
Elevated levels of total cholesterol and high-density lipoprotein cholesterol are associated with better prognosis in patients with lung adenocarcinoma: a retrospective cohort study
Highlight box
Key findings
• Our study finds that that in patients with lung adenocarcinoma (LUAD), increased total cholesterol levels may predict better overall survival, while decreased levels of high-density lipoprotein cholesterol may predict worse outcomes for both disease-free survival and overall survival. These findings may aid in the identification of high-risk patients and allow them to take necessary measures in advance.
What is known and what is new?
• It is widely known lipids are important for cellular metabolism and storage, they exert a critical influence on signal transduction in cancer cell processes, and regulate the growth, invasion, migration, metastasis, and survival of cancer cells through various signaling pathways. Shen et al. found anlotinib suppresses LUAD growth via inhibiting recombinant fatty acid synthase-mediated lipid metabolism. Our previous study found that preoperative lipid distribution has an important impact on the prognosis of patients with lung squamous cell carcinoma.
• However, there is little evidence about the association between blood lipid levels and clinical characteristics of patients with LUAD and the effect of lipid profiles on prognosis of patients with LUAD. Our current study shows changes in lipid metabolism and its potential to become a survival biomarker in patients with LUAD.
What is the implication, and what should change now?
• It may be a therapeutic option for patients with LUAD via regulating lipid metabolism related signaling pathways or gene targets, and combining preoperative blood lipid factors into nomogram models may improves the prediction of the prognosis of LUAD patients compared to using a simple tumor node metastasis staging system.
Introduction
Lung cancer has a poor prognosis and accounts for the majority of new cases and cancer deaths worldwide (1). Lung adenocarcinoma (LUAD), a type of non-small cell lung cancer, is the most common pathological type of lung cancer in humans and the most common type of lung cancer among non-smokers and younger patients. An epidemiological study has reported that adenocarcinoma accounts for approximately 40% of lung cancer cases (2). Due to the high risk of metastasis and recurrence, the prognosis of patients with LUAD is poor. Thus far, a variety of biomarkers have been studied to predict the prognosis of patients with LUAD (3,4). For example, Zhang et al. found that PSMD14 may serve as a potential prognostic marker and therapeutic target for LUAD patients (5), and Sun et al. suggested that downregulation of m6A reader YTHDC2 may promote tumor progression and predict poor prognosis in LUAD (6). Due to the high cost and complicated detection equipment required for existing methods, the development of accurate, fast, and convenient predictive biomarkers for the identification of patients with a high risk of metastasis and recurrence is emerging as a significant area of investigation, and it may present an opportunity for improving clinical prognosis and postoperative quality of life (7).
Findings from previous studies have demonstrated that patients with cancer often present with an altered serum lipid profile. A high level of serum triglycerides (TG) was reported to increase the risk of lung cancer (8), and Kitahara et al. showed a lower risk of lung cancer in males with higher serum total cholesterol (TC) than in the general population (9). Additionally, a study has indicated low high-density lipoprotein cholesterol (HDL-C) levels are associated with an increased risk of lung cancer (10), and a prospective cohort study of Chinese men showed that low levels of low-density lipoprotein cholesterol (LDL-C) are implicated in an increased risk of lung cancer (11). Several recent studies have confirmed that dyslipidemia is associated with an increased risk of tumor recurrence and reduced survival for multiple cancer types (7,12,13). The studies of lipid biomarkers provided clinical evidence for the treatment of tumors via lipid metabolism. It is widely known lipids are important for cellular metabolism and storage, they exert a critical influence on signal transduction in cancer cell processes, and regulate the growth, invasion, migration, metastasis, and survival of cancer cells through various signaling pathways (14,15). Shen et al. found anlotinib suppresses LUAD growth via inhibiting recombinant fatty acid synthase (FASN)-mediated lipid metabolism (16). Our previous study found that preoperative lipid distribution has an important impact on the prognosis of patients with lung squamous cell carcinoma (7), but there is little evidence about the effect of lipid profiles on prognosis of patients with LUAD, lipid metabolism changes and their potential to become biomarkers related to the survival of LUAD require further exploration.
Our current study aimed to develop nomograms to elucidate the effect of lipid distribution on predicting prognosis in patients with LUAD who have undergone complete lung resections. We present this article in accordance with the TRIPOD reporting checklist (available at https://tcr.amegroups.com/article/view/10.21037/tcr-24-1062/rc).
Methods
Patient selection
Records from a total of 304 patients with LUAD who underwent complete lung resections at Shaoxing People’s Hospital from January 2016 through December 2017 were collected and reviewed in our retrospective cohort study. We performed a prior sample size estimation by referencing our past research. Blood samples were collected on an empty stomach before surgery, and plasma was prepared and analyzed using an automated biochemical detection instrument, and blood lipids were determined via enzymatic method. All operations were completed by professional personnel with relevant qualifications from our hospital. The tumor node metastasis (TNM) staging criteria for LUAD referred to the latest 2023 9th edition of the TNM staging criteria for lung cancer. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). Approval of the study protocol was obtained from the Academic Ethics Committee of Shaoxing People’s Hospital (No. 078), and all of the methods were performed in accordance with the relevant guidelines and regulations. Written informed consent was not required due to the retrospective nature of this study. All of the patients met the following eligibility criteria: (I) pathological confirmation of LUAD (pathological diagnoses were confirmed by an independent experienced pathologist at Shaoxing People’s Hospital); (II) no previous history of cancer; and (III) had serum samples obtained before treatment (including anti-cancer treatment or any other treatment that may affect blood lipids).
