
Comparison of chemoport and Hickman central venous catheters in patients with hematological cancer
Highlight box
Key findings
• For immunocompromised patients with hematological malignancies, the usage of chemoport compared to that of Hickman central venous catheters (CVCs) may reduce the occurrence of infections, ensuring the long-term maintenance of vascular access.
• The routine management of CVCs by medical professionals can significantly reduce the risk of infections.
What is known and what is new?
• The evolution and diversification of CVCs and other device types has enabled the selection and application of the most appropriate devices tailored to the patient’s clinical needs. However, research on the optimal access selecting evidence remains relatively limited and replacing the Hickman catheter remains a challenge in hematopoietic stem cell transplantation.
• Chemoport-type CVCs can safely and effectively minimize the long-term occurrence of infections in immunocompromised patients with hematological malignancies. Furthermore, both types of CVCs had reinsertion rates of more than 1.
What is the implication, and what should change now?
• Performing the stem cell transplantation procedure using Hickman CVC and then changing to chemoport for subsequent chemotherapy can minimize infection risk and safely preserve long-term vascular access.
• The overall complication rate can be reduced through accurate CVC insertion methods using vascular imaging and routine management by medical professionals.
Introduction
Background
Most patients with advanced malignancies require chemotherapy as a fundamental component of their treatment plan (1,2). During chemotherapy, peripheral venous access is a challenge, necessitating reliable venous access to avoid repetitive venipuncture (1,2). A durable venous access device is essential to facilitate the repeated administration of chemotherapeutic agents as a part of the treatment plan and to address the need for long-term venous access for the delivery of blood products or antibiotics (2,3). The introduction of central venous catheters (CVCs) in the 1980s represents a significant turning point in the treatment of cancer patients, and the use of these catheters has rapidly increased since then (4,5). Despite the significant benefits of CVCs, their use was initially associated with many challenges attributable to insertion-related complications and mechanical issues associated with the device itself (2). However, advancements in ultrasound guidance and improvements in CVC design and materials have substantially reduced the incidence of such complications (6).
As insertion techniques and post-insertion management have become more data-based and clearer, they can effectively address a significant proportion of these issues (7-9). The major complications associated with the use of CVCs in current clinical practice are primarily late complications, such as catheter-related thrombosis or infection. Incidences of these complications vary according to CVC type, such as exit site opening type and catheter inner size (10). With the evolution and diversification of CVCs and other device types, medical professionals can now evaluate the advantages and drawbacks of these devices (1,2,11), enabling the selection and application of the most appropriate devices tailored to the patient’s clinical needs.
Rationale and knowledge gap
Despite these advancements, research on the selection of the optimal access methods remains relatively limited (12,13). No single access method has shown clear superiority, leading to varying preferences for different central venous access devices among centers and physicians (12,13). This variability has led to considerable debate (1,2). Currently, at Pusan National University Hospital, the chemoport and Hickman CVC are commonly used central venous access devices for patients with hematological malignancies.
Objective
We aimed to characterize the differences in clinical outcomes associated with these two types of catheters and recommend the selection of an optimal access method and management strategy. We present this article in accordance with the STROBE reporting checklist (available at https://tcr.amegroups.com/article/view/10.21037/tcr-24-2147/rc).
Methods
Patients and CVCs
This retrospective, single-center study was conducted at the Department of Thoracic and Cardiovascular Surgery at Pusan National University Hospital. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study was approved by the Institutional Review Board of the Pusan National University Hospital (No. 2406-018-140). Prior to all procedures, the potential risks and benefits were explained in detail to the patients, and written informed consent was obtained. Research was performed in accordance with relevant guidelines.
We collected the data of 2,113 patients with advanced cancer who underwent chemoport and Hickman CVC insertion between January 2014 and December 2022 to assess the treatment outcomes. A total of 653 patients with hematological malignancies who underwent CVC insertion, including 280 who underwent chemoport placement and 373 who underwent Hickman catheter placement, at Pusan National University Hospital were included in this study. We reviewed the electronic medical reports of each patient. The primary endpoint was the complication rate, which was determined by measuring the incidence of thrombosis, pulmonary embolism, infection, etc. Thrombosis was catheter-related, and deep vein thrombosis unrelated to the catheter was excluded. The secondary endpoints were the lifespan of the CVC, duration of maintenance, and reasons for removal. The inclusion criteria are summarized in Figure 1.
Patients with a platelet count less than 50,000/mm3, absolute neutrophil count less than 500/mm3, or prothrombin time-international normalized ratio of 1.5 or higher did not undergo CVC placement. Both types of CVCs were inserted in the operating room under local anesthesia and ultrasound guidance by three proficient vascular surgeons. After insertion, the position of the catheter tip was confirmed using a C-arm. Although both types of CVCs were tunneled through a subclavian incision, they differed in terms of the placement within the chest wall: the Hickman catheter was a double/triple-lumen catheter, whereas the chemoport had a single-lumen reservoir port. Among patients with hematological cancer, Hickman catheters were indicated for those undergoing hematopoietic stem cell transplantation (HSCT), whereas chemoports were inserted in those receiving chemotherapy without transplantation. When performing HSCT, a large-bore catheter is necessary to prevent cellular damage. Additionally, owing to the infusion of various medications and fluids after transplantation, a catheter with more than one lumen is advantageous. Therefore, for diseases requiring HSCT, such as leukemia, multiple myeloma, and aplastic anemia, a Hickman catheter was selected. For other cases, a chemoport was generally chosen. The selection of double- or triple-lumen Hickman catheters was based on the chemotherapy drugs used in the hematology-oncology department and the condition of the patient. Triple-lumen Hickman catheters were inserted in high-risk patients, whereas double-lumen catheters were predominantly used in other cases.
