
Neurofibromatosis with diffuse intestinal ganglioneuromatosis: a case report
Introduction
Neurofibromatosis type 1 (NF-1), a rare autosomal dominant hereditary disorder, has an incidence of 1 in 3,000 individuals. It is caused by germline mutations in the NF1 gene. The NF1 gene encodes neurofibromin, a crucial protein that functions as a Ras GTPase-activating protein. When mutations occur in the NF1 gene, the function of neurofibromin is impaired, leading to the persistent activation of Ras proteins. This abnormal activation triggers a cascade of complex physiological responses, ultimately manifesting in various clinical symptoms of NF-1, including café-au-lait spots, epilepsy, intractable chronic pain, vascular abnormalities, skeletal dysplasia, tumors of the central and peripheral nervous systems, and an increased risk of malignancies (1). Intestinal complications of NF1 have gained increasing attention, with gastrointestinal stromal tumors being the most common malignancies associated with NF1. However, diffuse intestinal ganglioneuromatosis of the intestine remains exceedingly rare (2,3). This paper reports a case of a young patient with neurofibromatosis accompanied by diffuse intestinal ganglioneuromatosis who had a massive neurofibroma of the right ankle and the rare complication of transmural diffuse intestinal ganglioneuromatosis which led to severe complications including bone destruction and intestinal involvement. We present this case in accordance with the CARE reporting checklist (available at https://tcr.amegroups.com/article/view/10.21037/tcr-2024-2266/rc).
Case presentation
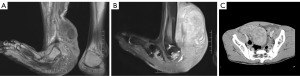
A 23-year-old male patient was admitted to our hospital in December 2020 with recurrent right lower abdominal pain for 1 week. Upon admission, multiple nodules were observed throughout the body, predominantly in the axilla, right elbow, and right ankle, along with café-au-lait spots (Figure 1A-1D). A computed tomography (CT) scan revealed a 7.3-cm mass in the cecum, which had already invaded the appendix. Magnetic resonance imaging (MRI) showed a tumor encasing and deforming the right ankle joint (Figure 2A-2C).

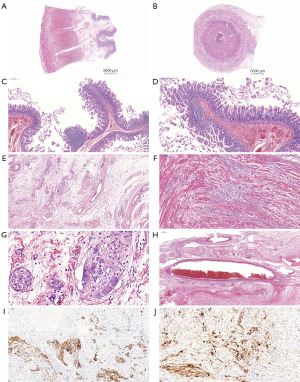
The patient underwent a right hemicolectomy and appendectomy. The postoperative pathology revealed significant and disorganized hyperplasia of ganglion cells within the muscularis propria of the colon and appendix (Figure 3A,3B). Additionally, the intestinal mucosa exhibited polypoid hyperplasia, accompanied by focal proliferation of lymphoid follicles (Figure 3C,3D). Disordered and hyperplastic ganglia can be clearly seen on the intestinal wall (Figure 3E-3G). Large blood vessels surrounding the hyperplastic nerve bundles were notably dilated and congested (Figure 3H). Immunohistochemistry showed that spindle cells and ganglion-like cells were positive for S-100, and ganglion-like cells were also positive for synaptophysin (Syn), confirming the diagnosis of diffuse intestinal ganglioneuromatosis (Figure 3I,3J).
In August 2021, the patient was readmitted due to the continued enlargement of a right lower limb mass that had been present for 20 years, with significant growth over the previous 6 months. Physical examination revealed a tumor approximately 20 cm in diameter at the ankle and another 5 cm mass on the inner side of the calf causing limited mobility. MRI showed a large, poorly defined, mass-like abnormal signal in the soft tissue of the right calf and foot, measuring approximately 23.0 cm × 9.0 cm × 8.9 cm in size.
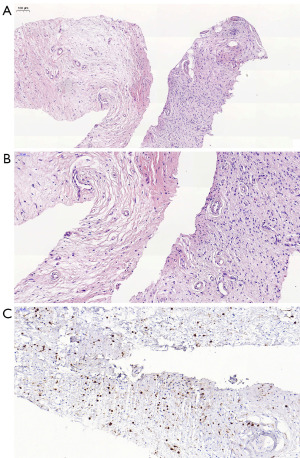
To further clarify the diagnosis, a biopsy was performed on the mass in the patient’s right lower limb. Microscopic examination revealed spindle-shaped and ovoid cells, with mild cellular morphology and pale nuclear staining present but no mitotic figures being observed. Immunohistochemical analysis indicated the following: positivity for vimentin (Vim) and S100; negativity for smooth muscle actin (SMA), desmin, and epithelial membrane antigen (EMA); and a Ki-67 positivity rate of approximately 1% (Figure 4A-4C). Based on these findings, the pathological diagnosis was consistent with a neurofibroma of the right lower limb. Two weeks later, the patient was transferred to another hospital for tumor resection surgery. Clinical diagnosis based on MRI results confirmed neurofibromas in the right calf, ankle, and subcutaneous tissue of both the dorsal and palmar sides of the thenar muscles of the left hand. In addition, the bone lesions in the right ankle and heel were attributed to disuse-related deformity and atrophy.