Patient follow-up
Follow-up on all patients was completed in the clinic or by telephone, and the study was discontinued after a total of 25 patients were unable to complete the designated follow-up. Physical examination and laboratory blood draws were conducted every 3–6 months in the first 2 years and every 6–12 months from years 3–5 (7). The main outcome of this study was overall survival (OS), which we defined as the time from curative resection to death. The secondary outcome was disease-free survival (DFS), which was calculated from the date of radical surgery to the date of disease recurrence or diagnosis of distant metastasis (13).
Nomogram construction
We determined the univariate prognostic factors of OS and DFS using log-rank test. Variables with a P<0.05 were entered into the multivariate Cox proportional hazards model. The final model was selected using a backward stepdown selection process based on the Akaike information criterion (17). The independent prognostic factors determined through multivariate analysis were then used to construct nomograms for OS and DFS.
Nomogram validation and calibration
The nomograms were subjected to 1,000 bootstrap resamples for validation. The concordance index (C-index) was used to assess the discrimination performance of the nomograms (17). The value of the C-index ranges from 0.5 to 1.0, with a higher C-index value indicating a better capacity to separate patients with different survival outcomes. We utilized previously introduced methods to compare the C-indexes of two different models (17). The TNM staging system in this study was the model, which included the tumor size, number of positive lymph nodes, and metastasis. Calibration represents the capacity of a model to accurately estimate outcomes (17). The observed rates were then compared to the nomogram-predicted probabilities of the models, and the differences between them were used to construct calibration curves. In a well-calibrated model, the predictions are expected to fall on a 45° diagonal line (17).
Statistical analysis
Data analyses were conducted on SPSS 20 and R software, version 3.5.0 (http://www.r-project.org). Continuous variables were displayed as mean ± standard deviation (SD). The optimal cut-off values for the lipid profile determined by X-Tile software were used to dichotomize lipids for the Chi-squared test and Cox proportional analyses. The relationship between lipid profile and survival rates was obtained with the Kaplan-Meier survival approach and the Log-rank (Mantel-Cox) test. The risk factors for survival were identified using the Cox proportional hazards model. The association between serum lipid levels and clinicopathological parameters, including age, gender, history of smoking, history of drinking alcohol, T stage, lymph node metastasis, TNM stage, recurrence, and death, was tested with the Chi-squared test (7). The R packages cmprsk21 and rms22 were used for modeling and developing the nomograms (17). The rcorrp.cens function in the R package Hmisc23 was used for comparing the C-index between two nomograms (17). In all statistical methods, P<0.05 indicated a statistically significant difference.
Results
Patient characteristics
After the eligibility review, 304 patients with LUAD who had undergone radical surgical resection were enrolled in the analysis, and their characteristics are presented in Table 1. The median follow-up time was 42 months (range, 1–70 months). The median age at resection was 59 years (range, 40–81 years); and 171 (56.3%) of the patients were male and the remaining 133 (43.8%) were female. Cancer staging was conducted according to the TNM classification for LUAD. A total of 213 patients (70.1%) were diagnosed with stage I–II disease and 91 (29.9%) were diagnosed with stage III–IV disease. A total of 117 patients (38.5%) died during the follow-up period and 125 patients (41.1%) had disease recurrence or distant metastasis.
Table 1
| Characteristics | Patients, n (%) |
|---|---|
| Age, years | |
| >60 | 136 (44.7) |
| ≤60 | 168 (55.3) |
| Gender | |
| Female | 133 (43.8) |
| Male | 171 (56.3) |
| Smoking state | |
| Yes | 138 (45.4) |
| No | 166 (54.6) |
| Drinking state | |
| Yes | 103 (33.9) |
| No | 201 (66.1) |
| T stage | |
| I–II | 263 (86.5) |
| III–IV | 41 (13.5) |
| Lymph node metastasis | |
| Yes | 139 (45.7) |
| No | 165 (54.3) |
| TNM stage | |
| I–II | 213 (70.1) |
| III–IV | 91 (29.9) |
| Recurrence | |
| Yes | 125 (41.1) |
| No | 154 (50.7) |
| Unknown | 25 (8.2) |
| Death | |
| Yes | 117 (38.5) |
| No | 174 (57.2) |
| Unknown | 13 (4.3) |
| TG (mmol/L) | |
| >1.03 | 199 (65.5) |
| ≤1.03 | 105 (34.5) |
| TC (mmol/L) | |
| >3.58 | 247 (81.3) |
| ≤3.58 | 57 (18.8) |
| HDL-C (mmol/L) | |
| >1.01 | 232 (76.3) |
| ≤1.01 | 72 (23.7) |
| LDL-C (mmol/L) | |
| >2.20 | 224 (73.7) |
| ≤2.20 | 80 (26.3) |
TNM, tumor node metastasis; TG, triglycerides; TC, total cholesterol; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol.