CVC insertion procedure
Hickman catheter insertion
Under ultrasound guidance, the Seldinger technique was used to insert a guidewire into the internal jugular vein. A 15-blade scalpel was then used to make a 0.5-cm incision in the right subclavian area, and the hole was widened using electrocauterization to match the catheter size. The subcutaneous area around the site where the guidewire was inserted was dissected using mosquito forceps to facilitate easy passage of the catheter. The catheter was then threaded through the chest wall hole using a tunneller and advanced to the internal jugular puncture site, where the guidewire was inserted. Following the guidewire placement, a sheath was inserted to guide the catheter tip toward the superior vena cava (SVC). After insertion, placement of the catheter tip was confirmed using a C-arm, and the incision was closed with simple nylon 3-0 sutures. Dermabond Advanced (Ethicon, Cincinnati, Ohio, USA) was applied to the internal jugular vein puncture site.
Chemoport insertion
Under ultrasound guidance, the Seldinger technique was used to insert a guidewire into the internal jugular vein. A 15-blade scalpel was used to create a chest wall incision of approximately 1.5–2 cm in the right subclavian area. Using electrocauterization, a pocket approximately 2 cm × 2 cm in size, matching the size of the chemoport, was created beneath the skin. The subcutaneous area around the site where the guidewire was inserted was dissected using mosquito forceps to facilitate easy passage of the catheter. The catheter was then threaded through the chest wall incision into the internal jugular puncture site where the guidewire was inserted. Following the guidewire, a sheath was inserted to guide the catheter tip toward the SVC. After insertion, catheter tip placement was confirmed using a C-arm. The incision was closed with continuous Vicryl 4-0 sutures. Dermabond Advanced (Ethicon) was applied to the incision site.
Maintenance care
Patients were administered prophylactic antibiotics only preoperatively, and they received no additional antibiotics unless there was an infection sign. Catheter management, including dressing changes, was performed primarily by experienced nurses. After the insertion of each CVC, all patients underwent dressing changes at the insertion site twice weekly using Hexidine (4% chlorhexidine gluconate). Before discharge, comprehensive education on wound and infection management was provided by specialized nurses and physicians. The Hickman catheter was disinfected with chlorhexidine once or twice a week, and if there were no allergies, it was dressed with Tegaderm™ film (3M, Maplewood, MN, USA). For the chemoport, when the incision wound was fully healed, no additional dressing was applied, except when the needle was inserted or removed. Patients with Hickman catheters were instructed to visit the hematology-oncology outpatient clinic once a week for flushing upon discharge. Patients with chemoports were instructed to flush their devices once every 4 weeks.
For patients with a Hickman catheter, immunoglobulin injections were administered every two weeks for 3 months post-treatment, and the catheter was typically removed once this period is complete. In patients with multiple myeloma, the Hickman catheter was inserted when collecting bone marrow, while in patients with leukemia patients, it was inserted at the start of treatment. If no complications arose and removal was not required, the Hickman catheter was typically retained for about 6–9 months.
Definitions of complications and management for complications
Infection
The Centers for Disease Control and Prevention (CDC) CLABSI criteria were used, including pocket infections and tunnel infections (14). In patients showing local symptoms of infection related to the insertion site or systemic symptoms of infection along with suspicious blood test results, the CVC was immediately removed, and empirical antibiotic therapy was initiated. Empirical antibiotic therapy typically involves an administration of vancomycin alone or in combination with cefepime. After identification of the bacteria from the blood cultures, the regimen was adjusted, after consultation with infectious disease specialists, to an appropriate antibiotic for intravenous administration for at least 2 weeks. When blood cultures returned negative on two consecutive occasions, antibiotics were discontinued after consultation with an infectious disease specialist. In cases where an abscess was observed at the CVC insertion site, the site was opened, and a betadine-soaked dressing was applied concurrently with the antibiotic therapy. After confirming negative culture results for bacteria on two consecutive occasions, the insertion site was closed using simple sutures. Patients requiring reinsertion underwent the reinsertion procedure after confirmation of negative bacterial culture results.
Catheter-related thrombosis
Most cases are asymptomatic, but if symptoms such as swelling of head/neck/limb, superficial venous distension, inflammation/phlebitis, erythema of limb, localized pain/numbness, jaw or shoulder pain, difficulty with infusion or aspiration are present, catheter-related thrombosis was suspected (15,16). Treatment strategy varies depending on whether the catheter is removed; the use of systemic anticoagulants for at least 3 months is generally recommended (10,17). In our institution, low molecular weight heparin infusion is administered according to activated partial thromboplastin time (range, 50–70 s). In cases where there was no problem with the function of the catheter, no infection, and continuous use was required, the catheter was maintained (10). Anticoagulant treatment should be administered for at least 3 months, and prophylactic anticoagulation should be maintained until removal (10,17). In case of catheter removal, anticoagulant treatment should be started 3–5 days before removal and maintained for at least 6 weeks (10). For patients with thrombocytopenia, anticoagulants were administered for 3 months when the risk of recurrence after thrombosis was high. If the risk of bleeding was higher, the treatment method was modified (10). In cases where anticoagulants were contraindicated, a temporary inferior vena cava filter was considered (10). After completion of anticoagulant treatment, the presence of deep venous thrombosis was confirmed by computed tomography or sonography. Anticoagulation was maintained if thrombosis remained. In cases where pulmonary thromboembolism (PTE) progressed, anticoagulants were used for 6 months, and computed tomography was performed. If PTE was not found, treatment was discontinued. If PTE remains, chronic PTE and pulmonary hypertension should be evaluated, and treatment options should be discussed (17).
