
Increased risk of colorectal adenoma and benign colorectal polyp associated with Helicobacter pylori infection: a systematic review and meta-analysis
Highlight box
Key findings
• In this systematic review and meta-analysis, we identified all reported risks for colorectal adenomas (CRAs) associated with Helicobacter pylori (H. pylori) infection.
What is known and what is new?
• The effects of sociodemographic covariates as well as moderators such as ethnicity, polyp type, and qualitative analysis of individual studies well recognized.
• This study findings revealed a significant association between H. pylori infection and benign colorectal polyp, CRAs, and advanced CRAs.
What is the implication, and what should change now?
• Our findings allow experts to assess the overall risk of CRAs following H. pylori infection by considering various covariates.
Introduction
Helicobacter pylori (H. pylori) is a predominant microorganism primarily inhabiting the stomach, and its infection is strongly linked to the development of gastrointestinal diseases such as gastric inflammation, peptic ulcer, gastric cancer, and gastric mucosa-associated lymphoid-tissue lymphoma (1). Afflicted individuals typically manifest chronic gastric inflammation and an immune-mediated inflammatory reaction towards host gastric cells, culminating in heightened gastrin secretion (2). Numerous studies on H. pylori have been conducted to elucidate the pathogenesis of upper gastrointestinal disorders, revealing its association not only with digestive tract diseases but also with systemic disorders extending beyond the stomach (3,4). Given the conceivable link between upper gastrointestinal disorders and the onset of colorectal neoplasms, scholars have long endeavored to probe the potential correlation between H. pylori infection and colorectal cancer (CRC) (5,6).
CRC ranks as the third most prevalent cancer-related causes of death globally (7). Despite sustained scholarly inquiry into diverse factors encompassing dietary habits, lifestyle adjustments, environmental pollutants, and pharmaceutical agents, the pathogenesis remains intricate and only partially elucidated. Additionally, recent research indicates that the incidence rate of CRC, traditionally acknowledged as highest among middle-aged and older adults, has exceeded that of the elderly population. Based on current findings the incidence rate of CRC, measured through annual percentage change, has demonstrated a more pronounced increase in the 10–40-year age group (1.6%) compared to the 50–74-year age group (0.6%) (8). The most potent preventive measure identified thus far is prior colonoscopy screening (9,10). Colorectal adenomas (CRAs) identified during such screenings manifest a broad array of cancer-related molecular alterations, with histological, morphological, and genetic changes occurring progressively through a stepwise process (11). Previous studies have implicated the association between CRA detected during colonoscopy and H. pylori infection (12,13).
Numerous meta-analyses have suggested an association between H. pylori infection and an increased risk of CRC; however, there is limited specific analysis regarding the varying associations based on the histological subtypes of colon polyps (14). The progression to cancer is not sudden; rather, colon polyps persist as benign growths over an extended period before transitioning to malignancy (15). Thus, investigating the impact of H. pylori infection at varying stages of polyps is imperative. The aim of our study was to conduct a meta-analysis to assess the impact of H. pylori infection on colon polyps according to their histological classification as benign colorectal polyp (BCP), CRA, and advanced CRA. We present this article in accordance with the MOOSE reporting checklist (available at https://tcr.amegroups.com/article/view/10.21037/tcr-24-795/rc).
Methods
This systematic review and meta-analysis was registered at PROSPERO (CRD42022308533).
Search strategy
For this study, we collected literature rigorously from PubMed, Embase, and Cochrane databases using Medical Subject Headings and text keywords through January 2024 (Table S1). The main key words and medical terms included those related to population of interest (i.e., individuals who received colonoscopy screening for BCP or CRA), exposure (H. pylori infection positive), and outcomes of interest (CRAs). The main key words were categorized using the Boolean operators. The study was not limited by language or study design. Two independent researchers (J.H.K. and S.R.S.) checked the clinical research registries and reference lists for additional studies to increase the completeness of the study.
Study selection
The study inclusion criteria were as follows: (I) the study population comprised patients who were diagnosed with colonic polyp after colonoscopy including BCP, CRA and advanced CRA, but excluded those diagnosed with cancer and carcinoma. Hyperplastic polyp and traditional serrated adenoma are classified as BCP, and sessile serrated adenoma, tubular adenoma, villous adenoma, non-advanced adenomatous polyp, adenomatous are classified as CRA. In the hyperplastic polyp and colorectal polyp, if there is no stage, it is defined as BCP, and if there is a stage notation, it is classified as CRA. Excluded from simple Juvenile polyposis adenoma and multiple colorectal polyps are excluded from adenoma unless there is a detailed stage. Sessile serrated adenoma is classified as advanced CRA if dysplasia exists or CRA if not. Tubular adenoma is classified as advanced CRA or CRA if not. Advanced CRA was defined as one of the following features according to the current guideline: villous component, ≥10 mm in size, and high-grade dysplasia (16); (II) the exposure included the positive detection of H. pylori infection in stomach; (III) the comparison group was those with the negative detection of H. pylori; and (IV) outcomes were the risk for colonic polyp [odds ratios (ORs)]. Two independent authors (J.H.K. and S.R.S.) checked the titles and abstracts of individual studies to ensure that they met our inclusion and exclusion criteria. Only eligible studies were then strictly selected through full text checking, and a predefined form was used for data extraction. When the two authors’ perspectives had discrepancies, individual cases were rigorously discussed and resolved in a meeting of all researchers. Finally, the studies selected for this meta-analysis were selected through a full researchers’ meeting. To ensure the absence of overlapping data and to maintain the meta-integrity, analysis’s references and data for each included study were carefully cross-checked.