Preoperative plasma profile levels in patients with LUAD
The relationship between preoperative plasma lipid profile levels and clinical parameters was analyzed (Table 2). TG level (P=0.009) and TC (P=0.04) level were higher in patients who were non-smokers. A higher TC level was closely correlated with females (P=0.002) and no current history of drinking alcohol (P=0.047). In addition, HDL-C level was identified to be down-regulated in patients with lymph node metastasis (P=0.03) or recurrence (P=0.03) or death status (P=0.004). Other clinical parameters were not significantly associated with preoperative plasma lipid profile levels, such as age, T stage, and TNM stage (P>0.05).
Table 2
| Characteristics | TG (mmol/L) | TC (mmol/L) | HDL-C (mmol/L) | LDL-C (mmol/L) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean ± SD | P value | Mean ± SD | P value | Mean ± SD | P value | Mean ± SD | P value | ||||
| Age, years | 0.55 | 0.65 | 0.52 | 0.70 | |||||||
| >60 | 1.472±0.831 | 4.410±0.841 | 1.287±0.411 | 2.645±0.750 | |||||||
| ≤60 | 1.541±1.101 | 4.363±0.947 | 1.259±0.351 | 2.610±0.790 | |||||||
| Gender | 0.051 | 0.002 | 0.10 | 0.19 | |||||||
| Female | 1.636±1.087 | 4.563±0.902 | 1.313±0.366 | 2.692±0.795 | |||||||
| Male | 1.412±0.896 | 4.244±0.876 | 1.240±0.386 | 2.574±0.751 | |||||||
| Smoking state | 0.009 | 0.04 | 0.26 | 0.71 | |||||||
| Yes | 1.357±0.651 | 4.265±0.868 | 1.245±0.403 | 2.608±0.748 | |||||||
| No | 1.638±1.185 | 4.483±0.917 | 1.294±0.356 | 2.641±0.792 | |||||||
| Drinking state | 0.15 | 0.047 | 0.72 | 0.20 | |||||||
| Yes | 1.395±0.777 | 4.241±0.876 | 1.260±0.453 | 2.546±0.743 | |||||||
| No | 1.569±1.078 | 4.457±0.906 | 1.278±0.335 | 2.667±0.784 | |||||||
| T stage | 0.46 | 0.17 | 0.44 | 0.30 | |||||||
| I–II | 1.527±1.014 | 4.412±0.918 | 1.278±0.385 | 2.644±0.792 | |||||||
| III–IV | 1.403±0.806 | 4.205±0.763 | 1.229±0.331 | 2.510±0.617 | |||||||
| Lymph node metastasis | 0.67 | 0.65 | 0.03 | 0.08 | |||||||
| Yes | 1.483±1.042 | 4.409±0.923 | 1.222±0.317 | 2.710±0.758 | |||||||
| No | 1.533±0.943 | 4.363±0.883 | 1.314±0.420 | 2.555±0.778 | |||||||
| TNM stage | 0.12 | 0.46 | 0.07 | 0.43 | |||||||
| I–II | 1.567±1.055 | 4.409±0.893 | 1.297±0.399 | 2.603±0.773 | |||||||
| III–IV | 1.376±0.800 | 4.325±0.919 | 1.212±0.317 | 2.679±0.768 | |||||||
| Recurrence | 0.91 | 0.09 | 0.03 | 0.21 | |||||||
| Yes | 1.494±0.883 | 4.287±0.812 | 1.215±0.338 | 2.569±0.721 | |||||||
| No | 1.507±0.988 | 4.468±0.936 | 1.317±0.403 | 2.686±0.800 | |||||||
| Death | 0.14 | 0.09 | 0.004 | 0.53 | |||||||
| Yes | 1.393±0.689 | 4.289±0.923 | 1.204±0.326 | 2.594±0.788 | |||||||
| No | 1.566±1.125 | 4.473±0.880 | 1.335±0.404 | 2.652±0.768 | |||||||
TG, triglycerides; TC, total cholesterol; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; SD, standard deviation; TNM, tumor node metastasis.
Optimal cut-off determination of lipid metabolism
The optimal cut-off value, derived with X-tile software analysis, for TG was 1.03 mmol/L, 3.58 mmol/L for TC, 1.01 mmol/L for HDL-C, and 2.20 mmol/L for LDL-C.
Association of TC and HDL-C levels with clinical characteristics
The relationships between the clinicopathological features of LUAD and the levels of TC and HDL-C are summarized in Tables 3,4. The Chi-squared test showed that the TC level was significantly related to the sex of the patient (P=0.001), history of smoking (P=0.04), and death (P=0.007) (Table 3). However, no significant associations were reported between TC levels and other clinicopathological parameters, including age, history of drinking alcohol, T stage, and tumor recurrence (all P>0.05; Table 3). The HDL-C level was significantly associated with sex (P=0.004), history of smoking (P=0.02), tumor recurrence (P=0.050), and death (P=0.002) (Table 4). T stage, lymph node metastasis, and other clinical parameters were not significantly associated with preoperative plasma lipid profile levels (all P>0.05; Table 4).
