
Development and validation of survival prediction nomograms for patients with early-stage rectal cancer: a population-based study
Highlight box
Key findings
• The developed nomograms are capable to predict overall survival (OS) and cancer-specific survival (CSS) of early-stage rectal cancer (ESRC) patients. Radiation and chemotherapy showed no benefit, while low-risk patients, especially younger individuals, may benefit from local resection.
What is known and what is new?
• The incidence of ESRC is increasing with few relative prognostic studies.
• Based on a large population-level data, and we developed well-performing nomograms to predict OS and CSS after dividing patients into two risk groups. Then we assessed the potential benefits of various therapies across subgroups after propensity score-matching.
What is the implication, and what should change now?
• Identifying high-risk patients with ESRC and make targeted medical management may improve outcomes of this population.
Introduction
The colorectal cancer (CRC) is the second leading cause of cancer-related mortality and the third most prevalent cancer worldwide. In 2022, it accounted for over 1.8 million new diagnoses and 881,899 deaths (1). Advances in imaging technology have led to a marked rise in the detection of early-stage rectal cancer (ESRC) in recent decades, including tumors classified as T1–2N0M0 (2). Although 5-year survival rates for ESRC have shown improvement, there has been a marked increase in ESRC incidence, particularly in individuals younger than 50 years (3). For patients with ESRC, radical resection is recognized as the gold standard for treatment option, significantly reducing the risk of local recurrence and metastasis (4). However, advancements in endoscopic techniques have led to widespread recognition of local resection via endoscopy (5). Therefore, radical resection may not be the optimal treatment for patients with ESRC. Radical resection may lead to loss of organ function, surgical complications, and short-term low anterior resection syndrome (LARS) in the rectum. Furthermore, 75% of patients reported different degrees of gastrointestinal and urinary dysfunction after the procedure (6). One study indicated that adjuvant chemoradiotherapy in conjunction with local excision effectively eliminated occult metastatic lymph nodes in the rectal mesentery and residual cancer cells in the rectal wall, thereby significantly reducing the risk of tumor recurrence in T1 stage rectal cancer (7). A meta-analysis revealed that neoadjuvant chemotherapy offered several advantages for locally advanced rectal cancer (LARC) patients, including the eradication of occult micrometastases, reduced ostomy restoration time, improved treatment adherence, a significant increase in the pathological complete response (pCR) rate and enhanced R0 resection and tumor downstaging rates (8). Local resection alone may serve as an effective treatment option for patients with ESRC who lack poor prognostic pathology (9). However, it is crucial to recognize that the risk of lymph node metastasis following simple local excision can range from 12% to 28% in patients with intermediate-stage disease (10). Trans-abdominal resection is generally recommended for T2N0M0 rectal cancer, as local excision alone has been associated with local recurrence rates ranging from 11% to 45% in patients with T2 lesions (11). Chemoradiotherapy is regarded as the standard treatment for LARC, having demonstrated a reduction in disease recurrence and an improvement in overall survival (OS) outcomes (12). In addition, a study underscored the significant disease burden of ESRC, which exhibited distinct clinicopathological and molecular features compared to late-onset cases, including differences in tumor differentiation, tumor-infiltrating lymphocytes, and molecular phenotypes (13). The treatment strategies and outcomes also differ between ESRC and the late-onset rectal cancers (14). Nonetheless, the prognostic implications and effects of additional chemoradiotherapy in patients with ESRC continue to be debated (11,15). Moreover, population-based studies on prognosis and treatment options for patients with ESCC are limited, highlighting the significance to evaluate the function of chemoradiotherapy in this cohort.
This study utilized data from the Surveillance, Epidemiology, and End Results (SEER) database to identify prognostic factors and develop nomograms for predicting OS and cancer-specific survival (CSS) in patients with ESRC. Furthermore, it aimed to evaluate treatment strategies across subgroups to identify the most effective therapeutic approaches for this population. We present this article in accordance with the TRIPOD reporting checklist (available at https://tcr.amegroups.com/article/view/10.21037/tcr-24-1888/rc).
