
Oncogenic combined calcineurin-nuclear factor of activated T cells and toll-like receptor signals in colon
Inflammation is emerging as one of the hallmarks of cancer pathogenesis and progression; its role in many tumors still remains unclear. Increasing evidences suggest that acute and chronic intestinal inflammation and activation of inflammation-associated pathways contribute to the pathogenesis of colorectal cancer (CRC) (1,2). Long-term treatment with non-steroidal anti-inflammatory drugs is able to remarkably reduce the CRC rate and death, and CRC development and progression are often associated with dysbiosis, a broad change of intestinal microbiota stratification and enrichment of certain microbial strains. However, the molecular mechanisms how inflammatory-related cascades promote CRC development are still uncovered.
Calcineurin is unique calcium activated protein, serine/threonine phosphatase with key functions in physiological and pathological cell fate, and required for activation of the nuclear factor of activated T cells (NFAT) transcription factors contribute to immune response, cell differentiation, and inflammation (3,4). NFAT dephosphorylation and activation by calcineurin induce its nuclear translocation and the expressions of diverse downstream targets obtaining a central role in myeloid and lymphoid cells. Therefore, pharmacological inhibition of calcineurin is widely used for suppression of undesired immune response, especially for solid organ transplantation. Interestingly, there might be several connections between NFAT, and tumor burden or progression. Cohort studies that patient received kidney transplantation with immune-suppressive treatment by normal-dose of calcineurin inhibitor, cyclosporin, revealed increased many types of cancer incidents compared with low-dose group (5). On the other hand, pharmacological or experimental blocking of calcineurin-related signaling suppressed CRC cell line proliferation in vitro (6-8). Thus, these reports dig up the question whether epithelial cells intrinsically respond to calcineurin-NFAT singling pathway in the oncogenic manner.
There is a growing appreciation for the role of the microbiota contributes to cancer pathogenesis including CRC (9,10). The dietary components have important and direct impact on the microbiota composition leads to global changes of microbial metabolism, reducing protective metabolites and producing a large number of cytotoxic mediators, result in constitutive activation of intestinal epithelial cells (IECs) giving consequence of the chronic inflammation, alteration of the micrbiota composition and dysfunction of stratification (10). These events enable to facilitate the certain population among the resident microbiota having oncogenic potential to predominantly proliferate and explore the IECs. It has been shown that inflammation-mediated molecules on microbiota are recognized by toll-like receptor (TLR) expressed on epithelial cells and activate NF-κB signaling which alters the expression of anti-apoptotic genes essential to the transformation of epithelial cells (9). Moreover, cancer-initiating cells, often called cancer stem cells (CSCs), in the CRC have been initially identified as a small population expressing CD133 (11). CRC development and progression, including recurrence after primary anti-cancer treatment, resistance to chemotherapy and escaping from innate immune reaction, are thought as mostly relied on the presence and function of CSCs. Thus, clarification of molecular mechanism underlying the CSCs survival and proliferation can be a promising therapeutic target for preventing CRC development and malignancy.
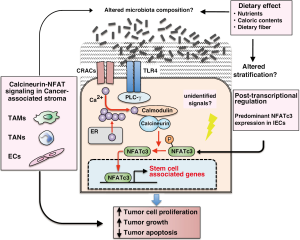
In this issue of Nature Medicine, Peuker, Muff, and colleagues explored novel relationships among epithelial cell-intrinsic calcineurin and NFAT pathway activation, microbiota-mediated stimulation of TLR expressing by IECs, and stem cell-associated gene expression controlled by calcium-dependent signaling pathway, which cooperatively functioned to the CRC development (12). In a mouse model of intestinal tumors (ApcMin/+), they demonstrated that IEC-specific deletion of the regulatory B1 subunit of calcineurin, which is required for calcineurin activation (CNB1∆IEC) resulted in fewer tumors and reduced tumor size. Notably, high expression of NFAT was observed in CRC cell lines and CRC-patient derived section, and IEC-specific deletion of NFAT in ApcMin/+ mouse model also resulted in reduced tumor size and number, similar to the effect seen in CNB1∆IEC mice. These results suggested that calcineurin promotes intestinal tumorigenesis through NFAT activation. TLR stimulation similarly activated calcineurin and increased NFAT-dependent transcriptional activity in CRC cells leads to progression of CNB1-wild type, but not CNB1∆IEC tumors. This tumorigenic effect required the impaired stratification following the recognition of the commensal microbiota by TLR4, as antibiotic treatment in ApcMin/+ tumors decreased NFAT-dependent transcription and reduced tumor growth. Importantly, they also found that epithelial calcineurin supports the survival and proliferation of CSCs via regulation of the epithelial response to tumor-associated changes in the commensal microbiota. Administration of antibiotic reagents or calcineurin inhibitor significantly suppressed the expression of stem cell-associated genes in IECs. Finally, consistent with these findings, somatic mutations that have been identified in human CRC are associated with constitutive activation of calcineurin, and NFAT nuclear translocation was observed in human CRC and correlated with increased CRC mortality.
