
Helicobacter pylori vacuolating cytotoxin and gastric cancer risk: reconsidered
Although Helicobacter pylori (H. pylori) cause gastric cancer, cancer develops in a fraction of H. pylori infected patients. Based on the notion that polymorphisms in the H. pylori vacuolating cytotoxin gene might be a determinant of clinical outcome, Abdi et al. used meta-analysis to examine the association between vacA gene subtypes and the risk of developing atrophic gastritis, intestinal metaplasia or gastric cancer (1).
H. pylori infection causes gastric mucosal inflammation which underlies the development of peptic ulcer disease and gastric cancer (2). The outcome of any H. pylori infection reflects complex interactions between the host, the bacterium and the environment. These interactions are evident clinically as marked geographic variation in the prevalence of H. pylori-related diseases (3).
The Abdi et al. meta-analysis focuses on one putative H. pylori virulence factor, vacA, the vacuolating cytotoxin. More than two decade ago, H. pylori were noted to differ in terms of ability to produce vacuolating cytotoxicity in vitro in cultured cells (4). The phenomena were subsequently related to the signal (s) and the middle (m) regions of the vacA gene (4). Changes in the signal peptide led in a shortened N-terminal portion and an attenuation of vacuolation ability. The most active cytotoxin was termed the s1 allele and the less active form, s2 allele. The m region of the gene encodes a vacA-to-host cell binding site; the m1 varient is more effective in binding than the m2 form. Different combinations resulted in different patterns of cytotoxicity: s1m1 strains produce the greatest level of cytotoxicity, s1m2 strains do not consistently induce vacuolation, and s2m2 strains are not cytotoxic (4).
The presence of marked geographic variation in the prevalence of vacA genotypes led to studies to test whether vacA genotyping might be usefully clinically. Abdi et al. meta-analysis included 33 studies with 1,446 cases and 2,697 controls and included European, Asian and American populations (1). The authors report that the vacA s1 genotype was associated with the risk of atrophic gastritis, intestinal metaplasia and gastric cancer [i.e., relative risk (RR) =1.116, 95% CI, 1.019–1.222; RR =1.418, 95% CI, 1.035–1.942; and RR =1.333, 95% CI, 1.115–1.593, respectively]. The vacA m1 genotype was associated with intestinal metaplasia and gastric cancer, but failed to statistically influence the risk of atrophic gastritis (RR =1.571, 95% CI, 1.247–1.980 and RR =1.431, 95% CI, 1.180–1.735, respectively). Here, we examine whether the association is a determinant of clinical outcome (i.e., causative) and whether vacA genotyping has clinical utility.
In 1965, Bradford Hill formulated nine criteria to distinguish simple association from causation (5) (Table 1). We use those criteria to evaluate the vacA associations.
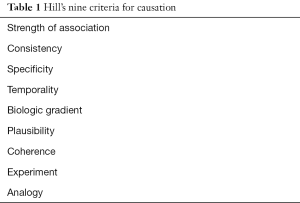
Full table
Strength: strong associations with a large increase in RR measured by the incidence (or prevalence) of the condition among the exposed relative to those unexposed are more likely to be causative than weak associations (e.g., the association of death from lung cancer and cigarette smoking is 20 times greater than for nonsmokers) (5,6). Although there are no value that clearly separates “strong” from “moderate” or “weak” associations, RR values below three are considered moderate or weak (7). The Abdi et al. meta-analysis (1) reported relatively weak associations (i.e., RR’s below 1.5) for both s1 and m1 and for the combination of s1m1.
Consistency: causal association (repeatability) should be consistent in different populations, places and times (5). Fifteen studies in the meta-analysis (1) confirmed the association of the s1m1 genotype with an increased risk for gastric cancer, however studies from Belgian and India only confirmed an association with vacA s1 but not with m1 (8,9). In Thailand (10), s, m or s, m, combination showed no statistically significant association with clinical outcome.
Specificity: causation is more certain when the association is limited to specific workers, anatomic sites, and type of disease (5). For example, an exposure that gives rise to a single outcome with no other explanation is likely specific (6). H. pylori related diseases are strongly influenced by environmental factor and this criterion is not applicable.
Temporality: temporality relates to exposure and outcome (i.e., the exposure must precede the outcome) (5). The temporality criteria is clear for H. pylori as a type I carcinogen (2).
Biologic gradient: biological gradient relates to a dose-response effect (5) (i.e., risk of cancer with an number of cigarettes smoked) (6). With vacA one might consider the association between degree of cytotoxicity and outcome. Comparison of area with high prevalence of s1m1 strains and cancer incidence fails to show either a strong or a consistent correlation. For example, s1m1 strains are common in China, Japan and Korea where gastric cancer is common (3). They are also common in India, Thailand, Bangladesh and Pakistan, sites where gastric cancer is rare. For example, in Taiwan vacA m2 strains are present in 87.4% (11) and the ASR of gastric cancer is considered high (i.e., 17 cases/100,000 population/year) (12).