Methods
Database source and patients selection
In the retrospective cohort study, clinicopathological data from ESRC patients in the SEER database was analyzed from January 1, 2010 to December 31, 2017 by use of SEER*Stat 8.4.0.1 software. Data information collected included age, race, tumor grade, sex, histological grade, the American Joint Committee on Cancer (AJCC) 7th stages (T, N, M), tumor size, regional nodes examined, treatment sequence (surgery before, after, or without chemoradiotherapy), preoperative carcinoembryonic antigen (CEA) levels, perineural invasion, and radiotherapy and chemotherapy information. The inclusion criteria were defined: (I) individuals with rectal cancer as their sole primary malignancy; (II) those diagnosed with T1–2N0M0 stage rectal cancer; and (III) patients who underwent surgical intervention with subsequent complete pathological examination. Exclusion criteria were as follows: (I) patients presenting with multiple primary tumors; (II) cases identified solely through autopsy or death certificate reports; (III) individuals with incomplete data on any of the inclusion parameters; (IV) patients aged under 18 years; and (V) those with zero survival months. The final cohort comprised 2,501 patients, stratified into a training set of 1,750 and a test set of 751 for validation of predictive nomograms (Figure 1). The primary outcomes of interest were OS and CSS, with OS defined as the interval from diagnosis to the date of death from any cause or last follow-up, and CSS as the period from diagnosis to death attributed to cancer or last follow-up. This study utilized publicly available data from the SEER database, with all patient data de-identified prior to collection, thus exempting it from ethics committee approval or informed consent. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013).
Propensity score-matching (PSM)
To reduce bias and confounding factors in subgroups among ESRC patients, we employed PSM. The method accounted for age, sex, race, T stage, surgical options, tumor grade, tumor size, CEA level, perineural invasion, and chemoradiotherapy. We used the logistic regression to get propensity scores. Subgroups of ESRC patients were matched in a 1:1 ratio after PSM. We compared clinicopathological variables before and after PSM by use of the χ2 test to evaluate the effectiveness of the matching method.
Statistics analysis
This study employed the Mann-Whitney U test to assess disparities in continuous variables, with categorical variables subjected to chi-squared analysis. Univariate and multivariate Cox regression analyses were performed to calculate odds ratios (ORs) and 95% confidence intervals (CIs). Prognostic variables identified as significant (P<0.05) from the multivariate Cox regression were integrated into a nomogram, facilitating a graphical representation for the estimation of 1-, 3-, and 5-year survival probabilities.
Hazard ratios (HRs) along with their 95% CIs were reported for all significant findings. The optimal threshold for tumor size was determined utilizing X-tile software (version 3.6.1, Yale University). Model performance was evaluated with the concordance index (C-index) and receiver operating characteristic (ROC) curves, with the area under the curve (AUC) being calculated. Calibration plots were constructed to compare predicted versus actual 1-, 3-, and 5-year survival outcomes. The clinical applicability of the predictive model was appraised through decision curve analysis (DCA). The study population was stratified into high- and low-risk categories on the basis of their total nomogram scores. Survival disparities, as measured by OS and CSS, were compared across subgroups using the Kaplan-Meier method complemented by the log-rank test. Statistical analysis was performed using the Statistical Package for the Social Sciences (SPSS) 22.0 (International Business Machines Corporation, Armonk, NY, USA) and R version 4.2.0.
Results
Basic characteristics of the patients
Our study comprised a total of 2,501 patients diagnosed with ESRC, with 1,750 patients designated to the training set and 751 to the validation set. The demographic and clinical characteristics of patients with ESRC in both the training and validation cohorts for OS were detailed in Table 1. The median age of the patient cohort was 63 years, with a median survival duration of 59 months. Notably, nearly half of the patients (43.14%) were younger than 60 years, and approximately one-fifth (21.99%) underwent local resection. Chemotherapy was administered to 575 patients (22.99%), while 576 patients (23.03%) received radiotherapy. Additionally, 393 patients (17.24%) underwent neoadjuvant therapy prior to surgery, and 151 patients (6.63%) received adjuvant therapy post-surgery. Statistical analyses, including the Mann-Whitney U and Chi-squared tests, revealed no significant differences in feature distributions between the training and validation sets. The demographic and clinical characteristics for CSS in both cohorts yielded comparable results (see Table S1).