Table 3
| Characteristics | TC >3.58 mmol/L | TC ≤3.58 mmol/L | P |
|---|---|---|---|
| Age, years | 0.88 | ||
| >60 | 111 | 25 | |
| ≤60 | 136 | 32 | |
| Gender | 0.001 | ||
| Female | 119 | 14 | |
| Male | 128 | 43 | |
| Smoking state | 0.04 | ||
| Yes | 105 | 33 | |
| No | 142 | 24 | |
| Drinking state | 0.08 | ||
| Yes | 78 | 25 | |
| No | 169 | 32 | |
| T stage | 0.77 | ||
| I–II | 213 | 50 | |
| III–IV | 34 | 7 | |
| Lymph node metastasis | 0.54 | ||
| Yes | 115 | 24 | |
| No | 132 | 33 | |
| TNM stage | 0.73 | ||
| I–II | 172 | 41 | |
| III–IV | 75 | 16 | |
| Recurrence | 0.33 | ||
| Yes | 99 | 26 | |
| No | 129 | 25 | |
| Death | 0.007 | ||
| Yes | 87 | 30 | |
| No | 151 | 23 |
a total of 25 patients were unable to complete the designated follow-up. TC, total cholesterol; TNM, tumor node metastasis.
Table 4
| Characteristics | HDL-C >1.01 mmol/L | HDL-C ≤1.01 mmol/L | P |
|---|---|---|---|
| Age, years | 0.83 | ||
| >60 | 103 | 33 | |
| ≤60 | 129 | 39 | |
| Gender | 0.004 | ||
| Female | 112 | 21 | |
| Male | 120 | 51 | |
| Smoking state | 0.02 | ||
| Yes | 97 | 41 | |
| No | 135 | 31 | |
| Drinking state | 0.01 | ||
| Yes | 70 | 33 | |
| No | 162 | 39 | |
| T stage | 0.61 | ||
| I–II | 202 | 61 | |
| III–IV | 30 | 11 | |
| Lymph node metastasis | 0.98 | ||
| Yes | 106 | 33 | |
| No | 126 | 39 | |
| TNM stage | 0.31 | ||
| I–II | 166 | 47 | |
| III–IV | 66 | 25 | |
| Recurrence | 0.050 | ||
| Yes | 89 | 36 | |
| No | 125 | 29 | |
| Death | 0.002 | ||
| Yes | 80 | 37 | |
| No | 146 | 28 | |
a total of 25 patients were unable to complete the designated follow-up. HDL-C, high-density lipoprotein cholesterol; TNM, tumor node metastasis.
Prognostic significance of clinical characteristics in LUAD
In the univariate analyses, a significant correlation between history of smoking, T stage, lymph node metastasis, TNM stage, levels of TC, HDL-C, and LDL-C, and OS were detected. Additionally, we found a correlation between lymph node metastasis, TNM stage, HDL-C levels, and DFS. In the multivariate analysis, we observed significant associations between age, T stage, lymph node metastasis, and levels of TC and HDL-C with OS (Table 5). In addition, associations between lymph node metastasis and HDL-C levels with DFS were observed (Table 6). The multivariate analysis was conducted based on the age at resection, sex, history of smoking, history of drinking alcohol, T stage, lymph node metastasis, TNM stage, and levels of TG, TC, HDL-C, and LDL-C.
Table 5
| Parameters | Univariate analysis | Multivariate analysis | |||
|---|---|---|---|---|---|
| HR (95% CI) | P | HR (95% CI) | P | ||
| Age (>60 vs. ≤60 years) | 1.421 (0.989–2.042) | 0.06 | 1.596 (1.105–2.306) | 0.01 | |
| Gender (female vs. male) | 0.735 (0.506–1.066) | 0.11 | |||
| Smoking state (yes vs. no) | 1.442 (1.003–2.074) | 0.048 | |||
| Drinking state (yes vs. no) | 1.388 (0.954–2.018) | 0.09 | |||
| T stage (I–II vs. III–IV) | 0.499 (0.321–0.778) | 0.002 | 0.575 (0.365–0.906) | 0.02 | |
| Lymph node metastasis (yes vs. no) | 2.773 (1.896–4.056) | <0.001 | 2.926 (1.975–4.335) | <0.001 | |
| TNM stage (I–II vs. III–IV) | 0.393 (0.273–0.567) | <0.001 | |||
| TG (>1.03 vs. ≤1.03 mmol/L) | 0.700 (0.484–1.010) | 0.06 | |||
| TC (>3.58 vs. ≤3.58 mmol/L) | 0.545 (0.359–0.826) | 0.004 | 0.504 (0.324–0.782) | 0.002 | |
| HDL-C (>1.01 vs. ≤1.01 mmol/L) | 0.557 (0.377–0.823) | 0.003 | 0.665 (0.443–0.999) | 0.049 | |
| LDL-C (>2.20 vs. ≤2.20 mmol/L) | 0.658 (0.446–0.971) | 0.04 | |||
HR, hazard ratio; CI, confidence interval; TNM, tumor node metastasis; TG, triglycerides; TC, total cholesterol; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol.