Plausibility: causation requires the presence of a biological plausible mechanism that links cause and effect (5). To date no convincing mechanism has been described that can link vacA to a particular H. pylori-related disease outcome.
Coherence: causality suggest the cause and effect interpretation should not seriously conflict with the generally known of natural history and biology of disease (5,6). The lack of a biologically plausible mechanism does not allow this factor to be tested.
Experiment: in vitro studies suggest that vacA-induced vacuolation can disrupt protein trafficking pathways to and from the plasma membrane and thus influence various cell capacities (13). However, the physiological role of vacuolation in vitro remains unclear and animal experiments have failed to confirm a vacA-gastric cancer association (14). Experiments using gnotobiotic piglets failed to confirm a role of vacA in bacterial colonization, epithelial vacuolation or clinical outcome (15). Although vacA mutants colonized less well than their wild type vacA counterparts, his loss of vacA did not cause any apparent disadvantage (16). A study of the interaction between H. pylori and human T84 epithelial cell polarized monolayers using 43,000 element-spotted human cDNA microarrays, failed to detect any gene was specifically related with the presence of the vacA gene (17).
Analogy: analogy means the effect of similar factors may be considered. The presence of the s1 vacA genotype is strongly associated with the presence other virulence genes particularly the cag pathogenicity island, oipA, and babA. Both CagA and OipA are directly associated with the production of mucosal inflammation which underlies both gastric cancer and peptic ulcer disease (2). Most likely, the associations ascribed to vacA genotypes reflect the presence of these other virulence factors (i.e., CagA, OipA, BabA positive) especially the proinflammatory virulence factors, CagA and OipA.
Summary
Gastric cancer is a multifactorial disease related to long-standing gastric mucosal inflammation enhanced by H. pylori-specific factors that result in genetic instability (2). Studies attempting to link individual putative H. pylori virulence factors to specific disease outcome while ignoring the presence of other factors (e.g., CagA) are likely to produce spurious findings. The outcome of an H. pylori infection reflects intimate interactions between the bacteria, the host and the environment. Changes in the environment (e.g., diet) can result in a marked change in disease prevalence (e.g., rapid fall in gastric cancer incidence). Such that any factor can potentially either enhance or reduce the risk of a particular clinical outcome of H. pylori related disease. Disease association studies have used an expanding group of vacA gene polymorphisms that include the i (intermediate), the d (which refers to a deletion, d, located between the i and m regions), and the c genotypes (a polymorphic site in the three end region of vacA) (10). The original predictions made for the s, m, and i regions failed as disease determinants in East Asian and Southeast Asian countries where gastric cancer is a major clinical problem (18). Predictions for d genotype (a deletion, d, located between the i and m regions) base on western countries, failed in East Asia (19). Overall, vacA genotyping has generally failed as a consistent and reliable marker of risk and cannot be recommended.
Early in H. pylori history, it was suggested that the presence of CagA might serve as a biomarker to identify those at highest risk (3). The Maastricht Consensus III conference (20) reviewed the role of biomarkers for clinical use in H. pylori and stated “The detection of H pylori pathogenic factors and the study of host genetic polymorphisms is currently not recommended in the management of H. pylori infection”. This recommendation was based on failure of identification of such facts to “allow a reliable prediction of the outcome at an individual level”. The fact that no H. pylori type has been discovered that does not carry a significant risk of development of gastric cancer and peptic ulcer has continued to confirm that recommendation. vacA genotype differences most likely reflect genetic drift in populations and thus describe the population and it diseases rather than playing a causative role. In particular vacA genotyping studies are often surrogates for the presence of other virulence factors especially, CagA.
Acknowledgments
Funding: Dr. Graham is supported in part by the Office of Research and Development Medical Research Service Department of Veterans Affairs, Public Health Service grant DK56338 which funds the Texas Medical Center Digestive Diseases Center. The contents are solely the responsibility of the authors and do not necessarily represent the official views of the VA or NIH. Professor Yamaoka is supported in part by grants from the National Institutes of Health (DK62813) and the Grants-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science, and Technology (MEXT) of Japan (24406015, 24659200, 25293104, 26640114 and 15H02657). Professor Yamaoka and Dr. Miftahussurur are also supported by the Japan Society for the Promotion of Science (JSPS) Institutional Program for Young Researcher Overseas Visits, the Strategic Funds for the Promotion of Science and Technology from Japan Science and Technology Agency.
Footnote
Provenance and Peer Review: This article was commissioned and reviewed by the Section Editor Xiaoying Zhou (Institute of Gastroenterology, the First Affiliated Hospital of Nanjing Medical University, Nanjing, China).