Table 1
| Characteristics | Total (N=2,501) | Training set (N=1,750) | Test set (N=751) | P value |
|---|---|---|---|---|
| Age | 0.08 | |||
| <60 years | 1,079 (43.14) | 735 (42.00) | 344 (45.81) | |
| ≥60 years | 1,422 (56.86) | 1,015 (58.00) | 407 (54.19) | |
| Sex | 0.10 | |||
| Female | 1,064 (42.54) | 726 (41.49) | 338 (45.01) | |
| Male | 1,437 (57.46) | 1,024 (58.51) | 413 (54.99) | |
| Race | 0.81 | |||
| White | 2,041 (81.61) | 1,426 (81.49) | 615 (81.89) | |
| Others | 460 (18.39) | 324 (18.51) | 136 (18.11) | |
| Grade | 0.67 | |||
| I/II | 2,300 (91.96) | 1,612 (92.11) | 688 (91.61) | |
| III/IV | 201 (8.04) | 138 (7.89) | 63 (8.39) | |
| Histology | 0.29 | |||
| Adenocarcinoma | 2,442 (97.64) | 1,705 (97.43) | 737 (98.14) | |
| Others | 59 (2.36) | 45 (2.57) | 14 (1.86) | |
| T stage | 0.37 | |||
| T1 | 1,171 (46.82) | 809 (46.23) | 362 (48.20) | |
| T2 | 1,330 (53.18) | 941 (53.77) | 389 (51.80) | |
| Surgery options | 0.14 | |||
| Local resection | 550 (21.99) | 399 (22.80) | 151 (20.11) | |
| Radical resection | 1,951 (78.01) | 1,351 (77.20) | 600 (79.89) | |
| Radiation | 0.92 | |||
| No | 1,925 (76.97) | 1,346 (76.91) | 579 (77.10) | |
| Yes | 576 (23.03) | 404 (23.09) | 172 (22.90) | |
| Chemotherapy | 0.97 | |||
| No | 1,926 (77.01) | 1,348 (77.03) | 578 (76.96) | |
| Yes | 575 (22.99) | 402 (22.97) | 173 (23.04) | |
| Treatment sequence | 0.88 | |||
| Only surgery | 1,925 (76.97) | 1,347 (76.97) | 578 (76.96) | |
| Adjuvant therapy | 165 (6.60) | 118 (6.74) | 47 (6.26) | |
| Neoadjuvant therapy | 411 (16.43) | 285 (16.29) | 126 (16.78) | |
| CEA | 0.89 | |||
| Negative | 1,985 (79.37) | 1,387 (79.26) | 598 (79.63) | |
| Positive | 516 (20.63) | 363 (20.74) | 153 (20.37) | |
| Perineural invasion | 0.68 | |||
| No | 2,443 (97.68) | 1,708 (97.60) | 735 (97.87) | |
| Yes | 58 (2.32) | 42 (2.40) | 16 (2.13) | |
| Tumor size | 0.85 | |||
| <18 mm | 889 (35.55) | 620 (35.43) | 269 (35.82) | |
| ≥18 mm | 1,612 (64.45) | 1,130 (64.57) | 482 (64.18) |
Data are presented as n (%). CEA, carcinoembryonic antigen.
Prognostic factors for OS and CSS
To identify prognostic indicators for OS, we performed univariate and multivariate Cox regression analyses on the training set (Table 2). These analyses identified several risk factors associated with OS, including age at diagnosis, T stage, tumor grade, CEA level, and surgical options. Notably, T2 stage (HR: 1.23; 95% CI: 0.94–1.63), poor tumor grade (HR: 1.81; 95% CI: 1.25–2.62), older age (HR: 2.55; 95% CI: 1.93–3.36), elevated CEA levels (HR: 1.80; 95% CI: 1.40–2.32), and larger tumor size (HR: 1.41; 95% CI: 1.08–1.84) were all linked to worse OS outcomes. In contrast, radical resection (HR: 0.64; 95% CI: 0.48–0.85) was associated with improved OS prognosis.