Table 6
| Parameters | Univariate analysis | Multivariate analysis | |||
|---|---|---|---|---|---|
| HR (95% CI) | P | HR (95% CI) | P | ||
| Age (>60 vs. ≤60 years) | 1.030 (0.724–1.465) | 0.87 | |||
| Gender (female vs. male) | 0.803 (0.562–1.148) | 0.23 | |||
| Smoking state (yes vs. no) | 1.283 (0.904–1.823) | 0.16 | |||
| Drinking state (yes vs. no) | 1.174 (0.816–1.689) | 0.39 | |||
| T stage (I–II vs. III–IV) | 0.711 (0.445–1.137) | 0.16 | |||
| Lymph node metastasis (yes vs. no) | 2.005 (1.402–2.867) | <0.001 | 2.026 (1.417–2.899) | <0.001 | |
| TNM stage (I–II vs. III–IV) | 0.572 (0.399–0.820) | 0.002 | |||
| TG (>1.03 vs. ≤1.03 mmol/L) | 0.884 (0.615–1.269) | 0.50 | |||
| TC (>3.58 vs. ≤3.58 mmol/L) | 0.767 (0.498–1.181) | 0.23 | |||
| HDL-C (>1.01 vs. ≤1.01 mmol/L) | 0.632 (0.429–0.931) | 0.02 | 0.619 (0.420–0.912) | 0.02 | |
| LDL-C (>2.20 vs. ≤2.20 mmol/L) | 0.808 (0.549–1.190) | 0.28 | |||
HR, hazard ratio; CI, confidence interval; TNM, tumor node metastasis; TG, triglycerides; TC, total cholesterol; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol.
Prognostic significance of the serum lipid profile in LUAD
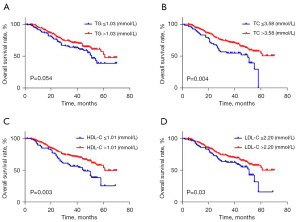
Among all 304 patients, 30 of 53 (56.6%) who had a TC level ≤3.58 mmol/L and 87 of 238 (36.6%) who had a TC level >3.58 mmol/L died (P=0.007). In addition, 37 of 65 (56.9%) patients who had an HDL-C level ≤1.01 mmol/L and 80 of 226 (35.4%) patients who had an HDL-C level >1.01 mmol/L (P=0.002) died. In the univariate Cox proportional analysis, a decreased TC [hazard ratio (HR), 0.545; 95% confidence interval (CI): 0.359–0.826, P=0.004] or HDL-C level (HR, 0.557; 95% CI: 0.377–0.823, P=0.003) was significantly associated with decreased OS (Table 5; Figure 1), and this finding remained significant in the multivariate analysis that included T stage and lymph node metastasis. In addition, an increased LDL-C level (HR, 0.658; 95% CI: 0.446–0.971, P=0.04; Table 5) and III–IV TNM stage (HR, 0.393; 95% CI: 0.273–0.567, P<0.001) were statistically linked with an increased OS in the univariate Cox proportional analysis. However, in the multivariate analysis, the LDL-C levels were not statistically significant, including TNM stage (P>0.05; Table 5). Other clinical parameters showed no significant difference in the results from either the univariate or multivariate analysis, including sex and history of drinking alcohol (P>0.05; Table 5).
Regarding DFS, local recurrence or distant metastasis after radical surgical resection was diagnosed in 26 of 51 (51.0%) patients with a TC level ≤3.58 mmol/L and in 99 of 228 (43.4%) patients with a TC level >3.58 mmol/L (P>0.05), as well as in 36 of 65 (55.4%) patients with an HDL-C level ≤1.01 mmol/L and in 89 of 214 (41.6%) patients with an HDL-C level >1.01 mmol/L (P=0.050). In the univariate Cox proportional analysis, a decreased HDL-C level (HR, 0.632; 95% CI: 0.429–0.931, P=0.02; Table 6) was significantly correlated with decreased DFS, and the significance of this finding remained in the multivariate analysis (HR, 0.619; 95% CI: 0.420–0.912, P=0.02), including the lymph node metastasis. In addition, III–IV TNM stage was statistically linked with increased DFS (HR, 0.572; 95% CI: 0.399–0.820, P=0.002), although there was no statistical significance of TNM stage in the multivariate analysis (P>0.05; Table 6). TG, TC, and LDL-C showed no significant difference in the results from either the univariate or multivariate analysis (Table 6, Figure 2), including age, gender, history of smoking, and history of drinking alcohol (P>0.05; Table 6).

Construction and validation of nomograms for OS and DFS
Nomograms including significant prognostic variables for the OS and DFS of LUAD patients at 1, 3, and 5 years are presented in Figure 3A,3B. Points in the nomograms were assigned based on the hierarchy of effects on survival. The highest points were assigned to lymph node metastasis status for both the OS and DFS nomograms. Although lymph node metastasis status contributed the most to the prognosis, preoperative blood lipid variables moderately impacted prognosis (Figure 3A,3B). Calibration plots revealed a high consistency between the predicted and actual observed 1-, 3-, and 5-year OS and DFS for LUAD patients (Figure 4A,4B). The C-index for the final nomograms for OS was higher than that for the TNM staging system (0.735 vs. 0.689; P=0.009; Table 7). A lower C-index was generated by the OS and DFS nomograms (0.699 vs. 0.735, P=0.03; 0.659 vs. 0.700, P=0.002, respectively; Table 7) that excluded all preoperative blood lipid factors.


