
High-frequency KRAS mutations in pancreatic adenocarcinoma: prognostic significance and potential co-targeting therapies
Highlight box
Key findings
• Kirsten rat sarcoma viral oncogene homolog (KRAS) displayed high frequency mutations in pancreatic adenocarcinoma (PAAD), colorectal cancer and lung adenocarcinoma.
• KRASG12C, G12D, G12V mutation is an independent prognostic risk factor for PAAD patients.
• Gefitinib, afatinib, erlotinib, and selumetinib may serve as potential co-targeted therapeutic agents against KRAS.
What is known and what is new?
• KRAS mutations are common across a spectrum of cancers, correlating with unfavorable prognosis. KRAS protein is deemed challenging for drug development due to its unique structural attributes and diverse mutational landscape.
• High-frequency KRAS mutations (G12C, G12D, G12V) are independent prognostic risk factors for PAAD patients. Gefitinib, afatinib, erlotinib and selumetinib small molecule compounds may serve as co-targeting agents for KRAS.
What is the implication, and what should change now?
• Detecting specific types of KRAS mutations and conducting combination therapy may help improve clinical outcomes in PAAD patients.
Introduction
The Rat sarcoma (RAS) gene family, which includes Kirsten rat sarcoma viral oncogene homolog (KRAS), Harvey rat sarcoma viral oncogene homolog (HRAS), and neuroblastoma rat sarcoma viral oncogene homolog (NRAS), is deeply implicated in the pathogenesis of various malignancies. Among these, KRAS mutations are the most prevalent and have been shown to drive tumorigenesis across a wide range of cancers, including pancreatic adenocarcinoma (PAAD), colorectal cancer (CRC), and lung adenocarcinoma (LUAD) (1-5). KRAS, a small GTPase encoded by the KRAS gene, functions as a molecular switch, alternating between an inactive GDP-bound state and an active GTP-bound state. Upon activation by growth factors, chemokines, calcium ions, or receptor tyrosine kinases (RTKs), KRAS triggers key oncogenic signaling pathways, such as PI3K-AKT-mTOR and RAF-MEK-ERK, which regulate cell proliferation, survival, and drug resistance (6-8). KRAS mutations, particularly in codons 12 and 13, are highly recurrent across cancers, with G12D, G12V, and G12C being the most frequent mutations. These mutations alter the protein’s GTPase activity, locking KRAS in a constitutively active state, which in turn promotes unchecked cellular growth and survival. Numerous studies have established that KRAS mutations are associated with poor clinical outcomes, underscoring the urgent need for effective therapeutic strategies to target this oncogene (9,10).
KRAS mutations also contribute to chemotherapeutic resistance, a major barrier to effective cancer treatment. For instance, a strong negative correlation has been observed between KRASG12 mutations and overall survival (OS) in CRC patients treated with trifluridine/tipiracil (FTD/TPI). Furthermore, preclinical studies have demonstrated that eliminating KRASG12 mutations reduces the efficacy of FTD, suggesting that these mutations play a pivotal role in DNA damage resistance (11). The development of KRAS-targeted therapies has been challenging due to the lack of well-defined binding pockets in the KRAS protein, rendering it a “non-druggable” target (12,13). However, recent advances have led to the identification of promising small molecule inhibitors. For example, sotorasib (AMG510), which targets the G12C variant, has shown significant efficacy in early clinical trials by irreversibly binding to the mutant Cys12 residue (14). Additionally, peptide inhibitors such as KS-58, derived from the screening of KRpep-2d, have exhibited specificity against KRASG12D, demonstrating anticancer activity in vivo (15,16). Despite these advances, targeting KRAS mutations remains a formidable challenge. Efforts to inhibit downstream molecules in the KRAS signaling pathway, such as RAF, ERK, and MEK, are yet to yield substantial clinical success (17). Thus, the development of novel inhibitors that can selectively and effectively target specific KRAS mutations remains an urgent clinical need.
In this study, we conducted a comprehensive pan-cancer analysis to evaluate the impact of specific KRAS mutations (G12C, G12D, and G12V) on clinical outcomes in patients with PAAD, CRC, and LUAD. Additionally, we performed targeted sequencing on a cohort of patients with these cancers to identify mutation profiles. Based on these findings, we propose potential small molecule compounds to guide the development of KRAS inhibitors targeting specific mutations or multiple KRAS variants simultaneously. We present this article in accordance with the REMARK reporting checklist (available at https://tcr.amegroups.com/article/view/10.21037/tcr-24-1832/rc).