Table 2
| Characteristics | Univariate analysis | Multivariate analysis | |||
|---|---|---|---|---|---|
| Hazard ratio (95% CI) | P value | Hazard ratio (95% CI) | P value | ||
| Age (years) | <0.001* | <0.001* | |||
| <60 | Ref | Ref | |||
| ≥60 | 2.72 (2.07–3.57) | 2.55 (1.93–3.36) | |||
| Sex | 0.82 | ||||
| Female | Ref | ||||
| Male | 0.97 (0.77–1.23) | ||||
| Race | 0.07 | ||||
| Others | Ref | ||||
| White | 1.37 (0.98–1.90) | ||||
| Grade | 0.002* | <0.001* | |||
| I/II | Ref | Ref | |||
| III/IV | 1.77 (1.23–2.54) | 1.81 (1.25–2.62) | |||
| Histology | 0.01* | 0.21 | |||
| Others | Ref | Ref | |||
| Adenocarcinoma | 0.50 (0.28–0.86) | 0.70 (0.39–1.23) | |||
| T stage | 0.02* | 0.13 | |||
| T1 | Ref | Ref | |||
| T2 | 1.34 (1.05–1.70) | 1.23 (0.94–1.63) | |||
| Surgery options | 0.005* | 0.003* | |||
| Local resection | Ref | Ref | |||
| Radical resection | 0.69 (0.53–0.90) | 0.64 (0.48–0.85) | |||
| Radiation | 0.14 | ||||
| No | Ref | Ref | 0.17 | ||
| Yes | 1.22 (0.94–1.58) | 1.57 (0.82–2.99) | |||
| Chemotherapy | 0.37 | ||||
| No | Ref | Ref | |||
| Yes | 1.13 (0.87–1.48) | 0.66 (0.34–1.27) | |||
| CEA | <0.001* | <0.001* | |||
| Negative | Ref | Ref | |||
| Positive | 1.86 (1.45–2.39) | 1.80 (1.40–2.32) | |||
| Perineural invasion | 0.69 | ||||
| No | Ref | Ref | |||
| Yes | 1.17 (0.55–2.47) | 1.11 (0.52–2.36) | |||
| Tumor size (mm) | 0.03* | 0.01* | |||
| <18 | Ref | Ref | |||
| ≥18 | 1.32 (1.02–1.71) | 1.41 (1.08–1.84) | |||
*, statistically significant. CEA, carcinoembryonic antigen; CI, confidence interval.
Additionally, the risk factors for CSS mirrored those for OS (Table S2). Poor tumor grade (HR: 1.90; 95% CI: 1.13–3.18), older age (HR: 1.77; 95% CI: 1.20–2.61), and positive CEA levels (HR: 1.59; 95% CI: 1.07–2.36) were similarly correlated with inferior CSS outcomes, while radical resection (HR: 0.61; 95% CI: 0.39–0.97) was linked to improved CSS prognosis.
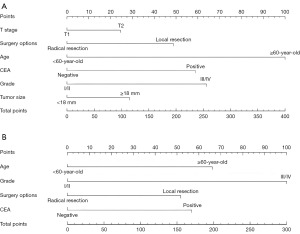
Development and validation of prognostic nomograms
Utilizing the multivariate analysis results from the training cohort, we constructed nomograms to predict OS and CSS in ESRC patients (Figure 2). Each variable was assigned a score ranging from 0 to 100, reflecting its contribution to model accuracy. By aggregating these scores for each patient, we calculated a total point value to estimate the probabilities of 1-, 3-, and 5-year OS and CSS. Importantly, higher scores were associated with poorer prognoses. In the training set, the nomogram for OS exhibited a C-index of 0.69 (95% CI: 0.64–0.74), surpassing the 7th edition of the AJCC staging system, which recorded a C-index of 0.60 (95% CI: 0.54–0.65). And validation in an independent testing set yielded a C-index of 0.65 (95% CI: 0.62–0.68). For CSS, the C-index was 0.68 (95% CI: 0.61–0.76) in the training set and 0.64 (95% CI: 0.59–0.68) in the validation set, both surpassing the 7th AJCC staging system.