Table 7
| Items | OS | DFS | |||
|---|---|---|---|---|---|
| C-index (95% CI) | P | C-index (95% CI) | P | ||
| Nomogram 1 | 0.735 (0.676–0.794) | Reference | 0.700 (0.639–0.757) | Reference | |
| TNM staging system | 0.689 (0.627–0.750) | 0.009 | 0.689 (0.627–0.750) | 0.60 | |
| Nomogram 2 (excluding preoperative plasma lipid profile factors) | 0.699 (0.638–0.760) | 0.03 | 0.659 (0.602–0.717) | 0.002 | |
| Nomogram 3 (excluding age factors) | 0.718 (0.658–0.779) | 0.08 | |||
TNM, tumor node metastasis; LUAD, lung adenocarcinoma; OS, overall survival; DFS, disease-free survival; CI, confidence interval.
Discussion
Exploring the risk factors of LUAD prognosis and recurrence can provide a clinical basis for postoperative monitoring and early intervention to reduce the tumor recurrence rate and prolong the survival time of patients (18,19). Research shows that blood lipid levels, which are easy and inexpensive to obtain, are closely related to tumor occurrence and recurrence. Therefore, we are interested in understanding the relationship between blood lipid levels and the prognosis of LUAD patients in China.
There is increasing evidence that dyslipidemia is a diagnostic feature of tumor patients. In this study, lower TC levels indicated a poor OS and was an independent risk factor for the prognosis of patients with LUAD. In addition, lower HDL-C levels were related to sex and history of smoking but not stage of cancer. Cholesterol is essential to maintain cell integrity and various biological functions, and its level changes can affect various signal pathways and related proteins (20,21), such as cell survival kinase Akt (11). Low cholesterol levels are thought to be associated with immunosuppression, up-regulation of mevalonate pathway activity or reactivity, and increase in nuclear factor kappa B (NF-κB) activity, thereby promoting the occurrence and development of cancer (7,11). A previous prospective cohort study showed that serum TC level is negatively correlated with total cancer mortality (22). In recent years, research on the correlation between serum cholesterol levels and the risk of lung cancer has gradually increased. Our recent previous study found that preoperative low cholesterol levels are associated with poor prognosis in lung squamous cell carcinoma (7), and Zhang et al. also reported that high cholesterol levels reduce the risk of death in patients with advanced non-small cell lung cancer (NSCLC) and who have the epidermal growth factor receptor (EGFR) gene mutation (23), thus aligning with our results.
HDL-C is a major indicator of lipoprotein cholesterol (24,25). We revealed that increased HDL-C levels predicted increased DFS and OS after radical surgery. Lower HDL-C levels were related to sex, history of drinking alcohol, and history of smoking but not stage of cancer. Based on data from previous studies, HDL-C may affect the occurrence of cancer by participating in the reverse transport of cholesterol (26), affecting cell cycle entry (27), and regulating apoptosis and the inflammatory response (7,28,29). Tumor cells increase their pathogenicity by using exogenous fatty acids to support their rapid division while generating large numbers of signal lipids, such as phosphatidylinositol (3,4,5)-triphoshpate (PIP3), ceramide-1-phosphate (CIP), and prostaglandins (30). HDL-C can remove excess cholesterol and reduce the consumption and storage of cholesterol in tumor tissues (31). Additionally, HDL may reduce proinflammatory cytokines, such as interleukin-6 (IL-6), interleukin-1 (IL-1), and tumor necrosis factor-α (TNF-α), and increase the level of anti-inflammatory cytokines, such as IL-10, thereby inhibiting cell proliferation and promoting cell apoptosis (13,32,33).
There are different research conclusions about the influence of LDL-C on the prognosis of lung cancer patients. In small cell lung cancer, lower low-density lipoprotein (LDL) levels were significantly associated with higher OS (34). In lung squamous cell carcinoma, a higher preoperative LDL level indicates a better prognosis (7). This may be due to different tissue types of lung cancer and different treatment options. In our present study, we found that LUAD patients with low LDL had a worse prognosis, although this may not be an independent risk factor.
The abnormality of tumor lipid metabolism is a new field of concern that has gathered attention in recent years. On the one hand, the increase of lipid uptake, storage, and metabolism can promote the growth of tumor cells in various cancers (8-10); while on the other hand, low levels of cholesterol may impair the immune system’s ability to weaken tumor inhibition (35). Our study showed that low levels of TC and HDL-C are independent risk factors for the poor prognosis of LUAD patients undergoing surgery.
Building on previous studies investigating the relationship between preoperative blood lipid levels and tumor patient prognosis, our study further attempted to establish a nomogram model to predict patient prognosis to facilitate more accurate decisions for subsequent treatment. Our research indicates that combining preoperative blood lipid factors into nomogram models improves the prediction of the prognosis of LUAD patients compared to using a simple TNM staging system.