Methods
Data collection and processing
Clinicopathologic data for patients with PAAD, CRC, and LUAD were retrieved from the cBioPortal database (https://www.cbioportal.org/). Additionally, we downloaded the Single Nucleotide Variation dataset for all The Cancer Genome Atlas (TCGA) samples processed using MuTect2 software (DOI: 10.1038/nature08822) from the Genomic Data Commons (GDC) portal (https://portal.gdc.cancer.gov/). Mutational data were integrated, and protein structural domain information was obtained via the R package maftools (v2.2.10). Visualizations for partial data analyses were performed using the Sangerbox 3.0 platform (http://sangerbox.com/).
Kaplan-Meier and Cox regression analysis
Patients were classified into two groups—altered and unaltered—based on the presence of KRAS mutations at the G12C, G12D, and G12V sites. Survival curves were visualized using the R package Survminer. Patients with a survival time of less than one month were excluded from the analysis. Clinicopathologic characteristics were integrated into univariate and multivariate Cox regression models to assess their association with OS. All data processing and analyses were performed using R (v4.1.3) and Zstats (v1.0, www.zstats.net).
Identification of small molecule compounds
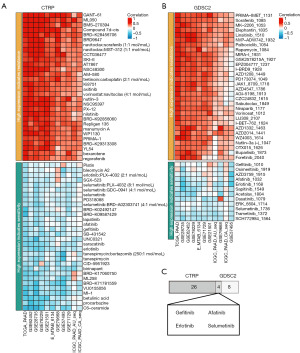
To identify potential small molecule compounds targeting KRAS mutations, we utilized the BEST database (https://rookieutopia.hiplot.com.cn/app_direct/BEST/). Briefly, BEST employed a ridge regression model to ascertain the correlations between various drugs and the KRAS gene across multiple cohorts. This was achieved by conducting a 10-fold cross-validation, utilizing drug response and gene expression data sourced from cancer cell lines in the GDSC_v1, GDSC_v2, CTRP, and PRISM databases. Drug efficacy was evaluated through ridge regression models built on drug response data and expression profiles of cancer cell lines from the Genomics of Drug Sensitivity in Cancer (GDSC) and the Cancer Therapeutics Response Portal (CTRP). Sensitivity predictions for individual drugs were made by integrating gene expression data from PAAD samples.
Patient sample collection
Between June 2022 and February 2024, we collected samples from 129 patients with PAAD, 40 patients with CRC, and 35 patients with LUAD at Qilu Hospital of Shandong University. Eligible patients had a postoperative pathological diagnosis of PAAD, CRC, or LUAD and had undergone enhanced chest or abdominal CT scans within 10 days prior to surgery. Collected clinicopathological data included gender, age, tumor location, differentiation grade, tumor invasion depth, tumor size, and lymph node metastasis status. Exclusion criteria were as follows: (I) patients who received preoperative radiotherapy, chemotherapy, or both; (II) patients with multiple concurrent malignancies. Tumor staging and grading were performed according to the 8th edition of the American Joint Committee on Cancer (AJCC)/Union for International Cancer Control (UICC) guidelines. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013) and was approved by the Clinical Research Ethics Committee of Qilu Hospital (No. KYLL-202203-029). Informed consent was obtained from all participants or their legal representatives.
KRAS mutation detection
KRAS mutation analysis was conducted at the Medical Laboratory Center of Qilu Hospital, using tissue samples from patients with PAAD, CRC, and LUAD. Histological evaluation was performed to select suitable regions for DNA extraction. Formalin-fixed paraffin-embedded (FFPE) samples are stored dry at room temperature. Approximately 30 mg of tissue was dissected from FFPE samples using a scalpel. DNA was extracted using the TIANamp FFPE DNA Kit (TIANGEN, Beijing, China) and amplified using the 2× Taq Master Mix kit (Nearshore, Shanghai, China) with primers KRAS-275F (TATCTGTATCAAAGAATGGTCCTG) and KRAS-275R (TTGTATTAAAAGGTACTGGTGGAG). Real-time fluorescence quantitative polymerase chain reaction (qPCR) was performed using a Bioer system (Hangzhou, China) following the manufacturer’s instructions. The ABI3730XL platform was employed for sequencing using the Sanger method and the BigDye v3.1 kit (Thermo Fisher Scientific, WA, USA). Both wild-type (control) and mutant KRAS variants (G12C, G12D, and G12V) were analyzed.