Occlusion
When administration through the catheter slowed or regurgitation did not occur as expected, thrombosis was suspected. In the Hickman catheter group, when thrombosis was suspected, the Hickman catheter was promptly removed. In the chemoport group, after explaining the risk of bleeding, urokinase was administered twice at an interval of 30 min–1 h through the reservoir port for thrombolysis. The chemoport was removed if functional failure persisted.
Statistical analysis
All statistical analyses were performed using SPSS for Windows (version 27; IBM Corp., Armonk, NY, USA). The characteristics of the study population were presented as mean ± standard deviation for continuous variables and as frequencies and proportions for categorical variables. Independent t-tests for continuous variables and the chi-squared test or Fisher’s exact test for categorical variables were performed to compare the Hickman and chemoport catheters. Statistical significance was defined as P<0.05. Kaplan-Meier survival curves and Cox proportional-hazard regression model analysis were employed to ascertain the risk at different catheter insertion durations for Hickman and chemoport catheters in relation to the outcomes of infection and thrombus status. The adjusted model also included sex, age, hypertension, diabetes mellitus, coronary artery disease, peripheral artery disease, cerebrovascular accidents, chronic obstructive pulmonary disease, and renal insufficiency as variables.
Results
Patients and CVC characteristics
Table 1 shows the patient characteristics in the two groups. The average patient age in the chemoport group was 10 years higher than that of the Hickman group (62.90±15.25 vs. 51.53±14.56 years, P<0.001), whereas the gender ratio showed a slight male predominance (56.8%) in both groups. The relatively high proportion of older patients may have increased the prevalence of comorbidities. The chemoport group showed significant differences in the incidence of underlying diseases such as hypertension, diabetes mellitus, coronary artery occlusive disease, cerebrovascular accidents, chronic obstructive pulmonary disease, and renal insufficiency, except peripheral arterial occlusive disease. Leukemia was the predominant hematological malignancy (58.2%) in the Hickman group, whereas lymphoma (86.8%) was predominant in the chemoport group. Table 2 presents the distribution of HSCT procedures in the Hickman catheter group. In our institution, nearly all patients undergo peripheral blood stem cell transplantation (PBSCT), and bone marrow transplantation (BMT) is rarely performed. According to the data, 219 patients (58.7%) underwent stem cell transplantation; of these, 101 patients (27.1%) received autologous PBSCT, 115 patients (30.8%) received allogeneic PBSCT, 5 patients (1.3%) received allogeneic BMT, and 3 patients (0.8%) underwent both autologous and allogeneic PBSCT.
Table 1
| Variables | Hickman (n=373) | Chemoport (n=280) | P |
|---|---|---|---|
| Age (years) | 51.53±14.56 | 62.90±15.25 | <0.001 |
| Sex (male) | 212 (56.8) | 159 (56.8) | 0.52 |
| Catheter tip location (mm) | 27.95±22.04 | 28.56±17.15 | 0.62 |
| Catheter angle (°) | 52.64±11.61 | 51.91±11.87 | 0.40 |
| Catheter size (Fr) | 10.49±1.41 | 6.11±0.21 | <0.001 |
| Left IJV approach | 11 (2.9) | 9 (3.2) | 0.051 |
| Disease | |||
| Leukemia | 217 (58.2) | 9 (3.2) | <0.001 |
| Lymphoma | 70 (18.8) | 243 (86.8) | |
| Multiple myeloma | 53 (14.2) | 20 (7.1) | |
| Myelodysplasia | 12 (3.2) | 6 (2.1) | |
| Et cetera | 21 (5.6) | 2 (0.7) | |
| Hypertension | 60 (16.1) | 99 (35.4) | <0.001 |
| DM | 50 (13.4) | 64 (22.9) | 0.001 |
| CAOD | 12 (3.2) | 18 (6.4) | 0.041 |
| PAOD | 2 (0.5) | 0 (0.0) | 0.32 |
| CVA | 5 (1.3) | 14 (5.0) | 0.006 |
| COPD | 5 (1.3) | 11 (3.9) | 0.03 |
| Renal insufficiency | 2 (0.5) | 23 (8.2) | <0.001 |
Data are presented as mean ± standard deviation or number (percentage). Catheter tip location: the distance from the carina to the catheter tip; catheter angle: the angle between the apex inserted into the port and the internal jugular vein and the catheter tip. IJV, internal jugular vein; DM, diabetes mellitus; CAOD, coronary artery occlusive disease; PAOD, peripheral arterial occlusive disease; CVA, cerebrovascular accident; COPD, chronic obstructive pulmonary disease.