Interestingly, Peuker, Muff, and colleagues demonstrated that CRC cell lines predominantly expressed NFATc3, compared to other NFAT family, NFATc1, c2, and c4. Indeed, intracellular NFAT expression and function depended on cell- or tissue-specific manner (13-15). NFAT c3 and c4 are expressed in the developing myocardium, whereas NFATc1 is expressed in developing heart valves (13). In chondrocytes, NFATc2 plays as an endogenous negative regulator for cartilage differentiation (14). In endothelial cells (ECs), NFATc1, c2, c3 are at least expressed and contributed the calcineurin signaling (16). Genome-wide screenings provided the evidence that NFATc1 could transduce vascular endothelial growth factor (VEGF)-mediated signaling and transcriptionally regulated angiogenesis-related genes (15). Thus, the mechanism which IECs predominantly expressing NFATc3 exhibit the calcineurin and NFAT signal-dependent CRC development may exist and give a hint to improving the therapeutic specificity in terms of NFATc3 targeting treatment. Accumulating evidences that not only cancer cells but also cancer-associated stromal cells including leukocytes, fibroblasts and ECs play crucial roles for tumor development and progression via providing pro-inflammatory and pro-tumorigenic microenvironment (17). From the sight of tumor microenvironment, the exposure of certain microbiota to IECs resulted in the activation of TLR and expression of NFAT-mediated gene can be strong pro-tumorigenic triggers such as infiltration and activation of neutrophils, macrophages, fibroblasts, and ECs in cancerous tissue. Whereas there are few reports approaching the role of calcineurin and NFAT signaling in these cancer-associated stromal cells, it has been shown the importance of NFAT-regulated gene expression in endothelium. Recently, we found that many of pro-angiogenic genes related to ECs activation in endothelium during the pathological condition including cancer were relied on calcineurin-NFAT pathways and transcriptional activity (16,18). Together these findings, NFAT family-mediated signaling pathways that are activated by calcineurin play specific roles in cancer development and progression not only in the cancer but also in the stromal tissue. In this issue, Peuker, Muff, and colleagues also demonstrated that the epithelial calcineurin–NFATc3 signal regulated CSCs even though they did not find evidences for roles of canonical or non-canonical Wnt signaling in the calcineurin-activated IECs. Recently, Kesselring et al. published the article to Cancer Cell; they demonstrated that IRAK-M, a negative regulator of TLR-MYD88-IRAK signaling, contributes to inflammation-associated CRC progression (19). IRAK-M expression was induced in epithelial cells through combined TLR2/4 and Wnt activation and was associated with altered microbiota composition and bacterial diversity. These findings indicated that survival and proliferation of CSCs in terms of stem cell-associated gene expression and functional cellular phenotypes are maintained by simultaneously activation of many intracellular signals including Wnt, TLR and calcineurin-mediated pathways. There are still undetermined mechanisms associated with composition and stratification of microbiota alterations, and whether the dietary components such as nutrients and fibers give any effects on microbial homeostasis in this oncogenic context. In fact, most CRC cases show a strong and complex association with diet and microbial metabolism (10). Some of metabolites produced from micrbiota affected by dietary components are responsible for the CRC development via secretion of proinflammatory mediator and DNA damage in IECs. There has been that differences between the composition of the microbial community in patients with CRC and that of healthy subjects, and are partially but definitely considered as a cause of the disease. Combined with targeting therapy of IEC-specific molecular mechanisms and changing diet intake might be more effective to prevent CRC development and progression.
In summary, as shown in Figure 1, Peuker, Muff, and colleagues revealed an epithelial-intrinsic oncogenic pathway that regulated by the calcium-dependent signal such as calcineurin and NFAT, synergized with TLR activation by the commensal microbiota result in the CRC development. The activation of this pathway promotes the CSC-associated gene expression contribute to the survival and proliferation of CSCs. Although there are still unsolved questions, this study clearly proposed a distinctive interaction between activation of IECs and microbiota which may be amenable to therapeutic targeting.