Statistical analysis
All variables had the same measurement units, and the outcomes were recorded using binary data with a frequency of improvement. To adequately analyze the overall effect sizes, ORs with their corresponding 95% confidence intervals (CIs) using a random-effects model were used. If individual studies had multiple or different follow-up periods, the longest was selected.
Cochran’s Q test and the I2 statistic were used to assess proportion of total heterogeneity due to within-and between-study variations. Each moderator was subjected to a meta-regression analysis. To analyze potential moderators (e.g., number of patients, ethnic groups, study designs, polyp types, testing methods for H. pylori infection, research periods, population sources, and quality assessments), we estimated the variance of the true effects using a restricted maximum likelihood (REML) estimator. A two-sided P value less than 0.05 was considered for statistical significance. R 4.1.3 software (R Foundation for Statistical Computing, Vienna, Austria) was used to conduct this analysis (17).
Assessment of methodological quality
To evaluate the quality of this study, we used the Newcastle-Ottawa Quality Assessment Scale (NOS), which is based on three main factors (18). The first is the proper selection of participants, the second is the use of appropriate statistical analysis methods to evaluate comparisons, and the third is the clarity of the outcome measures and the adequacy of the study process. Each criterion was given a star rating; a study could receive one star for each item in the selection and outcome categories, but two stars for comparability. A maximum of nine stars can be awarded for all parameters. According to specific conditions, the qualitative power of the evidence related to the evaluation of benefits and drawbacks was exhibited (18).
Assessment of potential publication bias
We measured publication bias using a funnel plot. A funnel plot is to check whether publication bias exists in the meta-analysis by displaying the number of samples and the risk as a figure, respectively. First of all, looking at the funnel plot, it was judged that there would be no publication bias if the effect sizes of the studies were evenly distributed from side to side. In addition, it was confirmed whether publication bias exists as a test to statistically test publication bias (the Begg and Mazumdar rank correlation test and Egger’s linear regression test).
Results
Study selection
The initial search identified a total of 153 articles from different electronic databases (PubMed, n=43; Cochrane, n=2; Embase, n=105) and Websites (n=3). Twenty studies were removed as duplicates because they were extracted from more than one database. At screening, 43 studies were removed because they were not relevant to the scope of the study, and 49 studies (no target diseases, n=15; cancer or carcinoma, n=16; no exposure of H. pylori, n=6; no outcome of interest, n=2; commentary and letter, n=5; and others, n=5) were excluded because they did not meet the study’s inclusion and exclusion criteria during data extraction. Finally, 40 studies (Table 1) were selected for inclusion in this systematic review and meta-analysis after a rigorous selection process (Figure 1).
Table 1
| Study | Country/region | Age, years (mean ± SD) | Male (%) | No. of participants | Study period | Study population | Study design | Testing for HP infection | Disease |
|---|---|---|---|---|---|---|---|---|---|
| Abbass 2011 (2) | USA | 59.1 | 39.6 | 192 | 2008–2009 | Hospital | Cross-sectional | RUT or histologic examination | CRA |
| Akalin 2019 (19) | Turkey | 60.54±10.69 | 61.2 | 831 | 2014–2018 | Hospital | Case-control | IgG anti-HP antibody | Benign hyperplasia, CRA |
| Aydin 1999 (20) | Turkey | 49 | 41.2 | 267 | 1996–1997 | Hospital | Case-control | IgG anti-HP antibody | CRA |
| Bae 2009 (21) | Korea | 61.2±9.7 | 72.9 | 346 | 2005–2008 | Hospital | Case-control | UBT, RUT, or histological examination | Advanced CRA |
| Basmaci 2023 (22) | Turkey | NA | 46.5 | 3,231 | 2015–2019 | Hospital | Case-control | IgG anti-HP antibody | Advanced CRA |
| Breuer-Katschinsk 1999 (23) | Germany | 62.2±9.1 | 62.2 | 196 | 1993–1996 | Hospital-based | Case-control | IgG anti-HP antibody | CRA |
| Brim 2014 (24) | USA | ≥60 | 44.0 | 1,256 | 2005–2009 | Hospital | Cross-sectional | Histologic examination or IgG anti-HP antibody | CRA |
| ChangxiChen 2019 (25) | China | 58.6±11.3 | 81.7 | 1,375 | 2013–2014 | Community-based | Cross-sectional | UBT | Benign hyperplasia |