Table 2
| Variables | Values (n=373) |
|---|---|
| None | 154 (41.3) |
| SCT | 219 (58.7) |
| Auto-SCT | 101 (27.1) |
| Allo-SCT | 115 (30.8) |
| Auto/allo-SCT | 3 (0.8) |
| PBSCT | 214 (57.4) |
| BMT | 5 (1.3) |
Data are presented as number (percentage). SCT, stem cell transplantation; PBSCT, peripheral blood stem cell transplantation; BMT, bone marrow transplantation.
Outcomes
Table 3 presents the results of the comparison between the two patient groups. The mean maintenance duration of the chemoport group was significantly longer than that of the Hickman group (366.30±367.48 vs. 168.33±118.76 days, P<0.001). The maintenance success rate was defined as catheter maintenance without other complications until death, maintenance after the completion of chemotherapy and discharge, or maintenance until catheter removal. The maintenance success rate was 91.8% in the chemoport group and 79.4% in the Hickman group, indicating a significant difference. Removal rate was 8.2% (n=23) in the chemoport group and 20.6% (n=77) in the Hickman group. Consequently, the reinsertion rate was significantly higher in the Hickman group than in the chemoport group (1.64±0.94 vs. 1.19±0.55, P<0.001). In this study, the rates of catheter-related infections were 6.1% (n=17) and 14.2% (n=53) in the chemoport and Hickman groups, respectively, with the infection rate in the Hickman catheter group being higher than that in the chemoport group. The two groups showed no significant differences in the occurrence of thrombosis [3 cases (0.8%) vs. 6 cases (2.1%), P=0.13] or pulmonary embolism [1 case (0.3%) vs. 2 cases (0.7%), P=0.39]. CVC occlusion occurrence was 2.5% (n=7) in the chemoport group and 2.1% (n=8) in the Hickman group, but the difference was not significant. The major reasons for CVC removal in both groups were infection, thrombosis, and malpositioning, in that order. The average catheter diameter was significantly larger in the Hickman group (10.49±1.41 vs. 6.11±0.21 Fr, P<0.001), whereas the two groups showed no significant difference in catheter angle. The catheter tip location was measured as the distance from the carina to the catheter tip, and the catheter angle was defined as the angle between the apex inserted into the port and the internal jugular vein and the catheter tip.
Table 3
| Variables | Hickman (n=373) | Chemoport (n=280) | P |
|---|---|---|---|
| Duration (days) | 168.33±118.76 | 366.30±367.48 | <0.001 |
| Reinsertion | 1.64±0.94 | 1.19±0.55 | <0.001 |
| Maintenance | 296 (79.4) | 257 (91.8) | <0.001 |
| Removal | 77 (20.6) | 23 (8.2) | <0.001 |
| Infection | 53 (14.2) | 17 (6.1) | <0.001 |
| Thrombosis | 3 (0.8) | 6 (2.1) | 0.13 |
| Occlusion | 8 (2.1) | 7 (2.5) | 0.48 |
| Malpositioning | 3 (0.8) | 1 (0.4) | 0.42 |
| Pulmonary embolism | 1 (0.3) | 2 (0.7) | 0.39 |
Data are presented as mean ± standard deviation or number (percentage).
Figure 2 shows the hazard ratios for the occurrence of infection/thrombosis based on the CVC type. The hazard ratio was 4.140 [95% confidence interval (CI): 2.305–7.436] for infection status and 0.971 (95% CI: 0.329–4.555) for thrombosis status. Figure 3 shows the CVC survival probabilities of the two groups determined using the presence of infection and thrombosis. The entire period was set to 1,000 days. The infection graph shows an increasing gap between the two groups. Both groups’ survival probability’s decreasing trend started from 200 days, but the Hickman group slope decreased more steeply. The thrombosis graph shows no significant difference between the two groups. However, the survival probability of Hickman started decreasing after 500 days. Table 4 lists the bacteria identified in each patient group. In the Hickman group, infections were confirmed in 55 patients, and 54 bacteria were identified, of which Corynebacterium striatum (n=9), Staphylococcus aureus (n=6), Enterococcus faecium (n=7) and Pseudomonas aeruginosa (n=5) were the most frequent. In the chemoport group, 13 bacterial species were identified in 18 infected patients, of which Staphylococcus epidermidis (n=3) and Candida tropicalis (n=3) were the most frequent.
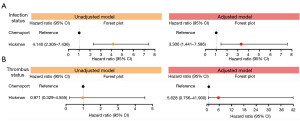

Table 4
| Type of pathogens | CVC type | |
|---|---|---|
| Hickman (n=55) | Chemoport (n=18) | |
| Not identified | 1 | 5 |
| Identified | 54 | 13 |
| Gram-positive | ||
| Staphylococcus spp. | ||
| Staphylococcus epidermidis | 3 | 3 |
| Staphylococcus aureus | 6 | 1 |
| Staphylococcus haemolyticus | 4 | 0 |
| Streptococcus spp. | ||
| Streptococcus anginosus | 1 | 0 |
| Streptococcus mitis | 2 | 0 |
| Corynebacterium striatum | 9 | 1 |
| Enterococcus faecium | 7 | 0 |
| Bacillus cereus | 1 | 0 |
| Propionibacterium acne | 1 | 0 |
| Gram-negative | ||
| Escherichia coli | 1 | 1 |
| Pseudomonas aeruginosa | 5 | 1 |
| Stenotrophomonas maltophilia | 2 | 0 |
| Enterobacter cloacea | 0 | 1 |
| Klebsiella pneumoniae | 1 | 0 |
| Serratia marcescens | 3 | 2 |
| Fungus | ||
| Candida spp. | ||
| Candida albicans | 2 | 0 |
| Candida parapsilosis | 1 | 0 |
| Candida tropicalis | 2 | 3 |
| Candida glabrata | 1 | 0 |
| Candida guilliermondii | 1 | 0 |
| Pichia kudriavzevii | 1 | 0 |
CVC, central venous catheter.