Conflicts of Interest: Dr. Graham is a paid consultant and has received research funding from RedHill Biopharma regarding novel H. pylori therapies and is a consultant to BioGaia regarding use of probiotics for H. pylori infections. And other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Abdi E, Latifi-Navid S, Latifi-Navid H, et al. Helicobacter pylori vacuolating cytotoxin genotypes and preneoplastic lesions or gastric cancer risk: a meta-analysis. J Gastroenterol Hepatol 2016;31:734-44. [Crossref] [PubMed]
- Graham DY. Helicobacter pylori update: gastric cancer, reliable therapy, and possible benefits. Gastroenterology 2015;148:719-31.e3. [Crossref] [PubMed]
- Yamaoka Y. Mechanisms of disease: Helicobacter pylori virulence factors. Nat Rev Gastroenterol Hepatol 2010;7:629-41. [PubMed]
- Atherton JC, Cao P, Peek RM Jr, et al. Mosaicism in vacuolating cytotoxin alleles of Helicobacter pylori. Association of specific vacA types with cytotoxin production and peptic ulceration. J Biol Chem 1995;270:17771-7. [Crossref] [PubMed]
- Hill AB. The Environment and Disease: Association or Causation? Proc R Soc Med 1965;58:295-300. [PubMed]
- Lucas RM, McMichael AJ. Association or causation: evaluating links between "environment and disease". Bull World Health Organ 2005;83:792-5. [PubMed]
- Boffetta P. Causation in the presence of weak associations. Crit Rev Food Sci Nutr 2010;50:13-16. [Crossref]
- Memon AA, Hussein NR, Miendje Deyi VY, et al. Vacuolating cytotoxin genotypes are strong markers of gastric cancer and duodenal ulcer-associated Helicobacter pylori strains: a matched case-control study. J Clin Microbiol 2014;52:2984-9. [Crossref] [PubMed]
- Saxena A, Shukla S, Prasad KN, et al. Virulence attributes of Helicobacter pylori isolates & their association with gastroduodenal disease. Indian J Med Res 2011;133:514-20. [PubMed]
- Chomvarin C, Namwat W, Chaicumpar K, et al. Prevalence of Helicobacter pylori vacA, cagA, cagE, iceA and babA2 genotypes in Thai dyspeptic patients. Int J Infect Dis 2008;12:30-6. [Crossref] [PubMed]
- Wang HJ, Kuo CH, Yeh AA, et al. Vacuolating toxin production in clinical isolates of Helicobacter pylori with different vacA genotypes. J Infect Dis 1998;178:207-12. [Crossref] [PubMed]
- Lee YC, Chiang TH, Liou JM, et al. Mass Eradication of Helicobacter pylorito Prevent Gastric Cancer: Theoretical and Practical Considerations. Gut Liver 2016;10:12-26. [Crossref] [PubMed]
- Li Y, Wandinger-Ness A, Goldenring JR, et al. Clustering and redistribution of late endocytic compartments in response to Helicobacter pylori vacuolating toxin. Mol Biol Cell 2004;15:1946-59. [Crossref] [PubMed]
- Ogura K, Maeda S, Nakao M, et al. Virulence factors of Helicobacter pylori responsible for gastric diseases in Mongolian gerbil. J Exp Med 2000;192:1601-10. [Crossref] [PubMed]
- Eaton KA, Cover TL, Tummuru MK, et al. Role of vacuolating cytotoxin in gastritis due to Helicobacter pylori in gnotobiotic piglets. Infect Immun 1997;65:3462-4. [PubMed]
- Salama NR, Otto G, Tompkins L, et al. Vacuolating cytotoxin of Helicobacter pylori plays a role during colonization in a mouse model of infection. Infect Immun 2001;69:730-6. [Crossref] [PubMed]
- El-Etr SH, Mueller A, Tompkins LS, et al. Phosphorylation-independent effects of CagA during interaction between Helicobacter pylori and T84 polarized monolayers. J Infect Dis 2004;190:1516-23. [Crossref] [PubMed]
- Ogiwara H, Graham DY, Yamaoka Y. vacA i-region subtyping. Gastroenterology 2008;134:1267-author reply 1268. [Crossref] [PubMed]
- Ogiwara H, Sugimoto M, Ohno T, et al. Role of deletion located between the intermediate and middle regions of the Helicobacter pylori vacA gene in cases of gastroduodenal diseases. J Clin Microbiol 2009;47:3493-500. [Crossref] [PubMed]
- Malfertheiner P, Megraud F, O'Morain C, et al. Current concepts in the management of Helicobacter pylori infection: the Maastricht III Consensus Report. Gut 2007;56:772-81. [Crossref] [PubMed]