| Dong 2019 (26) | China | – | – | 5,543 | 2012–2017 | Hospital | NA | UBT | CRA |
| Feng 2023 (27) | China | 48.3±12 | 58.3 | 7,700 | 2015–2021 | Hospital | Cross-sectional | UBT | Benign hyperplasia |
| Fujimori 2005 (28) | Japan | 61.1±9.7 | 74.4 | 669 | 1996–2003 | Hospital | Cross-sectional | UBT, RUT, or histological examination | CRA |
| Georgopoulos 2006 (29) | Greece | 64 | 53.8 | 156 | 2000–2001 | Hospital | Case-control | IgG anti-HP antibody | CRA |
| Hong 2012 (30) | Korea | 49.2 | 38.3 | 2,195 | 2010 | Community-based | Cross-sectional | UBT or histologic examination | CRA, advanced CRA |
| Hong 2020 (31) | Taiwan | 56.71 | 73.8 | 2,475 | 2006–2015 | Hospital | Case-control | EGD | CRA |
| Hu 2017 (32) | Taiwan | 53.29±0.31 | 68.1 | 2,475 | 2006–2015 | Community-based | Cross-sectional | RUT | CRA |
| Huang 2019 (33) | China | 50.10±8.29 | 86.8 | 493 | 2010–2014 | Hospital | Case-control | UBT | Benign hyperplasia |
| Inoue 2011 (34) | Japan | 49.9 | – | 478 | 1996–2004 | Community-based | Case-control | IgG anti-HP antibody | Advanced CRA |
| Jia 2023 (35) | China | NA | 73.2 | 191 | 2020–2022 | Hospital | Case-control | Immune-histochemical stain | Advanced CRA |
| Kim 2017 (36) | Korea | 51.6±7.9 | – | 8,916 | 2002–2010 | Community-based | Cross-sectional | IgG anti-HP antibody | CRA |
| Kumar 2018 (37) | USA | 64±12 | 38.8 | 8,963 | 2006–2016 | Hospital | Cross-sectional | Histologic examination | CRA |
| Lee 2022 (38) | Taiwan | 50 | 50.0 | 20,129 | 2005–2007 | National-based | Cross-sectional | UBT | Benign hyperplasia |
| Liou 2006 (39) | Taiwan | 50.27±0.55 | 57.5 | 462 | NA | Hospital | Cross-sectional | UBT | Advanced CRA |
| Meucci 1997 (40) | Italy | 62 | 56.3 | 156 | 1993–1994 | Hospital | Case-control | IgG anti-HP antibody | CRA |
| Mizuno 2005 (41) | Japan | 58.5±1.18 | 60.4 | 307 | NA | Hospital | Cross-sectional | IgG anti-HP antibody | CRA |
| Mohamed 2020 (42) | Sudan | 47.1±19.8 | 52.3 | 69 | 2017 | Hospital | NA | UBT | Benign hyperplasia |
| Nam 2017 (43) | Korea | 35 | 0 | 4,466 | 2007–2009 | Community-based | Cross-sectional | RUT | CRA, advanced CRA |
| Nam 2013 (44) | Korea | 56.9±9.5 | 67.5 | 597 | 2004–2005 | Community-based | Cross-sectional | IgG anti-HP antibody | CRA, advanced CRA |
| Patel 2014 (45) | USA | 53.7±11.0 | 42.8 | 798 | 2009–2011 | Hospital | Cross-sectional | Histologic examination | CRA |
| Ren 2021 (46) | China | 62.57±6.24 | 55.5 | 733 | NA | Hospital | NA | UBT | Benign hyperplasia |
| Selgrad 2014 (47) | Germany | 66.38±9.83 | 50.1 | 377 | 2008–2013 | Hospital | Cross-sectional | IgG anti-HP antibody | CRA |
| Shao-Hua 2023 (48) | China | 54.34±10.92 | 48.1 | 792 | 2021–2022 | Hospital | Case-control | UBT | Advanced CRA |
| Shen 2021 (49) | China | 63.18±10.10 | 71.6 | 301 | 2018–2019 | Hospital | Cross-sectional | UBT | Benign hyperplasia |
| Siddheshwar 2001 (50) | UK | 66 | 64.9 | 368 | 1997–1999 | Hospital | Case-control | IgG anti-HP antibody | CRA |
| Sonnenberg 2013 (51) | USA | 57.8 | 58.8 | 119,142 | 2008–2011 | Community-based | Cross-sectional | Histologic examination | Advanced CRA |
| Sonnenberg 2020 (6) | USA | 62.7 | 28.2 | 302,061 | 2008–2018 | National-based | Case-control | Immune-histochemical stain | CRA |
| Teimoorian 2018 (52) | Iran | ≥50 | 66.7 | 150 | 2015–2016 | Hospital | Case-control | IgG anti-HP antibody | CRA |
| Tongtawee 2016 (53) | Thailand | 23.5 | 0 | 303 | 2014–2015 | Hospital | Cross-sectional | RUT or histologic examination | CRA |
| Yan 2017 (54) | China | 53.17 | 81.1 | 1,641 | 2014–2016 | Community-based | Cross-sectional | UBT | Benign hyperplasia |
| Zhuang 2022 (55) | China | 55.01±12.20 | 57.2 | 1,622 | 2019–2021 | Hospital | Case-control | UBT | Advanced CRA |
| Zuniga 2015 (56) | USA | 62.6 | 52.8 | 943 | 2010–2012 | Community-based | Case-control | RUT, stool antigen test, or culture | CRA |
Traditional serrated adenoma, non-advanced adenomatous polyp, adenomatous are classified as CRA; in the hyperplastic polyp and colorectal polyp, if there is no stage, it is excluded from adenoma, and if there is a stage notation, it is classified as CRA; excluded from simple Juvenile polyposis adenoma; multiple colorectal polyps are excluded from adenoma unless there is a detailed stage; sessile serrated adenoma is classified as advanced CRA if dysplasia exists or CRA if not; tubular adenoma is classified as advanced CRA or CRA if not. CRA, colorectal adenoma; EGD, esophagogastroduodenoscopy; HP, Helicobacter pylori; IgG, immunoglobulin G; NA, not available; RUT, rapid urease test; SD, standard deviation; UBT, urea breath test.