Discussion
CVC insertion can enhance vascular access and is a crucial initial step in the treatment of advanced cancer, where securing vascular access for the administration of chemotherapy drugs and multiple medications becomes increasingly difficult as chemotherapy progresses (1,2). Also, patients undergoing chemotherapy experience compromised immune function, increasing their susceptibility to infections, with catheter-related infections being the most significant threat (3). This risk is further increased in patients with hematological malignancies because of pancytopenia or neutropenic state (3,18). The CVC type is chosen based on various factors to suit the patients’ needs. These factors include the treatment duration, insertion technique, patient compliance, complications, cost, and efficacy (19). In this study, we aimed to compare two types of CVCs commonly used in patients with hematological cancer: the chemoport and the Hickman catheter, and to recommend a more secure management strategy.
Patients in the Hickman group were statistically significantly younger than those in the chemoport group. The National Health Insurance in Korea limits the age for transplantation to <70 years, which may have influenced the age factor. However, our center does not have any specific age restrictions for HSCT.
The most important criterion for the choice between these two types of catheters was whether the patient requires HSCT. The predominance of leukemia in the Hickman group could be attributed to the indication for Hickman catheter insertion, which is necessary for HSCT. In contrast, lymphoma was particularly prevalent in the chemoport group, in which chemotherapy was the primary treatment modality. The Hickman catheter is a tunneled catheter with the tip positioned in the SVC and the catheter lumen located along the chest wall (11). The Hickman catheter can be used immediately without additional needling (11). The chemoport, similar to the Hickman catheter, is a tunneled catheter with an implanted port that acts as a reservoir beneath the skin of the chest wall (11). Both types of catheters were tunneled, and we anticipated that comparing them would be straightforward.
In previous reports, the incidence of infection was reported to be 0.2 infections per 1,000 catheter days for implantable ports and 1.4–2.2 infections per 1,000 catheter days for tunneled CVCs (20-22). Consistent with these findings, the infection rate of the chemoport group in the present study was lower than that of the Hickman group. The risk factors for catheter infection have been published in many studies. Patients with hematologic malignancies are more vulnerable to infection than patients with general solid cancers owing to weakened immunity and large amounts of blood transfusions (3,23). Kim et al. reported that the risk of infection with Hickman catheters increases when immunosuppressive drugs are used after stem cell transplantation (23). Zakhour et al. also reported that patients with hematological malignancies, especially those diagnosed with leukemia, are more vulnerable to infections than those with other hematological malignancies such as lymphoma or myeloma (3). Infectious complications related to CVC occur at a frequency of 0.02–3 per 1,000 catheter days in patients with solid cancer (24) and up to 5.2 infections per 1,000 catheter days in patients with hematological malignancies (24). Heidenreich et al. reported that male gender, insertion site, induction/consolidation chemotherapy, and long-lasting neutropenia > 25 days were identified as risk factors for local infection (25). Insertion of CVC in the internal jugular vein is a major risk factor of infection compared to the insertion in the SVC (25). A hallmark study on CVC complications also reported that SCV-CVCs were associated with a lower risk of infection compared to the insertion in the internal jugular vein or femoral vein (26). Likewise, the incidence of infection was reported to be higher when CVC was inserted in the neutropenic state (27). Neutropenia is known to be a major risk factor for catheter-related complications (25). In the study, significantly shorter times to infection in patients with neutropenia on the day of CVC insertion (27) and long-lasting neutropenia during hospitalization were found to be significant risk factors for infection (25). Male gender has been reported as a risk factor owing to beard growth and shaving processes around the insertion site (26). The infection rate may vary depending on the CVC insertion method (28). Zakhour et al. reported that the incidence of infectious complications has increased with the recent increase in performing CVC insertion at bedsides or in intervention rooms other than operating rooms (3). Additionally, although not a risk factor, over-diagnosis owing to differences in the criteria for defining infection in patients with hematologic malignancy has been attributed to the reported high risk of infection (29,30). Many of the multiple risk factors were controlled in our study. The inclusion criteria began with patients with hematologic malignancy, and both groups had comparable gender ratios, slightly favoring males, with no significant difference in sex ratio. As this was a study conducted at a single institution, procedures were performed in the operating room with the same insertion method and routine management. However, in the case of leukemia, requiring stem cell transplantation was an absolute indication for the Hickman catheter; hence, patients with leukemia patients vulnerable to infection inevitably predominated in the Hickman group. Consequently, the infection risk of the Hickman group was confirmed to be higher than that of the chemoport group, and thus the catheter removal rate would have been higher, and the maintenance duration would have been shorter.