Statistical analysis
Continuous variables were compared between groups using the Mann-Whitney U test, while categorical variables were compared using the Chi-squared or Fisher’s exact test. The association between KRAS mutations and patient prognosis was evaluated using the Kaplan-Meier method. Cox regression analyses were conducted to identify factors significantly associated with OS. A P value of less than 0.05 was considered statistically significant. All statistical analyses were performed using Prism (v9.0, GraphPad Software, SD, CA, USA) and SPSS (v26.0, SPSS Inc., Chicago, IL, USA).
Results
KRAS is widely mutated across several cancers
To investigate the mutation rate of KRAS across various cancers, we analyzed simple nucleotide variations in 32 cancer types from the TCGA dataset. The results indicated that KRAS mutations were prevalent in multiple cancers, with the highest mutation frequencies observed in LUAD (27.2%), rectum adenocarcinoma (READ) (36.7%), colon adenocarcinoma (COAD) (42.6%), and PAAD (77.4%) (Figure 1A). Focusing on these cancers, we further assessed the mutational characteristics of KRAS and compared the mutation frequency between high and low KRAS expression groups using the Chi-squared test. The predominant mutation type identified in KRAS was missense mutations (Figure 1B). Figure 1C illustrates the most frequent mutation hotspots were G12C, G12D, and G12V, consistent with previous findings (5).

Subsequently, we performed Sanger sequencing to detect KRAS mutations in a cohort of 129 PAAD, 40 CRC, and 35 LUAD patients. In PAAD cases, 65 KRAS mutations were identified, accounting for 50.4% of the samples, with G12C, G12D, and G12V mutations present in 83.1% (54/65) of the cases. An additional 8.5% (11/129) of the samples displayed other mutations, such as G13D and G12R. Among CRC patients, 33 KRAS mutations were detected, representing 82.5% (33/40) of the cases, with G12C, G12D, and G12V mutations found in 84.8% (28/33) of samples. Additionally, 15.2% (5/33) exhibited G12R mutations (Table S1). In LUAD, 24 KRAS mutations were identified in 68.6% (24/35) of patients, with G12C present in 33.3% (8/24) and G12D in 66.7% (16/24) of cases. No other mutations were detected in LUAD samples (Table S1). These findings suggest that G12C, G12D, and G12V mutations in KRAS may contribute to cancer progression.
KRAS mutations as independent prognostic risk factors in PAAD
Our previous analyses highlighted G12C, G12D, and G12V as the most prevalent KRAS mutations. We classified patients with these mutations into an “altered” group, while those without them were grouped as “unaltered”. Table S2 contains integrated clinical subgroup analysis for the three cancers: PAAD, CRC, and LUAD. In PAAD patients, the altered group exhibited significantly shorter OS (P<0.001), progression-free survival (PFS, P=0.004), disease-free survival (DFS, P=0.014), and disease-specific survival (DSS, P=0.005) compared to the unaltered group (Figure 2A-2D). Nevertheless, no significant difference in survival outcomes was observed between patients with CRC and LUAD patients (Figure 2E-2L).

To further investigate the prognostic impact of KRAS mutations in PAAD, we performed a Cox regression analysis. Univariate Cox proportional hazards analysis revealed that several clinical factors, including age [hazards ratio (HR) =1.03, P=0.02], mutation type (HR =2.33, P<0.001), pN stage (HR =2.23, P=0.007), Grade 2 (HR =1.92, P=0.049), Grade 3 (HR =2.42, P=0.01), locoregional recurrence (HR =2.34, P=0.008), and radiotherapy (HR =0.36, P=0.01), were significantly associated with prognosis. Multivariate Cox regression analysis confirmed that lymph node metastasis, locoregional recurrence, tumor grade, and KRAS mutations (G12C, G12D, G12V) were independent prognostic risk factors for PAAD, while radiotherapy emerged as a protective factor (Table 1). Sequencing revealed the mutation sites in PAAD patients, and representative sequence maps for both wild-type and mutated KRAS (G12C, G12D, G12V) are shown in Figure 3A-3D. Interestingly, PAAD patients harboring these mutations were more likely to develop distant metastasis and exhibited higher tumor differentiation, although no significant differences were found in age, gender, T stage, N stage, or overall stage between the altered and unaltered groups (Figure 3E-3L and Table S2).