The 40 studies ultimately selected for systematic review and meta-analysis included 503,365 patients, and detailed demographic characteristics of the individual studies are shown in the Table 1. All of the studies were conducted in global region, and the research period included 1 to 10 years. The range of the calendar years of study and sample sizes in the quantitative meta-analysis were from 1993 to 2022 and 69 to 302,061 patients, respectively. Fifteen studies (37.5%) included Caucasian patients only and 25 studies (62.5%) had both Asian and other racial/ethnic minority groups. Further, seventeen studies used (42.5%) a case-control design and 20 studies (50.0%) used a cross-sectional design (Table 1).
Quality assessment
The quality assessment was conducted thoroughly using NOS by all authors. The authors strictly collected and evaluated each quality assessment domain. Thirty-two studies (80.0%) showed good quality, and the remaining eight studies (20.0%) had relatively small samples. It was judged that the confounding covariates were not properly controlled and evaluated as fair or poor (Table S2).
Outcomes
In CRA and advanced CRA, H. pylori positive was found to be associated with an increase (OR, 1.711; 95% CI: 1.408–2.080). Cochran’s Q test and Higgin’s I2 showed high heterogeneity (I2=90.0%). In subgroup analysis by ethnicity, Western group had lower OR compared to Asian, with a statistically significant difference observed (Western OR, 1.369; 95% CI: 1.222–1.535 vs. Asian OR, 1.990; 95% CI: 1.416–2.796, P=0.04) (Figure 2).
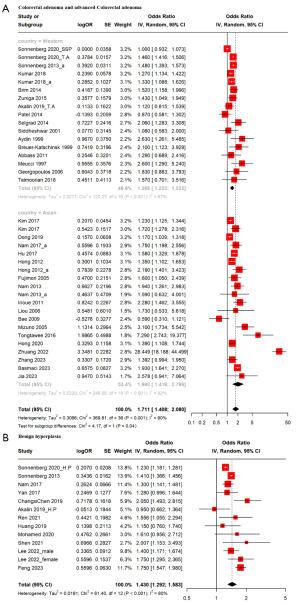
In BCP, H. pylori positive was found to be associated with an increase (OR, 1.430; 95% CI: 1.292–1.583). Cochran’s Q test and Higgin’s I2 showed high heterogeneity (I2=80.0%) (Figure 2).
Effect size modifiers
The moderating effect of covariates using meta-regression analysis for each of BCP, CRA, and advanced CRA is shown in Table 2. The subgroup analysis was categorized according to sample size group (≥10,000 vs. <10,000), ethnic group (Western vs. Asian), study designs (case-control vs. cross-sectional), polyp types (CRA vs. advanced CRA), testing methods (invasive vs. non-invasive), research period (≥3 vs. <3 years), population source, and quality assessment criteria (poor & fair vs. good). Upon analyzing ethnic groups, H. pylori infection was found to be border line P value or significantly associated with an increased ORs at all stages among Asian compared to Western [BCP: Western OR, 1.26 (95% CI: 1.08–1.48), Asian OR, 1.52 (95% CI: 1.35–1.71), P=0.059; CRA and advanced CRA: Western OR, 1.37 (95% CI: 1.22–1.53), Asian OR, 1.99 (95% CI: 1.42–2.80), P=0.04]. When conducted using biopsy protocols as per the testing method, there was a significant association in promoting the detection of colorectal polyps in BCP [invasive method OR, 1.29 (95% CI: 1.17–1.42), non-invasive method OR, 1.58 (95% CI: 1.40–1.78), P=0.01]. In addition, the smaller the study period (P=0.03) were identified as risk factors in CRA and advanced CRA. However, in the subgroup analysis of polyp type, population source, and quality assessment, statistically significant differences in the association between H. pylori infection and the promotion of CRA were not found (Table 2).
Table 2
| Variables | CRA and advanced CRA | Benign hyperplasia | |||||||
|---|---|---|---|---|---|---|---|---|---|
| k | Odds ratio | 95% CI | P | k | Odds ratio | 95% CI | P | ||
| No. of total patients | 0.06 | 0.23 | |||||||
| ≥10,000 | 3 | 1.29 | 1.01–1.66 | 3 | 1.33 | 1.21–1.47 | |||
| <10,000 | 34 | 1.78 | 1.43–2.18 | 10 | 1.49 | 1.28–1.72 | |||
| Ethnic group | 0.04 | 0.059 | |||||||
| Western | 17 | 1.37 | 1.22–1.53 | 3 | 1.26 | 1.08–1.48 | |||
| Asian | 20 | 1.99 | 1.42–2.80 | 10 | 1.52 | 1.35–1.71 | |||
| Study design | 0.40 | <0.001 | |||||||
| Case-control | 18 | 1.79 | 1.24–2.60 | 4 | 1.23 | 1.18–1.28 | |||
| Cross-sectional | 19 | 1.52 | 1.37–1.69 | 9 | 1.52 | 1.36–1.69 | |||
| Polyp type | 0.20 | ||||||||
| Colorectal adenoma | 25 | 1.45 | 1.30–1.61 | ||||||
| Advanced colorectal adenoma | 12 | 2.06 | 1.21–3.50 | ||||||
| Test method for HP | 0.57 | 0.01 | |||||||
| Invasive method | 35 | 1.73 | 1.42–2.13 | 4 | 1.29 | 1.17–1.42 | |||
| Non-invasive method | 2 | 1.32 | 0.53–3.29 | 9 | 1.58 | 1.40–1.78 | |||
| Research period | 0.03 | 0.29 | |||||||
| ≥3 years | 18 | 1.41 | 1.27–1.56 | 5 | 1.33 | 1.10–1.60 | |||
| <3 years | 19 | 2.13 | 1.48–3.07 | 8 | 1.50 | 1.32–1.71 | |||
| Population source | 0.24 | 0.93 | |||||||
| National | 2 | 1.21 | 0.84–1.75 | 3 | 1.38 | 1.15–1.65 | |||
| Community | 11 | 1.54 | 1.38–1.72 | 4 | 1.40 | 1.34–1.47 | |||
| Hospital | 24 | 1.83 | 1.35–2.49 | 6 | 1.46 | 1.16–1.83 | |||
| Quality assessment* | 0.96 | ||||||||
| Poor & fair | 7 | 1.69 | 1.21–2.36 | ||||||
| Good | 30 | 1.71 | 1.36–2.15 | ||||||