In Table 4, the causative bacteria identified in these cases were predominantly skin commensals. These bacteria adhere well to host proteins and are particularly resistant to silicone materials (31). Therefore, Hickman catheters, which have a silicone tube directly exposed to the chest wall, are believed to be more susceptible to infection. Post-HSCT, when the gut microbiota translocates into the bloodstream owing to gut barrier dysfunction, these bacteria can circulate through the blood, form biofilms on the surface of CVC, and then re-enter systemic circulation by the catheter, potentially leading to sepsis and multiple organ failure. Several previous studies have identified gut microbiota translocation as a major route for bloodstream infections, suggesting a deep connection with the increased occurrence of gut microbiota and Candida infections observed in the Hickman catheter group in our study.
The risk of infection with chemoport insertion is relatively low, necessitating a reduced frequency of heparin flushing and direct contact avoidance (11). However, the chemoport, like the Hickman catheter, requires needle access for administration only when needed (11). After infection, thrombosis was the next most significant catheter-related complication in the present study. No significant differences in thrombosis rates were observed between the two types of CVCs. In the thrombosis Kaplan-Meier graph, the survival probability of the two groups did not differ significantly until 500 days. Thereafter, there was a tendency for a decrease in the Hickman group, but it was not statistically significant. For thrombosis, varying study parameters and the scarcity of comparative research between the two CVC types have made comparisons between the groups difficult. The incidence of thrombosis varies widely across CVC types, with ranges such as 0.3–66% (15,32-34). More recent studies have reported rates ranging from 4–8% (34-36). A study showed that the larger the diameter of the catheter, the higher the rate of thrombosis, as the catheter is in the central area where blood flow is fastest (37). Contrastingly, despite the larger average catheter size of the Hickman catheter in comparison with the chemoport, the two groups showed no difference in the occurrence of thrombosis owing to this size disparity. There is a paucity of evidence-based treatments for catheter-related thrombosis, and recommendations are based on extrapolation from lower extremity deep venous thrombosis and small non-randomized controlled studies (38). Prevention of catheter-related thrombosis is considered better than treatment. The thrombosis incidence can be reduced by controlling several risk factors such as location of insertion and type of CVC (10). The International Society of Thrombosis and Hemostasis guidelines recommend that CVCs should be inserted on the right side, in the jugular vein, with the tip located at the junction of the SVC and the right atrium (38,39). A catheter that is too short increases the risk of thrombosis; therefore, proper insertion technique and confirmation of catheter tip placement are important (11). In this study, both groups had similar catheter location and angle outcomes. The use of prophylactic anticoagulants remains a topic of ongoing debate, with recent studies reporting negative findings regarding their efficacy (2,40-43). Malposition is another complication that occurs frequently after infection and thrombosis (2,11). Malposition of the catheter tip can cause difficulties with blood withdrawal and contribute to catheter occlusion (11). Although research on malposition is limited, Babu et al. reported that it occurred in 10.19% of the patients in their study (1). In this study, we observed occurrence rates of 0.4% (n=1) in the chemoport group and 0.8% (n=3) in the Hickman group, with no significant difference between the two groups.
Maintenance duration ultimately depends on the infection. In the Kaplan-Meier graph in Figure 3, the survival probability of the Hickman group decreases rapidly from around 200 days in the case of infection and from 500 days in the case of thrombosis. The reason for the shorter maintenance period with Hickman CVC may also be because it is often removed early during recovery from stem cell transplantation or upon discharge (3). In the case of chemoport, since it is often used for long-term chemotherapy; hence, the period of use itself is longer than that of Hickman.
In our study, both types of CVCs showed lower incidence rates of infection and thrombotic complications than those described in other reports. The reasons for this lower rate are unclear, but improvement in catheter materials, better insertion practices, and better catheter maintenance may be contributory (11). No complications, such as bleeding, pneumothorax, or hemothorax, occurred; this was attributed to the safety of performing all surgeries under ultrasound guidance, as highlighted by Babu et al. and Rockholt et al. (1,44). In addition to ultrasound guidance, the use of vascular imaging immediately before procedure completion allowed for immediate adjustment or reinsertion of the catheters. Regarding other possible reasons for these outcomes, only a speculative hypothesis can be made, including the presence of asymptomatic thrombosis not accounted for in the data and specialized management of CVCs by trained nursing staff. However, the important point is that routine management by medical professionals reduces infection, and accurate CVC insertion/tip location through vascular imaging reduces thrombosis and malposition.
This study had some limitations. First, as this was a single-center study, the number of enrolled patients was limited. Second, this was a retrospective review of data spanning 9 years, which may have led to unavoidable data loss or inaccuracies. Although the inclusion of all patients diagnosed with hematological malignancies was a strength of the study, the differences in the predominant disease types and underlying diseases, such as cerebrovascular accident and coronary artery occlusive disease, between the two groups may have been a limitation. Another potential limitation is the variability in management practices, as the care provided by specialized nurses during hospitalization may differ from that provided after discharge. Finally, the inclusion of patients requiring reinsertion in the same patient should be considered.