Given the patient’s severe difficulty in walking, including marked limping and frequent inability to walk, coupled with the high risk of recurrence following simple tumor excision, a decision was made to perform an amputation of the right lower limb and complete tumor removal. A prosthesis was fitted after surgery. Postoperative pathology results were consistent with the previous diagnosis.
It has been 24 months since the surgery, and the patient is currently in good condition with no recurrence of the tumor. According to the NF1-related tumor surveillance guidelines (4), it is recommended to conduct assessments of psychosocial well-being and quality of life. During the follow-up period, the patient’s family reported that the patient has regained the ability to walk, albeit with some instability. Additionally, the patient is experiencing mild gastrointestinal issues, which may be the result of a combination of psychological factors, skeletal developmental deficiencies, osteoporosis, and dietary factors.
All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Declaration of Helsinki (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal
Discussion
Neurofibromatosis is primarily classified into peripheral neurofibromatosis (NF1) and central neurofibromatosis (NF2). NF1 is an autosomal dominant hereditary disorder caused by mutations in the NF1 tumor-suppressor gene (5), with its origins traceable to developmental abnormalities of neural crest cells arising during embryogenesis. NF1 predominantly affects the skin and nervous system (6-11) but is rarely observed in the abdominal cavity or gastrointestinal tract, with the occurrence of giant neurofibromas being even more uncommon (3,12).
According to the strict diagnostic criteria for NF1 (13,14), a diagnosis can be confirmed if any two or more of the following conditions are met: (I) six or more café-au-lait spots, with diameters greater than 5 mm before puberty and greater than 15 mm after puberty; (II) two or more neurofibromas of any type or one plexiform neurofibroma; (III) freckling in the axillary or inguinal regions; (IV) two or more Lisch nodules (iris hamartomas); (V) optic glioma; (VI) characteristic skeletal dysplasia, such as sphenoid wing dysplasia or thinning of long bone cortex with or without pseudarthrosis; and (VII) a first-degree relative (parent, sibling, or offspring) diagnosed with NF1. This patient clearly met criteria 2 and 6, and given the histopathological and clinical findings, was ultimately diagnosed with NF1 with a giant neurofibroma of the right ankle and diffuse intestinal ganglioneuromatosis of the colon, ileocecal region, and appendix.
Notably, this patient’s NF1 had progressed over 20 years, with the neurofibroma gradually enlarging, eventually leading to bone destruction and disuse deformity around the ankle joint. There remains ongoing debate in the academic community regarding this phenomenon (15), specifically concerning whether bone deformity is primarily caused by skeletal developmental abnormalities or whether the long-standing periarticular tumor impedes normal bone growth. Further investigation through the collection of imaging data and genetic sequencing is required to address these issues and guide treatment strategies (16,17).
Additionally, this patient presented with an exceedingly rare intestinal complication—transmural diffuse intestinal ganglioneuromatosis, which is one of the tumors associated with NF1—primarily affecting the gastrointestinal tract (3). DG originates from primitive neural crest cells, is slow-growing and well-differentiated, and often involves subtle, early symptoms. In this case, the colon, ileocecal region, and appendix were fully infiltrated by DG, causing transmural invasion, which could lead to life-threatening acute abdominal complications, such as massive hemorrhage. This underscores the complexity and diversity of NF1 and its associated complications.
Conclusions
In conclusion, this case not only illustrates the progression of NF1 and its rare complications but also raises significant questions concerning the relationship between bone development and NF1, warranting further exploration. For NF1 patients and their families, early detection and intervention, along with genetic testing, are crucial in preventing severe complications and improving quality of life. The discovery and reporting of this rare case can enhance the clinical awareness of neurofibromatosis (particularly with regard to rare complications), enrich the knowledge regarding the clinical spectrum of NF1 and its complications, and provide new insights and therapeutic approaches for similar cases in the future.