k, number of effect sizes; traditional serrated adenoma, non-advanced adenomatous polyp, adenomatous are classified as CRA; in the hyperplastic polyp and colorectal polyp, if there is no stage, it is excluded from adenoma, and if there is a stage notation, it is classified as CRA; excluded from simple Juvenile polyposis adenoma; multiple colorectal polyps are excluded from adenoma unless there is a detailed stage; sessile serrated adenoma is classified as advanced CRA if dysplasia exists or CRA if not; tubular adenoma is classified as advanced CRA or CRA if not; test methods for HP were divided into invasive (immune-histochemical stain, histologic examination, IgG anti-HP antibody, RUT) and non-invasive (UBT) methods; P value from meta-regression analysis using the restricted maximum likelihood. *, quality assessment follows Newcastle-Ottawa Quality Assessment. CI, confidence interval; CRA, colorectal adenoma; HP, Helicobacter pylori; RUT, rapid urease test; UBT, urea breath test.
Publication bias
The distribution of OR funnel plots appear to be symmetrical (Figure 3). The P values for the Begg and Mazumdar correlation test (P=0.14 of CRA and advanced CRA; P=0.90 of BCP) and Egger’s regression coefficient test (P=0.15 of CRA and advanced CRA; P=0.42 of BCP) in this meta-analysis indicate that there was no indication of publication bias or a small-study effect.

Discussion
This systematic review and meta-analysis findings revealed a significant association between H. pylori infection and BCP, CRA, and advanced CRA. In particular, meta-regression analysis confirmed that ethnicity acts as a risk factor in both BCP and CRA.
Although most studies have focused on analyzing CRA stages that affect CRC among the various colon polyp types, our meta-analysis has been performed over a range of disease severity ranging from BCP, CRA, and advanced CRA associated with H. pylori infection. Furthermore, meta-regression analysis of various potential covariates (ethnicity, sample sizes, study designs, polyp types, test methods, technical, test methods, research period, population source, and quality assessment group) affecting risk was used to confirm whether they act as risk factors.
Previous research has consistently suggested a favorable correlation between H. pylori and CRA, particularly advanced CRA, supported by numerous significant findings (51,57), aligning with the outcomes of our meta-analysis. Hyperplastic polyps represent the most prevalent polypoid lesions encountered during colonoscopy (57). While lacking a notable association with CRC, their presence may signify a colonic microenvironment conducive to the genesis of sessile serrated polyps, whose increased size and multiplicity can heighten CRC risk (58,59). H. pylori infection fosters a positive relationship between diverse gastric histopathological abnormalities and the development of colonic polyps, attributed to diminished gastric acid barrier function and impaired gastric motility, which inhibit bacterial infiltration into the lower intestine (6). Moreover, prolonged suppression of gastric acid secretion may induce alterations in the bacterial composition of the lower gastrointestinal tract, potentially fostering the proliferation of colonic tumors (6). Additionally, chronic H. pylori infection may modulate the overall composition and responsiveness of the immune system to external stimuli (60). Our meta-analysis consistently revealed an association between H. pylori infection, spanning from BCP to CRA and advanced CRA.
In this study, it was confirmed that H. pylori infection in Asian increased the risk of BCP and CRA compared to Western. It is thought that this is probably the difference in the environment that makes up various diets and behaviors. For example, it has been suggested that H. pylori infection is associated with alterations and disparities in the gut microbial community, potentially induced through direct stimulation leading to hypergastrinemia (61-63). Moreover, the diversity of gut microbiota is subject to influence by a spectrum of environmental factors, notably including dietary parameters, which exert a notable influence (59). Previous studies analyzing the association between individual dietary patterns and overall gut bacteria uncovered no statistically significant distinctions in meal times or meal regularity between cohorts afflicted with CRC and control subjects. However, substantial disparities emerged concerning late-night snack consumption (64). Additionally, deleterious lifestyle habits such as nocturnal eating and prolonged wakefulness have been identified as risk factors for colorectal polyps (65). While consensus on whether H. pylori infection is independently associated with colorectal polyps and whether this association varies contingent on the histological subtype of colorectal polyps, the linkage between H. pylori and colorectal neoplasms is gaining traction. Our findings intimate that H. pylori infection exhibits associations commencing from the stage of BCP, with the significance of these associations amplifying concomitantly with the progression of polyp stages.