Conclusions
We found that chemoport-type CVCs can safely and effectively minimize the development of long-term infections in immunocompromised patients with hematological malignancies. Furthermore, the reinsertion rate was statistically higher in the Hickman catheter group, and the average reinsertion rate of both groups was confirmed to be more than 1. As is known, replacing the Hickman catheter for HSCT remains a challenge. Therefore, performing the stem cell transplantation procedure using Hickman CVC and then changing to chemoport for subsequent chemotherapy can minimize infection risk and safely preserve long-term vascular access. Additionally, the overall complication rate can be reduced through accurate CVC insertion methods using vascular imaging and routine management by medical professionals.
Acknowledgments
We would like to thank Editage (www.editage.co.kr) for English language editing.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://tcr.amegroups.com/article/view/10.21037/tcr-24-2147/rc
Data Sharing Statement: Available at https://tcr.amegroups.com/article/view/10.21037/tcr-24-2147/dss
Peer Review File: Available at https://tcr.amegroups.com/article/view/10.21037/tcr-24-2147/prf
Funding: This study was supported by
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tcr.amegroups.com/article/view/10.21037/tcr-24-2147/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study was approved by the Institutional Review Board of the Pusan National University Hospital (No. 2406-018-140). Prior to all procedures, the potential risks and benefits were explained in detail to the patients, and written informed consent was obtained.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Babu KG, Suresh Babu MC, Lokanatha D, et al. Outcomes, cost comparison, and patient satisfaction during long-term central venous access in cancer patients: Experience from a Tertiary Care Cancer Institute in South India. Indian J Med Paediatr Oncol 2016;37:232-8. [Crossref] [PubMed]
- Kim HJ, Yun J, Kim HJ, et al. Safety and effectiveness of central venous catheterization in patients with cancer: prospective observational study. J Korean Med Sci 2010;25:1748-53. [Crossref] [PubMed]
- Zakhour R, Chaftari AM, Raad II. Catheter-related infections in patients with haematological malignancies: novel preventive and therapeutic strategies. Lancet Infect Dis 2016;16:e241-50. [Crossref] [PubMed]
- Cameron GS. Central venous catheters for children with malignant disease: surgical issues. J Pediatr Surg 1987;22:702-4. [Crossref] [PubMed]
- Iannacci L, Piomelli S. Supportive care for children with cancer. Guidelines of the Childrens Cancer Study Group. Use of venous access lines. Am J Pediatr Hematol Oncol 1984;6:277-81. [Crossref] [PubMed]
- Mansfield PF, Hohn DC, Fornage BD, et al. Complications and failures of subclavian-vein catheterization. N Engl J Med 1994;331:1735-8. [Crossref] [PubMed]
- McGee DC, Gould MK. Preventing complications of central venous catheterization. N Engl J Med 2003;348:1123-33. [Crossref] [PubMed]
- Vescia S, Baumgärtner AK, Jacobs VR, et al. Management of venous port systems in oncology: a review of current evidence. Ann Oncol 2008;19:9-15. [Crossref] [PubMed]
- Gallieni M, Pittiruti M, Biffi R. Vascular access in oncology patients. CA Cancer J Clin 2008;58:323-46. [Crossref] [PubMed]
- Wall C, Moore J, Thachil J. Catheter-related thrombosis: A practical approach. J Intensive Care Soc 2016;17:160-7. [Crossref] [PubMed]
- Schiffer CA, Mangu PB, Wade JC, et al. Central venous catheter care for the patient with cancer: American Society of Clinical Oncology clinical practice guideline. J Clin Oncol 2013;31:1357-70. [Crossref] [PubMed]
- Kim JT, Oh TY. Clinical review of totally implantable venous catheter. J Chest Surg 2007;40:691-5.
- Cho SG, Kim SH, Song HH, et al. Radiologic placement of subcutaneous infusion ports in cancer patients: analysis of 45 cases. J Korean Cancer Assoc 2000;32:1115-21.
- Centers for Disease Control and Prevention. Centers for Disease Control and Prevention [Internet]. Centers for Disease Control and Prevention. U.S. Department of Health & Human Services; 2024. Available online: https://www.cdc.gov/
- Kuter DJ. Thrombotic complications of central venous catheters in cancer patients. Oncologist 2004;9:207-16. [Crossref] [PubMed]
- Baskin JL, Pui CH, Reiss U, et al. Management of occlusion and thrombosis associated with long-term indwelling central venous catheters. Lancet 2009;374:159-69. [Crossref] [PubMed]
- Kucher N. Clinical practice. Deep-vein thrombosis of the upper extremities. N Engl J Med 2011;364:861-9. [Crossref] [PubMed]
- Romano V, Castagnola E, Dallorso S, et al. Bloodstream infections can develop late (after day 100) and/or in the absence of neutropenia in children receiving allogeneic bone marrow transplantation. Bone Marrow Transplant 1999;23:271-5. [Crossref] [PubMed]
- Jain SA, Shukla SN, Talati SS, et al. A retrospective study of central venous catheters GCRI experience. Indian J Med Paediatr Oncol 2013;34:238-41. [Crossref] [PubMed]
- Bouza E, Burillo A, Muñoz P. Catheter-related infections: diagnosis and intravascular treatment. Clin Microbiol Infect 2002;8:265-74. [Crossref] [PubMed]