Table 1
| Variables | Univariate | Multivariate | |||
|---|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | ||
| Age | 1.03 (1.01–1.05) | 0.02 | 1.02 (0.99–1.05) | 0.12 | |
| Sex | |||||
| Female | 1.00 (reference) | ||||
| Male | 0.77 (0.50–1.20) | 0.25 | |||
| Mutation type | |||||
| Unaltered | 1.00 (reference) | 1.00 (reference) | |||
| Altered | 2.33 (1.45–3.74) | <0.001 | 2.09 (1.12–3.91) | 0.02 | |
| Stage | |||||
| I | 1.00 (reference) | 1.00 (reference) | |||
| II | 2.81 (1.12–7.04) | 0.03 | 0.43 (0.09–2.00) | 0.28 | |
| III | 2.01 (0.38–10.51) | 0.41 | |||
| IV | 2.91 (0.68–12.45) | 0.15 | |||
| Race | |||||
| Asian | 1.00 (reference) | ||||
| White | 1.20 (0.44–3.30) | 0.73 | |||
| Black or African American | 1.45 (0.39–5.44) | 0.58 | |||
| M | |||||
| M0 | 1.00 (reference) | ||||
| M1 | 1.18 (0.36–3.90) | 0.79 | |||
| N | |||||
| N0 | 1.00 (reference) | 1.00 (reference) | |||
| N1-N3 | 2.23 (1.24–4.00) | 0.007 | 3.35 (1.26–8.89) | 0.02 | |
| T | |||||
| T1 | 1.00 (reference) | ||||
| T2 | 0.93 (0.19–4.67) | 0.93 | |||
| T3 | 2.20 (0.53–9.11) | 0.28 | |||
| T4 | 1.65 (0.23–11.90) | 0.62 | |||
| Grade | |||||
| G1 | 1.00 (reference) | 1.00 (reference) | |||
| G2 | 1.92 (1.01–3.69) | 0.049 | 2.38 (0.95–5.93) | 0.06 | |
| G3 | 2.42 (1.20–4.90) | 0.01 | 3.02 (1.17–7.77) | 0.02 | |
| G4 | 1.60 (0.21–12.41) | 0.65 | |||
| Tumor type | |||||
| Primary | 1.00 (reference) | 1.00 (reference) | |||
| Distant metastasis | 1.30 (0.80–2.13) | 0.29 | |||
| Locoregional recurrence | 2.34 (1.25–4.38) | 0.008 | 2.49 (1.08–5.75) | 0.03 | |
| Alcohol history | |||||
| No | 1.00 (reference) | ||||
| Yes | 1.24 (0.77–2.02) | 0.38 | |||
| Family history of cancer | |||||
| No | 1.00 (reference) | ||||
| Yes | 1.03 (0.57–1.85) | 0.93 | |||
| History of chronic pancreatitis | |||||
| No | 1.00 (reference) | ||||
| Yes | 2.15 (0.92–5.03) | 0.08 | |||
| History of diabetes | |||||
| No | 1.00 (reference) | ||||
| Yes | 1.10 (0.60–1.99) | 0.76 | |||
| Radiotherapy | |||||
| No | 1.00 (reference) | 1.00 (reference) | |||
| Yes | 0.36 (0.16–0.79) | 0.01 | 0.29 (0.12–0.71) | 0.006 | |
| Chemotherapy | |||||
| No | 1.00 (reference) | ||||
| Yes | 0.68 (0.43–1.07) | 0.10 | |||
P<0.05 indicates a statistical difference. KRAS, Kirsten rat sarcoma viral oncogene homolog; HR, hazard ratio; CI, confidence interval.