Although this study was the largest and most recent meta-analysis to date, some limitations in this study deserve comment. First, since this study is a systematic literature review and meta-analysis, it is assumed that individual studies are mixed without applying new pathological diagnostic criteria. For example, BCP is judged to have no malignant potential, but the possibility that BCP was significant in H. pylori cannot be excluded from the study results as new colon cancer pathways such as Sessile serrated adenoma is mixed. Therefore, readers should consider this in the interpretation of BCP. Second, significant heterogeneity has been confirmed in meta-analysis. This may inevitably be due to diverse research settings across the globe and/or methodological differences when synthesizing various studies. However, the validity of the results was tested using subgroup analyses or meta-regression analyses. And also, in some cases, there was a publication bias graphically; however, two publication bias tests to quantify the amount of bias captured by funnel plots reveal insignificant bias. Third, there were typical biases in observational studies. In CRA, the difference in risk according to the research period is indicated, which is presumed to be an overestimation bias commonly found in observational studies (66). In addition, the bias of these observational studies can also be confirmed in BCP, and the risk is higher when the test method is non-invasive, which is presumed to be an intended bias to increase sensitivity rather than specificity during diagnostic tests, and in the study design, it is estimated that these are general biases with a high risk of simple cross-sectional studies with a low-evidence level.
Conclusions
This systematic review and meta-analysis showed that there is a positive association between the H. pylori infection and CRA. In particular, special attention should be paid to some covariates, such as ethnicity, because they can act as risk factors. Further studies are still needed to establish a causal relationship between environmental factors or multifactorial causes and adenoma progression. In addition, prospective, long-term follow-up studies are needed for comprehensive and sufficient consideration.
Acknowledgments
None.
Footnote
Reporting Checklist: The authors have completed the MOOSE reporting checklist. Available at https://tcr.amegroups.com/article/view/10.21037/tcr-24-795/rc
Peer Review File: Available at https://tcr.amegroups.com/article/view/10.21037/tcr-24-795/prf
Funding: None.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tcr.amegroups.com/article/view/10.21037/tcr-24-795/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Yang JC, Lu CW, Lin CJ. Treatment of Helicobacter pylori infection: current status and future concepts. World J Gastroenterol 2014;20:5283-93. [PubMed]
- Abbass K, Gul W, Beck G, et al. Association of Helicobacter pylori infection with the development of colorectal polyps and colorectal carcinoma. South Med J 2011;104:473-6. [PubMed]
- Gasbarrini A, Franceschi F, Armuzzi A, et al. Extradigestive manifestations of Helicobacter pylori gastric infection. Gut 1999;45:I9-I12. [PubMed]
- Majka J, Róg T, Konturek PC, et al. Influence of chronic Helicobacter pylori infection on ischemic cerebral stroke risk factors. Med Sci Monit 2002;8:CR675-84. [PubMed]
- Hong EJ, Park DI, Son HJ, et al. Correlations between the prevalence of colonic neoplasia and Helicobacter pylori infection. Korean J Med 2008;74:605-10.
- Sonnenberg A, Turner KO, Genta RM. Associations between gastric histopathology and the occurrence of colonic polyps. Colorectal Dis 2020;22:814-7. [Crossref] [PubMed]
- Stintzing S. Management of colorectal cancer. F1000Prime Rep 2014;6:108. [PubMed]
- Wang Y, Huang X, Cheryala M, et al. Global increase of colorectal cancer in young adults over the last 30 years: an analysis of the Global Burden of Disease Study 2019. J Gastroenterol Hepatol 2023;38:1552-8. [PubMed]
- Kahi CJ, Imperiale TF, Juliar BE, et al. Effect of screening colonoscopy on colorectal cancer incidence and mortality. Clin Gastroenterol Hepatol 2009;7:770-5; quiz 711. [PubMed]
- Pox CP, Altenhofen L, Brenner H, et al. Efficacy of a nationwide screening colonoscopy program for colorectal cancer. Gastroenterology 2012;142:1460-7.e2. [PubMed]
- Jeong YH, Kim KO, Park CS, et al. Risk Factors of Advanced Adenoma in Small and Diminutive Colorectal Polyp. J Korean Med Sci 2016;31:1426-30. [PubMed]
- Shmuely H, Passaro D, Figer A, et al. Relationship between Helicobacter pylori CagA status and colorectal cancer. Am J Gastroenterol 2001;96:3406-10. [PubMed]
- Zhang Y, Hoffmeister M, Weck MN, et al. Helicobacter pylori infection and colorectal cancer risk: evidence from a large population-based case-control study in Germany. Am J Epidemiol 2012;175:441-50. [PubMed]
- Zumkeller N, Brenner H, Zwahlen M, et al. Helicobacter pylori infection and colorectal cancer risk: a meta-analysis. Helicobacter 2006;11:75-80. [PubMed]
