.e., WBRT) is not balanced between the arms could confound the attribution of any apparent drug benefit in randomized trials (9,10).
And from the first-line ALK positive patient perspective?
Together with the existing data on the limited activity of crizotinib in untreated brain metastases, the new data from PROFILE 1014 in the setting of treated CNS disease, helps us to sketch out a practical decision tree with regard to appropriate action plans for a treatment naïve ALK positive NSCLC patient with parenchymal CNS metastases at diagnosis (Figure 1). In the setting of asymptomatic CNS disease it may be reasonable to commence crizotinib treatment and watch the CNS closely, given that the activity of crizotinib in the CNS is modest, but not zero. On the other hand, if the patient were symptomatic from parenchymal CNS disease, local CNS treatment should probably be utilized up front rather than relying on the crizotinib to do the job, when it will not be sufficient in most cases.
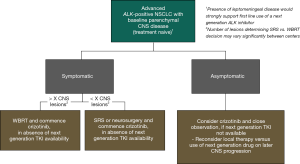
An ongoing debate relates to the number of CNS lesions that should prompt a decision for WBRT rather than SRS, which may be influenced by many different factors including access to specific equipment, health economic analyses, regional or national guidelines and general medical philosophies relating to the need to treat more than a certain number of deposits as if a field effect were present versus the utility (or futility) of treating each site individually. However, in the setting of ALK positive disease, the WBRT versus SRS decision also has to consider the emerging data on the marked longevity of these patients. Among 90 patients with CNS disease from ALK positive NSCLC, the median overall survival was 49.5 months, more than enough time to manifest significant cognitive side-effects from WBRT (13). Consequently, a proposal for WBRT might give us pause for thought and, beyond pushing the upper limit of the number of lesions considered appropriate for SRS, prompt us to look for other options (Figure 1). Fortunately, such options are becoming increasingly available. A number of next generation ALK inhibitors with significant activity against disease in the CNS are now either FDA approved in the USA post-crizotinib, approved in the first line setting in other countries, or are being explored in clinical trials across several different lines of therapy, including in the first line setting. For example, both alectinib and brigatinib have shown CNS response rates over 50% in the post-crizotinib setting and are being explored in the first line setting compared to crizotinib (14,15). In addition, in the J-ALEX study conducted in Japan, alectinib has already shown a significantly longer progression free survival compared to crizotinib in the treatment naive or post-chemotherapy (but ALK inhibitor naïve) setting, solidifying its existing first line license in that country [HR =0.34 (95% CI: 0.17–0.71)] (16). Among those with CNS disease at baseline, the magnitude of benefit from alectinib was even more marked [HR =0.08 (95% CI: 0.01–0.61)]. Whether the absolute difference in PFS will justify transitioning next generation drugs into the front-line for all ALK positive patients, rather than keeping them for sequential use post-crizotinib remains to be determined. However, when they are available, either because they are licensed, or through off-label or trial use, the presence of CNS disease at baseline is likely to be a key factor driving their first-line use over crizotinib, allowing any CNS radiotherapy, but especially WBRT, to be avoided, at least for a while.
Summary
PROFILE 1014 is the first phase III, randomized controlled trial that has prospectively studied the CNS efficacy of crizotinib compared to platinum-pemetrexed chemotherapy in ALK positive NSCLC, including among those with stable, treated CNS disease. Overall, PROFILE 1014 has given us valuable information to inform our optimal first line treatment decision for those with CNS disease at baseline, reassuring us about the efficacy of local treatment and use of crizotinib in these patients (Figure 1). It also highlights some of the design aspects that still need to be addressed for future clinical trials if we are to most informatively assess the activity of drugs in the CNS. Many next generation ALK inhibitors have been associated with significantly increased CNS activity compared to crizotinib and are now entering the clinic. Their CNS activity is so significant that their initial use could potentially allow the use of local CNS therapies, such as radiotherapy, to be deferred for those with CNS disease at baseline. Capturing robust CNS endpoints and learning the lessons from PROFILE 1014 will be vital if we are to determine the optimal use of these new drugs, among those both with and without CNS disease at baseline, in the future.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned and reviewed by the Section Editor Shaohua Cui (Department of Pulmonary Medicine, Shanghai Chest Hospital, Shanghai Jiao Tong University, Shanghai, China).