As a retrospective study, our article has some shortcomings. For example, a lipid panel was not rechecked after surgery. In addition, some patients with dyslipidemia were administered statins following surgery. A previous study has shown that statins can reduce the risk of death and improve the prognosis of lung cancer patients (36). A systematic review and meta-analysis suggested that statins may be associated with reduced mortality risk and improved OS in observational studies, but such results have yet to be confirmed by randomized controlled clinical trials (37).
Conclusions
In conclusion, our study showed that preoperative blood lipid levels are closely related to the occurrence and development of LUAD, revealing their potential as a predictor of poor prognosis. Therefore, preoperative TC and HDL-C levels can be considered prognostic factors of LUAD, and dyslipidemia may become a new topic of interest for lung cancer research.
Acknowledgments
We thank LetPub (www.letpub.com) for its linguistic assistance during the preparation of this manuscript.
Footnote
Reporting Checklist: The authors have completed the TRIPOD reporting checklist. Available at https://tcr.amegroups.com/article/view/10.21037/tcr-24-1062/rc
Data Sharing Statement: Available at https://tcr.amegroups.com/article/view/10.21037/tcr-24-1062/dss
Peer Review File: Available at https://tcr.amegroups.com/article/view/10.21037/tcr-24-1062/prf
Funding: This study was funded by
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tcr.amegroups.com/article/view/10.21037/tcr-24-1062/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study was approved by the Academic Ethics Committee of Shaoxing People’s Hospital (No. 078), and all of the methods were performed in accordance with the relevant guidelines and regulations. Written informed consent was not required due to the retrospective nature of this study.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Bischoff P, Trinks A, Obermayer B, et al. Single-cell RNA sequencing reveals distinct tumor microenvironmental patterns in lung adenocarcinoma. Oncogene 2021;40:6748-58. [Crossref] [PubMed]
- Li F, Niu Y, Zhao W, et al. Construction and validation of a prognostic model for lung adenocarcinoma based on endoplasmic reticulum stress-related genes. Sci Rep 2022;12:19857. [Crossref] [PubMed]
- Li X, Zhai S, Zhang J, et al. Interferon Regulatory Factor 4 Correlated With Immune Cells Infiltration Could Predict Prognosis for Patients With Lung Adenocarcinoma. Front Oncol 2021;11:698465. [Crossref] [PubMed]
- Gao S, Guo W, Liu T, et al. Plasma extracellular vesicle microRNA profiling and the identification of a diagnostic signature for stage I lung adenocarcinoma. Cancer Sci 2022;113:648-59. [Crossref] [PubMed]
- Zhang L, Xu H, Ma C, et al. Upregulation of deubiquitinase PSMD14 in lung adenocarcinoma (LUAD) and its prognostic significance. J Cancer 2020;11:2962-71. [Crossref] [PubMed]
- Sun S, Han Q, Liang M, et al. Downregulation of m(6) A reader YTHDC2 promotes tumor progression and predicts poor prognosis in non-small cell lung cancer. Thorac Cancer 2020;11:3269-79. [Crossref] [PubMed]
- Li Z, Xu J, Feng W, et al. The clinical significance of preoperative serum triglyceride, high-density lipoprotein, and low-density lipoprotein levels in lung squamous cell carcinoma. Sci Rep 2022;12:16828. [Crossref] [PubMed]
- Lin X, Lu L, Liu L, et al. Blood lipids profile and lung cancer risk in a meta-analysis of prospective cohort studies. J Clin Lipidol 2017;11:1073-81. [Crossref] [PubMed]
- Kitahara CM, Berrington de González A, Freedman ND, et al. Total cholesterol and cancer risk in a large prospective study in Korea. J Clin Oncol 2011;29:1592-8. [Crossref] [PubMed]
- Siemianowicz K, Gminski J, Stajszczyk M, et al. Serum HDL cholesterol concentration in patients with squamous cell and small cell lung cancer. Int J Mol Med 2000;6:307-11. [Crossref] [PubMed]
- Lyu Z, Li N, Wang G, et al. Independent and joint associations of blood lipids and lipoproteins with lung cancer risk in Chinese males: A prospective cohort study. Int J Cancer 2019;144:2972-84. [Crossref] [PubMed]
- Chen X, Zhou T, Chen M. Meta analysis of the association of cholesterol with pancreatic carcinoma risk. J BUON 2015;20:109-13. [PubMed]
- Jiang SS, Weng DS, Jiang L, et al. The clinical significance of preoperative serum cholesterol and high-density lipoprotein-cholesterol levels in hepatocellular carcinoma. J Cancer 2016;7:626-32. [Crossref] [PubMed]
- Cao X, Wu B, Hou Y, et al. Lipid metabolism-related gene signatures for predicting the prognosis of lung adenocarcinoma. Transl Cancer Res 2023;12:2099-114. [Crossref] [PubMed]