- Simon K. Colorectal cancer development and advances in screening. Clin Interv Aging 2016;11:967-76. [Crossref] [PubMed]
- Lieberman DA, Rex DK, Winawer SJ, et al. Guidelines for colonoscopy surveillance after screening and polypectomy: a consensus update by the US Multi-Society Task Force on Colorectal Cancer. Gastroenterology 2012;143:844-57. [Crossref] [PubMed]
- Shim SR, Kim SJ. Intervention meta-analysis: application and practice using R software. Epidemiol Health 2019;41:e2019008. [Crossref] [PubMed]
- Cochrane handbook for systematic reviews of interventions version 5.1.0 [updated March 2011]. Edited by: Higgins JPT, Green S. Available online: http://www.cochrane-handbook.org
- Akalin C, Ozdemir O. Evalution of the Patients with Colon Polyps in Terms of Helicobacter pylori with Sydney Criteria. Turk J Colorectal Dis 2019;29:111-7. [Crossref]
- Aydin A, Karasu Z, Zeytinoglu A, et al. Colorectal adenomateous polyps and Helicobacter pylori infection. Am J Gastroenterol 1999;94:1121-2. [Crossref] [PubMed]
- Bae RC, Jeon SW, Cho HJ, et al. Gastric dysplasia may be an independent risk factor of an advanced colorectal neoplasm. World J Gastroenterol 2009;15:5722-6. [Crossref] [PubMed]
- Basmaci N, Karataş A, Ergin M, et al. Association between Helicobacter pylori infection and colorectal polyps. Medicine (Baltimore) 2023;102:e35591. [Crossref] [PubMed]
- Breuer-Katschinski B, Nemes K, Marr A, et al. Helicobacter pylori and the risk of colonic adenomas. Colorectal Adenoma Study Group. Digestion 1999;60:210-5. [Crossref] [PubMed]
- Brim H, Zahaf M, Laiyemo AO, et al. Gastric Helicobacter pylori infection associates with an increased risk of colorectal polyps in African Americans. BMC Cancer 2014;14:296. [Crossref] [PubMed]
- ChangxiChen. Helicobacter pylori infection associated with an increased risk of colorectal adenomatous polyps in the Chinese population. BMC Gastroenterol 2019;19:14. [Crossref] [PubMed]
- Dong YF, Guo T, Yang H, et al. Correlations between gastric Helicobacter pylori infection and colorectal polyps or cancer. Zhonghua Nei Ke Za Zhi 2019;58:139-42. [PubMed]
- Feng L, Zhao K, Wang G, et al. Relationship between endoscopic gastric abnormalities and colorectal polyps: a cross-sectional study based on 33439 Chinese patients. Int J Med Sci 2023;20:219-24. [Crossref] [PubMed]
- Fujimori S, Kishida T, Kobayashi T, et al. Helicobacter pylori infection increases the risk of colorectal adenoma and adenocarcinoma, especially in women. J Gastroenterol 2005;40:887-93. [Crossref] [PubMed]
- Georgopoulos SD, Polymeros D, Triantafyllou K, et al. Hypergastrinemia is associated with increased risk of distal colon adenomas. Digestion 2006;74:42-6. [Crossref] [PubMed]
- Hong SN, Lee SM, Kim JH, et al. Helicobacter pylori infection increases the risk of colorectal adenomas: cross-sectional study and meta-analysis. Dig Dis Sci 2012;57:2184-94. [Crossref] [PubMed]
- Hong TC, Yang HC, Chen CL, et al. Relationship between serum gamma-glutamyl transferase level and colorectal adenoma. PLoS One 2020;15:e0240445. [Crossref] [PubMed]
- Hu KC, Wu MS, Chu CH, et al. Synergistic Effect of Hyperglycemia and Helicobacterpylori Infection Status on Colorectal Adenoma Risk. J Clin Endocrinol Metab 2017;102:2744-50. [Crossref] [PubMed]
- Huang L, Wu L, Qiao Q, et al. Correlation between Colon Polyps and Metabolic Syndrome and HP Infection Status. Gastroenterol Res Pract 2019;2019:3916154. [Crossref] [PubMed]
- Inoue I, Mukoubayashi C, Yoshimura N, et al. Elevated risk of colorectal adenoma with Helicobacter pylori-related chronic gastritis: a population-based case-control study. Int J Cancer 2011;129:2704-11. [Crossref] [PubMed]
- Jia Z, Wan X. Correlation and influencing factors analysis of colorectal polyps with Helicobacter pylori Infection and p-S6K1 expression. BMC Infect Dis 2023;23:794. [Crossref] [PubMed]
- Kim TJ, Kim ER, Chang DK, et al. Helicobacter pylori infection is an independent risk factor of early and advanced colorectal neoplasm. Helicobacter 2017; [Crossref] [PubMed]
- Kumar A, Kim M, Lukin DJ. Helicobacter pylori is associated with increased risk of serrated colonic polyps: Analysis of serrated polyp risk factors. Indian J Gastroenterol 2018;37:235-42. [Crossref] [PubMed]
- Lee C, Lin TH, Lin CJ, et al. A Noninvasive Risk Stratification Tool Build Using an Artificial Intelligence Approach for Colorectal Polyps Based on Annual Checkup Data. Healthcare (Basel) 2022;10:169. [Crossref] [PubMed]
- Liou JM, Lin JW, Huang SP, et al. Helicobacter pylori infection is not associated with increased risk of colorectal polyps in Taiwanese. Int J Cancer 2006;119:1999-2000. [Crossref] [PubMed]
- Meucci G, Tatarella M, Vecchi M, et al. High prevalence of Helicobacter pylori infection in patients with colonic adenomas and carcinomas. J Clin Gastroenterol 1997;25:605-7. [PubMed]
- Mizuno S, Morita Y, Inui T, et al. Helicobacter pylori infection is associated with colon adenomatous polyps detected by high-resolution colonoscopy. Int J Cancer 2005;117:1058-9. [PubMed]