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2016.09.38). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Solomon BJ, Cappuzzo F, Felip E, et al. Intracranial Efficacy of Crizotinib Versus Chemotherapy in Patients With Advanced ALK-Positive Non-Small-Cell Lung Cancer: Results From PROFILE 1014. J Clin Oncol 2016;34:2858-65. [Crossref] [PubMed]
- Shaw AT, Yeap BY, Solomon BJ, et al. Effect of crizotinib on overall survival in patients with advanced non-small-cell lung cancer harbouring ALK gene rearrangement: a retrospective analysis. Lancet Oncol 2011;12:1004-12. [Crossref] [PubMed]
- Yokoi K, Kamiya N, Matsuguma H, et al. Detection of brain metastasis in potentially operable non-small cell lung cancer: a comparison of CT and MRI. Chest 1999;115:714-9. [Crossref] [PubMed]
- Schuler M, Wu YL, Hirsh V, et al. First-Line Afatinib versus Chemotherapy in Patients with Non-Small Cell Lung Cancer and Common Epidermal Growth Factor Receptor Gene Mutations and Brain Metastases. J Thorac Oncol 2016;11:380-90. [Crossref] [PubMed]
- Doebele RC, Lu X, Sumey C, et al. Oncogene status predicts patterns of metastatic spread in treatment-naive nonsmall cell lung cancer. Cancer 2012;118:4502-11. [Crossref] [PubMed]
- Solomon BJ, Mok T, Kim DW, et al. First-line crizotinib versus chemotherapy in ALK-positive lung cancer. N Engl J Med 2014;371:2167-77. [Crossref] [PubMed]
- Weickhardt AJ, Scheier B, Burke JM, et al. Local ablative therapy of oligoprogressive disease prolongs disease control by tyrosine kinase inhibitors in oncogene-addicted non-small-cell lung cancer. J Thorac Oncol 2012;7:1807-14. [Crossref] [PubMed]
- Costa DB, Shaw AT, Ou SH, et al. Clinical Experience With Crizotinib in Patients With Advanced ALK-Rearranged Non-Small-Cell Lung Cancer and Brain Metastases. J Clin Oncol 2015;33:1881-8. [Crossref] [PubMed]
- Noonan SA, Camidge DR. PROFILE 1014: lessons for the new era of lung cancer clinical research. Transl Lung Cancer Res 2015;4:642-8. [PubMed]
- McCoach CE, Berge EM, Lu X, et al. A Brief Report of the Status of Central Nervous System Metastasis Enrollment Criteria for Advanced Non-Small Cell Lung Cancer Clinical Trials: A Review of the ClinicalTrials.gov Trial Registry. J Thorac Oncol 2016;11:407-13. [Crossref] [PubMed]
- Lin NU, Lee EQ, Aoyama H, et al. Challenges relating to solid tumour brain metastases in clinical trials, part 1: patient population, response, and progression. A report from the RANO group. Lancet Oncol 2013;14:e396-406. [Crossref] [PubMed]
- Lin NU, Lee EQ, Aoyama H, et al. Response assessment criteria for brain metastases: proposal from the RANO group. Lancet Oncol 2015;16:e270-8. [Crossref] [PubMed]
- Johung KL, Yeh N, Desai NB, et al. Extended Survival and Prognostic Factors for Patients With ALK-Rearranged Non-Small-Cell Lung Cancer and Brain Metastasis. J Clin Oncol 2016;34:123-9. [Crossref] [PubMed]
- Gadgeel SM, Gandhi L, Riely GJ, et al. Safety and activity of alectinib against systemic disease and brain metastases in patients with crizotinib-resistant ALK-rearranged non-small-cell lung cancer (AF-002JG): results from the dose-finding portion of a phase 1/2 study. Lancet Oncol 2014;15:1119-28. [Crossref] [PubMed]
- Camidge DR, Bazhenova L, Salgia R, et al. Safety and efficacy of brigatinib (AP26113) in advanced malignancies, including ALK+ non–small cell lung cancer (NSCLC). J Clin Oncol 2015;33:abstr 8062.
- Rossi A. Alectinib for ALK-positive non-small-cell lung cancer. Expert Rev Clin Pharmacol 2016;9:1005-13. [Crossref] [PubMed]