Acknowledgments
The authors would like to thank the patient.
Funding: None.
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://tcr.amegroups.com/article/view/10.21037/tcr-2024-2266/rc
Peer Review File: Available at https://tcr.amegroups.com/article/view/10.21037/tcr-2024-2266/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tcr.amegroups.com/article/view/10.21037/tcr-2024-2266/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Declaration of Helsinki (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Na B, Shah SR, Vasudevan HN. Past, Present, and Future Therapeutic Strategies for NF-1-Associated Tumors. Curr Oncol Rep 2024;26:706-13. [Crossref] [PubMed]
- Dupuis H, Chevalier B, Cardot-Bauters C, et al. Prevalence of Endocrine Manifestations and GIST in 108 Systematically Screened Patients With Neurofibromatosis Type 1. J Endocr Soc 2023;7:bvad083. [Crossref] [PubMed]
- Iwamuro M, Omote R, Tanaka T, et al. Diffuse Intestinal Ganglioneuromatosis Showing Multiple Large Bowel Ulcers in a Patient with Neurofibromatosis Type 1. Intern Med 2017;56:3287-91. [Crossref] [PubMed]
- Carton C, Evans DG, Blanco I, et al. ERN GENTURIS tumour surveillance guidelines for individuals with neurofibromatosis type 1. EClinicalMedicine 2023;56:101818. [Crossref] [PubMed]
- Cai S, Yang Y, Jia B, et al. Transcriptome-wide Sequencing Reveals Molecules and Pathways Involved in Neurofibromatosis Type I Combined With Spinal Deformities. Spine (Phila Pa 1976) 2020;45:E489-98. [Crossref] [PubMed]
- Liu ZM, Deng X, Jiang L. A case report of neurofibromatosis type 1 with precocious puberty and short stature. Sichuan Medical Journal 2023;44:334-6.
- Zhao Q, Xie Q, Lu J. Case reports of two children with neurofibromatosis type 1. China Tropical Medicine 2023;23:672-5.
- Xu JN, Guo Y, Wang S, et al. Neurofibromatosis Type 1 in a Child with Plexiform Neurofibroma Pressing the Urinary System. Journal of Rare Diseases 2023;2:186-90.
- Li Feng, Ni Xinhai, Xiong Changming, et al. A case of pulmonary hypertension secondary to neurofibromatosis type 1. Chinese Journal of Tuberculosis and Respiratory Diseases 2006;500-1.
- Wu X, Guo RB, Zhou E, et al. A case of giant nose syndrome with diffuse hyperplasia of the laryngeal mucosa in neurofibromatosis type 1. Chinese Journal of Otorhinolaryngology-skull Base Surgery 2023;29:50-2.
- Qu XD, Zhang SH, Cao L, et al. A case of neurofibromatosis with multiple meningiomas and a trigeminal schwannoma. Chinese Journal of Clinical Neurosurgery 2022;27:348.
- Stella A, Lastella P, Viggiano L, et al. Clinical presentation and genetic analyses of neurofibromatosis type 1 in independent patients with monoallelic double de novo closely spaced mutations in the NF1 gene. Hum Mutat 2022;43:1354-60. [Crossref] [PubMed]
- Kanaan C, Cotteret S, Khneisser P, et al. NF1-Associated Inflammatory Polyp of the Colon: First Report of a Sporadic Case. Int J Surg Pathol 2022;30:823-7. [Crossref] [PubMed]
- Guo D, Gao RX, Yao ZM, et al. Surgical treatments of the intraspinal rib head in pediatric patients with dystrophic scoliosis secondary to type 1 neurofibromatosis. Chinese Journal of Bone and Joint Surgery 2020;13:561-8.
- Shofty B, Barzilai O, Khashan M, et al. Spinal manifestations of Neurofibromatosis type 1. Childs Nerv Syst 2020;36:2401-8. [Crossref] [PubMed]
- Zhang H, Deng A, Guo C, et al. Halo traction combined with posterior-only approach correction for cervical kyphosis with Neurofibromatosis-1: minimum 2 years follow-up. BMC Musculoskelet Disord 2021;22:973. [Crossref] [PubMed]
- Desmond FJ, Buture A, Kavanagh EC, et al. Ischemic stroke with extensive vasculopathy in a patient with neurofibromatosis type 1. Radiol Case Rep 2022;17:3370-2. [Crossref] [PubMed]
(English Language Editor: J. Gray)