Prediction of small molecule compounds targeting KRAS
Due to its complex spatial conformation, KRAS has long been considered one of the most challenging mutant genes to target therapeutically. Recently, several regulatory inhibitors targeting KRAS mutations have shown promising therapeutic effects. However, clinical application of these inhibitors may be limited by the emergence of secondary mutations in KRAS, leading to drug resistance (18). The efficacy of these inhibitors can also be compromised due to the activation of alternative metabolic pathways upon KRAS mutation (19). To address the challenge of drug resistance, we aimed to predict potential small-molecule compounds targeting KRAS. Using data from the GDSC and CTRP databases, we identified several compounds with predicted efficacy against high KRAS expression in PAAD (Figure 4A,4B). Our analysis revealed that patients with elevated KRAS expression may be sensitive to gefitinib, afatinib, erlotinib, and selumetinib (Figure 4C). These compounds, in conjunction with KRAS-specific inhibitors, may offer a viable therapeutic strategy for patients with KRAS mutations.
Discussion
This study leverages bioinformatics to explore the landscape of KRAS mutations across various cancers. Our analysis underscores the elevated frequency of KRAS mutations in PAAD, CRC, and LUAD, with G12C, G12D, and G12V being the predominant mutation types. We investigated these mutations in a cohort comprising 129 PAAD, 40 CRC, and 35 LUAD patients. Our results revealed a substantial prevalence of KRAS mutations in PAAD (49.6%), CRC (70%), and LUAD (68.6%), with G12D emerging as the most frequent mutation. These observations align with previous reports, reinforcing the role of KRAS mutations as a critical factor in these cancers.
Our study confirms that KRAS mutations, particularly G12C, G12D, and G12V, are significant prognostic indicators for PAAD. Patients harboring these mutations experience markedly reduced OS, PFS, DFS, and DSS. These findings corroborate previous study that highlight KRAS mutations as independent prognostic risk factors for PAAD (20). Notably, our results also revealed a strong association between KRAS mutations and lymph node metastasis and locoregional recurrence in PAAD patients. This is consistent with Kong et al.’s observation that KRASG12D mutations drive PAAD lymph node metastasis through aberrant circRNA synthesis and signaling modulation (21). Such insights suggest that the specific type of KRAS mutation may confer distinct phenotypic characteristics and impact disease progression. In contrast, KRAS mutations did not show a significant impact on prognosis in CRC and LUAD patients. This discrepancy may be attributed to the diversity of KRAS mutation subtypes and their interactions with other co-mutations in these cancers. Previous research has identified age, gender, and primary lesion site as independent risk factors for KRAS mutations in CRC (22), yet our study did not find a direct prognostic effect of KRAS mutations in CRC and LUAD. This highlights the need for further investigation into the specific roles of KRAS mutations and their interactions with other genetic factors.
KRAS is often deemed non-druggable due to its unique structural attributes and mutational landscape. The protein’s smooth exterior, absence of a deep binding pocket, and its high affinity for GTP/GDP, which are abundant within cells, pose significant challenges for the development of competitive inhibitors. Additionally, the GTP binding site of KRAS exhibits variability among different mutants, such as G12C, G12D, G12V, G13D, and Q61H, further complicating inhibitor design (23). Consequently, the scientific community has shifted its focus towards the development of inhibitors targeting the downstream effects of KRAS signaling. Despite the promising therapeutic efficacy of certain KRAS-targeted inhibitors, resistance remains a significant challenge in clinical settings. Resistance mechanisms include secondary KRAS mutations, feedback regulation of upstream and downstream pathways, and immune-related factors (8). Our study contributes to addressing this challenge by predicting several potential small-molecule compounds—gefitinib, afatinib, selumetinib, and erlotinib—based on KRAS expression profiles. These compounds target upstream signaling pathways, such as epidermal growth factor receptors (EGFRs), which enhance cell proliferation and migration via KRAS signaling (24). Gefitinib, a first-generation EGFR tyrosine kinase inhibitor, blocks receptor phosphorylation and downstream signaling (25), while afatinib, an irreversible ErbB family inhibitor, has shown efficacy in treating advanced EGFR mutation-positive non-small cell lung carcinoma (NSCLC) (26). Erlotinib, another EGFR inhibitor, induces cell cycle arrest and apoptosis in tumors with high EGFR expression (27). However, KRAS mutations can diminish the effectiveness of EGFR-TKIs, leading to reduced PFS and OS (28). Selumetinib, a targeted MEK1/2 inhibitor, has demonstrated clinical benefits, especially when used in combination with EGFR inhibitors in KRAS-mutant NSCLC (29-31). This combination therapy approach offers a promising strategy for overcoming resistance and improving treatment outcomes in patients with KRAS-mutated cancers.