- Safdar N, Maki DG. Use of vancomycin-containing lock or flush solutions for prevention of bloodstream infection associated with central venous access devices: a meta-analysis of prospective, randomized trials. Clin Infect Dis 2006;43:474-84. [Crossref] [PubMed]
- Maki DG, Kluger DM, Crnich CJ. The risk of bloodstream infection in adults with different intravascular devices: a systematic review of 200 published prospective studies. Mayo Clin Proc 2006;81:1159-71. [Crossref] [PubMed]
- Kim DH, Bae NY, Sung WJ, et al. Hickman catheter site infections after allogeneic stem cell transplantation: single-center experience. Transplant Proc 2004;36:3203-7. [Crossref] [PubMed]
- Cortelezzia A, Fracchiolla NS, Maisonneuve P, et al. Central venous catheter-related complications in patients with hematological malignancies: a retrospective analysis of risk factors and prophylactic measures. Leuk Lymphoma 2003;44:1495-501. [Crossref] [PubMed]
- Heidenreich D, Hansen E, Kreil S, et al. The insertion site is the main risk factor for central venous catheter-related complications in patients with hematologic malignancies. Am J Hematol 2022;97:303-10. [Crossref] [PubMed]
- Parienti JJ, Mongardon N, Mégarbane B, et al. Intravascular Complications of Central Venous Catheterization by Insertion Site. N Engl J Med 2015;373:1220-9. [Crossref] [PubMed]
- Tölle D, Hentrich M, Pelzer BW, et al. Impact of neutropenia on central venous catheter-related bloodstream infections in patients with hematological malignancies at the time of central venous catheter insertion: A matched-pair analysis. Infect Control Hosp Epidemiol 2019;40:1204-6. [Crossref] [PubMed]
- Skaff ER, Doucette S, McDiarmid S, et al. Vascular access devices in leukemia: a retrospective review amongst patients treated at the Ottawa Hospital with induction chemotherapy for acute leukemia. Leuk Lymphoma 2012;53:1090-5. [Crossref] [PubMed]
- Steinberg JP, Coffin SE. Improving the central line-associated bloodstream infection surveillance definition: a work in progress. Infect Control Hosp Epidemiol 2013;34:777-9. [Crossref] [PubMed]
- See I, Iwamoto M, Allen-Bridson K, et al. Mucosal barrier injury laboratory-confirmed bloodstream infection: results from a field test of a new National Healthcare Safety Network definition. Infect Control Hosp Epidemiol 2013;34:769-76. [Crossref] [PubMed]
- Kim GM, Song S, Kim DY, et al. Impact of insertion into the left internal jugular vein in chemoport-associated infections: a retrospective single-center study of 1690 cases. Sci Rep 2024;14:8925. [Crossref] [PubMed]
- Verso M, Agnelli G. Venous thromboembolism associated with long-term use of central venous catheters in cancer patients. J Clin Oncol 2003;21:3665-75. [Crossref] [PubMed]
- Akl EA, Kamath G, Yosuico V, et al. Thromboprophylaxis for patients with cancer and central venous catheters: a systematic review and a meta-analysis. Cancer 2008;112:2483-92. [Crossref] [PubMed]
- Lee AY, Levine MN, Butler G, et al. Incidence, risk factors, and outcomes of catheter-related thrombosis in adult patients with cancer. J Clin Oncol 2006;24:1404-8. [Crossref] [PubMed]
- Akl EA, Rohilla S, Barba M, et al. Anticoagulation for the initial treatment of venous thromboembolism in patients with cancer: a systematic review. Cancer 2008;113:1685-94. [Crossref] [PubMed]
- Linenberger ML. Catheter-related thrombosis: risks, diagnosis, and management. J Natl Compr Canc Netw 2006;4:889-901. [Crossref] [PubMed]
- Sharp R, Cummings M, Fielder A, et al. The catheter to vein ratio and rates of symptomatic venous thromboembolism in patients with a peripherally inserted central catheter (PICC): a prospective cohort study. Int J Nurs Stud 2015;52:677-85. [Crossref] [PubMed]
- Debourdeau P, Farge D, Beckers M, et al. International clinical practice guidelines for the treatment and prophylaxis of thrombosis associated with central venous catheters in patients with cancer. J Thromb Haemost 2013;11:71-80. [Crossref] [PubMed]
- Fletcher SJ, Bodenham AR. Safe placement of central venous catheters: where should the tip of the catheter lie? Br J Anaesth 2000;85:188-91. [PubMed]
- Fallouh N, McGuirk HM, Flanders SA, et al. Peripherally Inserted Central Catheter-associated Deep Vein Thrombosis: A Narrative Review. Am J Med 2015;128:722-38. [Crossref] [PubMed]
- Debourdeau P, Elalamy I, de Raignac A, et al. Long-term use of daily subcutaneous low molecular weight heparin in cancer patients with venous thromboembolism: why hesitate any longer? Support Care Cancer 2008;16:1333-41. [Crossref] [PubMed]
- Bishop L, Dougherty L, Bodenham A, et al. Guidelines on the insertion and management of central venous access devices in adults. Int J Lab Hematol 2007;29:261-78. [Crossref] [PubMed]
- Kahn SR, Lim W, Dunn AS, et al. Prevention of VTE in nonsurgical patients: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 2012;141:e195S-226S.
- Rockholt MM, Thorarinsdottir HR, Lazarevic V, et al. Central venous catheter-related complications in hematologic patients: An observational study. Acta Anaesthesiol Scand 2022;66:473-82. [Crossref] [PubMed]