- Chen S, Huang F, He C, et al. Peripheral blood monocytes predict clinical prognosis and support tumor invasiveness through NF-κB-dependent upregulation of Snail in pancreatic cancer. Transl Cancer Res 2021;10:4773-85. [Crossref] [PubMed]
- Shen J, Huang J, Huang Y, et al. Anlotinib suppresses lung adenocarcinoma growth via inhibiting FASN-mediated lipid metabolism. Ann Transl Med 2022;10:1337. [Crossref] [PubMed]
- Ji P, Gong Y, Jiang CC, et al. Association between socioeconomic factors at diagnosis and survival in breast cancer: A population-based study. Cancer Med 2020;9:1922-36. [Crossref] [PubMed]
- Yu Y, Wang Z, Zheng Q, et al. GREB1L overexpression correlates with prognosis and immune cell infiltration in lung adenocarcinoma. Sci Rep 2021;11:13281. [Crossref] [PubMed]
- Zhang C, Zhang Z, Sun N, et al. Identification of a costimulatory molecule-based signature for predicting prognosis risk and immunotherapy response in patients with lung adenocarcinoma. Oncoimmunology 2020;9:1824641. [Crossref] [PubMed]
- Liu J, Cao L, Qu JZ, et al. NVD-BM-mediated genetic biosensor triggers accumulation of 7-dehydrocholesterol and inhibits melanoma via Akt1/NF-ĸB signaling. Aging (Albany NY) 2020;12:15021-36. [Crossref] [PubMed]
- Pilely K, Rosbjerg A, Genster N, et al. Cholesterol Crystals Activate the Lectin Complement Pathway via Ficolin-2 and Mannose-Binding Lectin: Implications for the Progression of Atherosclerosis. J Immunol 2016;196:5064-74. [Crossref] [PubMed]
- Law MR, Thompson SG. Low serum cholesterol and the risk of cancer: an analysis of the published prospective studies. Cancer Causes Control 1991;2:253-61. [Crossref] [PubMed]
- Zhang Y, Xu J, Lou Y, et al. Pretreatment direct bilirubin and total cholesterol are significant predictors of overall survival in advanced non-small-cell lung cancer patients with EGFR mutations. Int J Cancer 2017;140:1645-52. [Crossref] [PubMed]
- Greenhill C. Cancer: HDL cholesterol and cancer risk. Nat Rev Gastroenterol Hepatol 2011;8:299. [Crossref] [PubMed]
- Zhao W, Guan J, Horswell R, et al. HDL cholesterol and cancer risk among patients with type 2 diabetes. Diabetes Care 2014;37:3196-203. [Crossref] [PubMed]
- Pommier AJ, Alves G, Viennois E, et al. Liver X Receptor activation downregulates AKT survival signaling in lipid rafts and induces apoptosis of prostate cancer cells. Oncogene 2010;29:2712-23. [Crossref] [PubMed]
- Nofer JR, Junker R, Pulawski E, et al. High density lipoproteins induce cell cycle entry in vascular smooth muscle cells via mitogen activated protein kinase-dependent pathway. Thromb Haemost 2001;85:730-5. [Crossref] [PubMed]
- von Eckardstein A, Hersberger M, Rohrer L. Current understanding of the metabolism and biological actions of HDL. Curr Opin Clin Nutr Metab Care 2005;8:147-52. [Crossref] [PubMed]
- Nofer JR, Levkau B, Wolinska I, et al. Suppression of endothelial cell apoptosis by high density lipoproteins (HDL) and HDL-associated lysosphingolipids. J Biol Chem 2001;276:34480-5. [Crossref] [PubMed]
- Louie SM, Roberts LS, Mulvihill MM, et al. Cancer cells incorporate and remodel exogenous palmitate into structural and oncogenic signaling lipids. Biochim Biophys Acta 2013;1831:1566-72. [Crossref] [PubMed]
- Dessì S, Batetta B, Pulisci D, et al. Cholesterol content in tumor tissues is inversely associated with high-density lipoprotein cholesterol in serum in patients with gastrointestinal cancer. Cancer 1994;73:253-8. [Crossref] [PubMed]
- Esteve E, Ricart W, Fernández-Real JM. Dyslipidemia and inflammation: an evolutionary conserved mechanism. Clin Nutr 2005;24:16-31. [Crossref] [PubMed]
- Yvan-Charvet L, Wang N, Tall AR. Role of HDL, ABCA1, and ABCG1 transporters in cholesterol efflux and immune responses. Arterioscler Thromb Vasc Biol 2010;30:139-43. [Crossref] [PubMed]
- Zhou T, Zhan J, Fang W, et al. Serum low-density lipoprotein and low-density lipoprotein expression level at diagnosis are favorable prognostic factors in patients with small-cell lung cancer (SCLC). BMC Cancer 2017;17:269. [Crossref] [PubMed]
- Muldoon MF, Marsland A, Flory JD, et al. Immune system differences in men with hypo- or hypercholesterolemia. Clin Immunol Immunopathol 1997;84:145-9. [Crossref] [PubMed]
- Cardwell CR, Mc Menamin Ú, Hughes CM, et al. Statin use and survival from lung cancer: a population-based cohort study. Cancer Epidemiol Biomarkers Prev 2015;24:833-41. [Crossref] [PubMed]
- Xia DK, Hu ZG, Tian YF, et al. Statin use and prognosis of lung cancer: a systematic review and meta-analysis of observational studies and randomized controlled trials. Drug Des Devel Ther 2019;13:405-22. [Crossref] [PubMed]