Nevertheless, there are several limitations in our study. The analysis is predominantly retrospective and relies on public data, necessitating validation in larger, multicenter cohorts. Additionally, the lack of comprehensive follow-up data limits our ability to assess the impact of KRAS mutations on survival in PAAD patients fully. Further in vivo and in vitro experiments are required to validate the efficacy of the predicted small-molecule compounds in targeting specific KRAS mutations.
Conclusions
In summary, KRAS mutations at G12C, G12D, and G12V are prevalent and serve as independent prognostic risk factors for patients with PAAD. Targeting these specific KRAS mutations with a combination of small-molecule inhibitors presents a promising strategy to enhance clinical outcomes for affected patients. Integrating multiple targeted therapies could offer a more effective approach to managing KRAS-mutant PAAD and potentially improve patient prognosis.
Acknowledgments
We thank the current and former members of our laboratories and collaborators for their contributions to the publications cited in this article.
Footnote
Reporting Checklist: The authors have completed the REMARK reporting checklist. Available at https://tcr.amegroups.com/article/view/10.21037/tcr-24-1832/rc
Data Sharing Statement: Available at https://tcr.amegroups.com/article/view/10.21037/tcr-24-1832/dss
Peer Review File: Available at https://tcr.amegroups.com/article/view/10.21037/tcr-24-1832/prf
Funding: This work was supported by a grant from
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tcr.amegroups.com/article/view/10.21037/tcr-24-1832/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013) and was approved by the Clinical Research Ethics Committee of Qilu Hospital (No. KYLL-202203-029). Informed consent was obtained from all participants or their legal representatives.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Tomoshige K, Stuart WD, Fink-Baldauf IM, et al. FOXA2 Cooperates with Mutant KRAS to Drive Invasive Mucinous Adenocarcinoma of the Lung. Cancer Res 2023;83:1443-58. [Crossref] [PubMed]
- Hu H, Cheng R, Wang Y, et al. Oncogenic KRAS signaling drives evasion of innate immune surveillance in lung adenocarcinoma by activating CD47. J Clin Invest 2023;133:e153470. [Crossref] [PubMed]
- Taparra K, Wang H, Malek R, et al. O-GlcNAcylation is required for mutant KRAS-induced lung tumorigenesis. J Clin Invest 2018;128:4924-37. [Crossref] [PubMed]
- Li Z, Zhuang X, Pan CH, et al. Alveolar Differentiation Drives Resistance to KRAS Inhibition in Lung Adenocarcinoma. Cancer Discov 2024;14:308-25. [Crossref] [PubMed]
- Hofmann MH, Gerlach D, Misale S, et al. Expanding the Reach of Precision Oncology by Drugging All KRAS Mutants. Cancer Discov 2022;12:924-37. [Crossref] [PubMed]
- Prior IA, Hood FE, Hartley JL. The Frequency of Ras Mutations in Cancer. Cancer Res 2020;80:2969-74. [Crossref] [PubMed]
- Punekar SR, Velcheti V, Neel BG, et al. The current state of the art and future trends in RAS-targeted cancer therapies. Nat Rev Clin Oncol 2022;19:637-55. [Crossref] [PubMed]
- Zhu C, Guan X, Zhang X, et al. Targeting KRAS mutant cancers: from druggable therapy to drug resistance. Mol Cancer 2022;21:159. [Crossref] [PubMed]
- Li C, Vides A, Kim D, et al. The G protein signaling regulator RGS3 enhances the GTPase activity of KRAS. Science 2021;374:197-201. [Crossref] [PubMed]
- Zhao Y, Murciano-Goroff YR, Xue JY, et al. Diverse alterations associated with resistance to KRAS(G12C) inhibition. Nature 2021;599:679-83. [Crossref] [PubMed]
- van de Haar J, Ma X, Ooft SN, et al. Codon-specific KRAS mutations predict survival benefit of trifluridine/tipiracil in metastatic colorectal cancer. Nat Med 2023;29:605-14. [Crossref] [PubMed]