- Mohamed AK, Elhassan NM, Awhag ZA, et al. Prevalence of Helicobacter pylori among Sudanese patients diagnosed with colon polyps and colon cancer using immunohistochemistry technique. BMC Res Notes 2020;13:322. [Crossref] [PubMed]
- Nam JH, Hong CW, Kim BC, et al. Helicobacter pylori infection is an independent risk factor for colonic adenomatous neoplasms. Cancer Causes Control 2017;28:107-15. [Crossref] [PubMed]
- Nam KW, Baeg MK, Kwon JH, et al. Helicobacter pylori seropositivity is positively associated with colorectal neoplasms. Korean J Gastroenterol 2013;61:259-64. [Crossref] [PubMed]
- Patel S, Lipka S, Shen H, et al. The association of H. pylori and colorectal adenoma: does it exist in the US Hispanic population? J Gastrointest Oncol 2014;5:463-8. [PubMed]
- Ren JF, Feng P, Zhang QS, et al. Correlation between Helicobacter pylori infection and recurrence of colorectal adenoma. World Chinese Journal of Digestology 2021;29:952-9. [Crossref]
- Selgrad M, Bornschein J, Kandulski A, et al. Helicobacter pylori but not gastrin is associated with the development of colonic neoplasms. Int J Cancer 2014;135:1127-31. [Crossref] [PubMed]
- Shao-Hua Z, Lin-Lin R, Shen S, et al. Atrophic gastritis rather than Helicobacter pylori infection can be an independent risk factor of colorectal polyps: a retrospective study in China. BMC Gastroenterol 2023;23:213. [Crossref] [PubMed]
- Shen L, Bian R, Wang W, et al. Association of Helicobacter pylori infection with colorectal adenoma in the Chinese urban population: A cross-sectional study. Microb Pathog 2021;158:105111. [Crossref] [PubMed]
- Siddheshwar RK, Muhammad KB, Gray JC, et al. Seroprevalence of Helicobacter pylori in patients with colorectal polyps and colorectal carcinoma. Am J Gastroenterol 2001;96:84-8. [Crossref] [PubMed]
- Sonnenberg A, Genta RM. Helicobacter pylori is a risk factor for colonic neoplasms. Am J Gastroenterol 2013;108:208-15. [Crossref] [PubMed]
- Teimoorian F, Ranaei M, Hajian Tilaki K, et al. Association of Helicobacter pylori Infection With Colon Cancer and Adenomatous Polyps. Iran J Pathol 2018;13:325-32. [PubMed]
- Tongtawee T, Kaewpitoon S, Kaewpitoon N, et al. Helicobacter Pylori Associated Gastritis Increases Risk of Colorectal Polyps: a Hospital Based-Cross-Sectional Study in Nakhon Ratchasima Province, Northeastern Thailand. Asian Pac J Cancer Prev 2016;17:341-5. [Crossref] [PubMed]
- Yan Y, Chen YN, Zhao Q, et al. Helicobacter pylori infection with intestinal metaplasia: An independent risk factor for colorectal adenomas. World J Gastroenterol 2017;23:1443-9. [Crossref] [PubMed]
- Zhuang Z, Yu B, Xie M, et al. Association of Helicobacter pylori enrichment in colorectal adenoma tissue on clinical and pathological features of adenoma. Clin Res Hepatol Gastroenterol 2022;46:101961. [Crossref] [PubMed]
- Zuniga R, Bautista J, Sapra K, et al. Combination of Triple Therapy and Chronic PPI Use May Decrease Risk of Colonic Adenomatous Polyps in Helicobacter pylori Infection. Gastroenterol Res Pract 2015;2015:638547. [Crossref] [PubMed]
- Monachese M, Mankaney G, El-Khider F, et al. Association between baseline hyperplastic polyps and metachronous serrated lesions. Gastrointest Endosc 2021;93:1401-1407.e1. [Crossref] [PubMed]
- Anderson JC, Robinson CM, Butterly LF. Increased risk of metachronous large serrated polyps in individuals with 5- to 9-mm proximal hyperplastic polyps: data from the New Hampshire Colonoscopy Registry. Gastrointest Endosc 2020;92:387-93. [PubMed]
- O'Brien MJ, Winawer SJ, Zauber AG, et al. The National Polyp Study. Patient and polyp characteristics associated with high-grade dysplasia in colorectal adenomas. Gastroenterology 1990;98:371-9. [PubMed]
- Pachathundikandi SK, Müller A, Backert S. Inflammasome Activation by Helicobacter pylori and Its Implications for Persistence and Immunity. Curr Top Microbiol Immunol 2016;397:117-31. [PubMed]
- Choi DS, Seo SI, Shin WG, et al. Risk for Colorectal Neoplasia in Patients With Helicobacter pylori Infection: A Systematic Review and Meta-analysis. Clin Transl Gastroenterol 2020;11:e00127. [PubMed]
- Qing Y, Wang M, Lin YM, et al. Correlation between Helicobacter pylori-associated gastric diseases and colorectal neoplasia. World J Gastroenterol 2016;22:4576-84. [PubMed]
- Ryoo SK, Kim TJ, Kim ER, et al. Helicobacter pylori Infection and the Development of Advanced Colorectal Neoplasia. J Clin Gastroenterol 2020;54:696-700. [PubMed]
- Shen W, Sun J, Li Z, et al. Food intake and its effect on the species and abundance of intestinal flora in colorectal cancer and healthy individuals. Korean J Intern Med 2021;36:568-83. [PubMed]
- Xu N, Cong X, Sun R, et al. Metabolic risk factors link unhealthy lifestyles to the risk of colorectal polyps in China. Prev Med Rep 2023;35:102314. [PubMed]
- Moore DA, Healy PJ. The trouble with overconfidence. Psychol Rev 2008;115:502-17. [PubMed]