Acknowledgments
Funding: This study was supported in part by the Fund for Science and Technology of the Ministry of Education, Culture, Sports, Science and Technology-Japan (26290035 and 15H01348 to Takashi Minami), and the Daiichi Sankyo Life Science Foundation, Japan.
Footnote
Provenance and Peer Review: This article was commissioned and reviewed by the Section Editor Fengbo Tan (Department of Gastrointestinal Surgery, Xiangya Hospital, Central South University, Changsha, China).
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2016.09.36). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Terzić J, Grivennikov S, Karin E, et al. Inflammation and colon cancer. Gastroenterology 2010;138:2101-2114.e5. [Crossref] [PubMed]
- Lasry A, Zinger A, Ben-Neriah Y. Inflammatory networks underlying colorectal cancer. Nat Immunol 2016;17:230-40. [Crossref] [PubMed]
- Li H, Rao A, Hogan PG. Interaction of calcineurin with substrates and targeting proteins. Trends Cell Biol 2011;21:91-103. [Crossref] [PubMed]
- Wu H, Peisley A, Graef IA, et al. NFAT signaling and the invention of vertebrates. Trends Cell Biol 2007;17:251-60. [Crossref] [PubMed]
- Dantal J, Hourmant M, Cantarovich D, et al. Effect of long-term immunosuppression in kidney-graft recipients on cancer incidence: randomised comparison of two cyclosporin regimens. Lancet 1998;351:623-8. [Crossref] [PubMed]
- Jauliac S, López-Rodriguez C, Shaw LM, et al. The role of NFAT transcription factors in integrin-mediated carcinoma invasion. Nat Cell Biol 2002;4:540-4. [Crossref] [PubMed]
- Masuo T, Okamura S, Zhang Y, et al. Cyclosporine A inhibits colorectal cancer proliferation probably by regulating expression levels of c-Myc, p21(WAF1/CIP1) and proliferating cell nuclear antigen. Cancer Lett 2009;285:66-72. [Crossref] [PubMed]
- Tripathi MK, Deane NG, Zhu J, et al. Nuclear factor of activated T-cell activity is associated with metastatic capacity in colon cancer. Cancer Res 2014;74:6947-57. [Crossref] [PubMed]
- Kostic AD, Chun E, Meyerson M, et al. Microbes and inflammation in colorectal cancer. Cancer Immunol Res 2013;1:150-7. [Crossref] [PubMed]
- Louis P, Hold GL, Flint HJ. The gut microbiota, bacterial metabolites and colorectal cancer. Nat Rev Microbiol 2014;12:661-72. [Crossref] [PubMed]
- Ricci-Vitiani L, Lombardi DG, Pilozzi E, et al. Identification and expansion of human colon-cancer-initiating cells. Nature 2007;445:111-5. [Crossref] [PubMed]
- Peuker K, Muff S, Wang J, et al. Epithelial calcineurin controls microbiota-dependent intestinal tumor development. Nat Med 2016;22:506-15. [Crossref] [PubMed]
- Schulz RA, Yutzey KE. Calcineurin signaling and NFAT activation in cardiovascular and skeletal muscle development. Dev Biol 2004;266:1-16. [Crossref] [PubMed]
- Sitara D, Aliprantis AO. Transcriptional regulation of bone and joint remodeling by NFAT. Immunol Rev 2010;233:286-300. [Crossref] [PubMed]
- Suehiro J, Kanki Y, Makihara C, et al. Genome-wide approaches reveal functional vascular endothelial growth factor (VEGF)-inducible nuclear factor of activated T cells (NFAT) c1 binding to angiogenesis-related genes in the endothelium. J Biol Chem 2014;289:29044-59. [Crossref] [PubMed]
- Minami T, Yano K, Miura M, et al. The Down syndrome critical region gene 1 short variant promoters direct vascular bed-specific gene expression during inflammation in mice. J Clin Invest 2009;119:2257-70. [PubMed]
- Quail DF, Joyce JA. Microenvironmental regulation of tumor progression and metastasis. Nat Med 2013;19:1423-37. [Crossref] [PubMed]
- Minami T, Jiang S, Schadler K, et al. The calcineurin-NFAT-angiopoietin-2 signaling axis in lung endothelium is critical for the establishment of lung metastases. Cell Rep 2013;4:709-23. [Crossref] [PubMed]
- Kesselring R, Glaesner J, Hiergeist A, et al. IRAK-M Expression in Tumor Cells Supports Colorectal Cancer Progression through Reduction of Antimicrobial Defense and Stabilization of STAT3. Cancer Cell 2016;29:684-96. [Crossref] [PubMed]