- Chang EH, Gonda MA, Ellis RW, et al. Human genome contains four genes homologous to transforming genes of Harvey and Kirsten murine sarcoma viruses. Proc Natl Acad Sci U S A 1982;79:4848-52. [Crossref] [PubMed]
- Gao W, Jin J, Yin J, et al. KRAS and TP53 mutations in bronchoscopy samples from former lung cancer patients. Mol Carcinog 2017;56:381-8. [Crossref] [PubMed]
- Canon J, Rex K, Saiki AY, et al. The clinical KRAS(G12C) inhibitor AMG 510 drives anti-tumour immunity. Nature 2019;575:217-23. [Crossref] [PubMed]
- Sakamoto K, Kamada Y, Sameshima T, et al. K-Ras(G12D)-selective inhibitory peptides generated by random peptide T7 phage display technology. Biochem Biophys Res Commun 2017;484:605-11. [Crossref] [PubMed]
- Sakamoto K, Masutani T, Hirokawa T. Generation of KS-58 as the first K-Ras(G12D)-inhibitory peptide presenting anti-cancer activity in vivo. Sci Rep 2020;10:21671. [Crossref] [PubMed]
- Parikh K, Banna G, Liu SV, et al. Drugging KRAS: current perspectives and state-of-art review. J Hematol Oncol 2022;15:152. [Crossref] [PubMed]
- Awad MM, Liu S, Rybkin II, et al. Acquired Resistance to KRAS(G12C) Inhibition in Cancer. N Engl J Med 2021;384:2382-93. [Crossref] [PubMed]
- Akhave NS, Biter AB, Hong DS. Mechanisms of Resistance to KRAS(G12C)-Targeted Therapy. Cancer Discov 2021;11:1345-52. [Crossref] [PubMed]
- Yousef A, Yousef M, Chowdhury S, et al. Impact of KRAS mutations and co-mutations on clinical outcomes in pancreatic ductal adenocarcinoma. NPJ Precis Oncol 2024;8:27. [Crossref] [PubMed]
- Kong Y, Luo Y, Zheng S, et al. Mutant KRAS Mediates circARFGEF2 Biogenesis to Promote Lymphatic Metastasis of Pancreatic Ductal Adenocarcinoma. Cancer Res 2023;83:3077-94. [Crossref] [PubMed]
- Rudiman R, Alfarisy AN, Lukman K, et al. Associations of KRAS Mutations and Clinical Characteristics of Colorectal Cancer Patients in Indonesia. Asian Pac J Cancer Prev 2024;25:3457-61. [Crossref] [PubMed]
- Huang L, Guo Z, Wang F, et al. KRAS mutation: from undruggable to druggable in cancer. Signal Transduct Target Ther 2021;6:386. [Crossref] [PubMed]
- Mangiapane LR, Nicotra A, Turdo A, et al. PI3K-driven HER2 expression is a potential therapeutic target in colorectal cancer stem cells. Gut 2022;71:119-28. [Crossref] [PubMed]
- Muhsin M, Graham J, Kirkpatrick P. Gefitinib. Nat Rev Drug Discov 2003;2:515-6. [Crossref] [PubMed]
- Wecker H, Waller CF. Afatinib. Recent Results Cancer Res 2018;211:199-215. [Crossref] [PubMed]
- Abdelgalil AA, Al-Kahtani HM, Al-Jenoobi FI. Erlotinib. Profiles Drug Subst Excip Relat Methodol 2020;45:93-117. [Crossref] [PubMed]
- Guibert N, Barlesi F, Descourt R, et al. Characteristics and Outcomes of Patients with Lung Cancer Harboring Multiple Molecular Alterations: Results from the IFCT Study Biomarkers France. J Thorac Oncol 2017;12:963-73. [Crossref] [PubMed]
- Yang JC, Ohe Y, Chiu CH, et al. Osimertinib plus Selumetinib in EGFR-Mutated Non-Small Cell Lung Cancer After Progression on EGFR-TKIs: A Phase Ib, Open-Label, Multicenter Trial (TATTON Part B). Clin Cancer Res 2022; Epub ahead of print. [Crossref] [PubMed]
- Chen W, Yu D, Sun SY, et al. Nanoparticles for co-delivery of osimertinib and selumetinib to overcome osimertinib-acquired resistance in non-small cell lung cancer. Acta Biomater 2021;129:258-68. [Crossref] [PubMed]
- Della Corte CM, Ciaramella V, Cardone C, et al. Antitumor Efficacy of Dual Blockade of EGFR Signaling by Osimertinib in Combination With Selumetinib or Cetuximab in Activated EGFR Human NCLC Tumor Models. J Thorac Oncol 2018;13:810-20. [Crossref] [PubMed]