The ROC analysis revealed AUC values of 0.70, 0.70, and 0.67 in the training set for 1-, 3-, and 5-year OS, respectively. In the validation set, the AUC values for 1-, 3-, and 5-year were 0.67, 0.64, and 0.62, respectively (Figure S1A-S1C). For CSS, ROC analysis also yielded promising results, with AUCs of 0.72, 0.71, and 0.63 for 1-, 3-, and 5-year survival in the training set, and 0.63, 0.69, and 0.62 in the validation set (Figure S1D-S1F). In the training set and validation set for both OS and CSS, calibration plots demonstrated strong concordance between predicted and observed outcomes (Figure S2). Furthermore, both in the training sets and validation sets, the DCA curves of OS and CSS illustrated enhanced clinical utility relative to the 7th AJCC staging system (Figure S3).
The effects of different treatment therapy in various subgroups
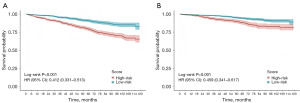
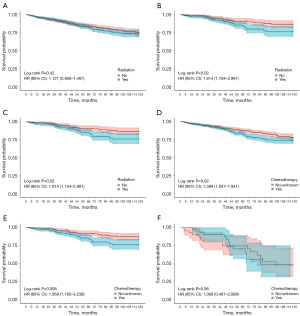
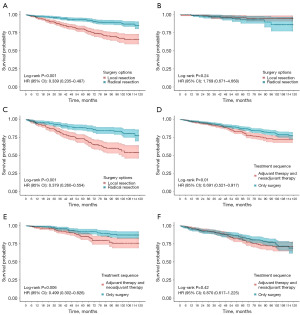
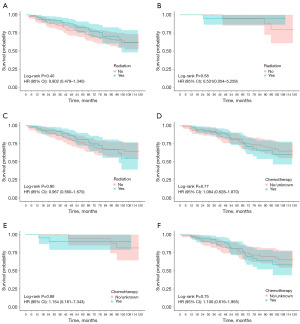
Patients were divided into two subgroups according to their prediction model scores: the high-risk group (≥128 points) and the low-risk group (<128 points) in the OS nomogram, as well as the low-risk group (<66 points) and the high-risk group (≥66 points) in the CSS nomogram. Kaplan-Meier survival curves revealed significant differences in both OS and CSS between these groups (Figure 3). Following PSM, we assessed various treatment options across subgroups. Notably, radiation and chemotherapy provided no survival benefits for ESRC patients in any subgroup and were associated with worse OS and CSS in the low-risk group (Figure 4, Figure S4). Additionally, local resection did not yield significant survival differences compared to radical resection in the low-risk group, while poorer OS and CSS were observed in the high-risk group (Figure 5, Figure S5). In subgroups undergoing local resection, neither radiotherapy nor chemotherapy enhanced OS or CSS outcomes (Figure 6, Figure S6). Importantly, the subset of patients potentially benefiting from local resection appeared limited to younger individuals (Figure S7). Furthermore, the age subgroup analysis was influenced by biases inherent in the retrospective study, as all elderly patients were classified into the high-risk group.
Discussion
Radical resection remains the cornerstone treatment for ESRC and is widely endorsed in clinical practice (11,14). However, this conventional approach carries considerable risks, including stoma formation, anastomotic leaks, genitourinary dysfunction, fecal incontinence, and sexual dysfunction (16). Such complications significantly impair patients’ quality of life post-resection. With the advent of advanced endoscopic techniques, local resection has emerged as a promising alternative for patients with ESRC (17). Furthermore, studies on prognostic outcomes in ESRC are limited, and comprehensive analyses of treatment options across various subgroups remain scarce (18,19).
In this study, we examined prognostic factors and devised predictive nomograms for OS and CSS in ESRC patients. Our analysis of 2,501 ESRC patients from the SEER database corroborated previous findings, identifying T stage, poor tumor grade, age, tumor size, elevated CEA levels, and surgical options as independent prognostic factors for OS and CSS. In rectal cancer, advanced T stage and lower tumor grade were well-established independent risk factors that significantly impact tumor survival, as evidenced by numerous studies (20,21). Likewise, elevated CEA level has been identified as critical to survival prognosis in patients with rectal cancer (22). Additionally, a recent study suggested that tumor size was associated with early metastasis and poor prognosis (23). What’s more, we identified local resection as an independent risk factor for OS and CSS in ESRC patients, consistent with findings from previous studies (24,25). However, a study has found that local resection was safe and beneficial for selected patients (26). One possible reason is that neoadjuvant chemoradiotherapy significantly improves survival outcomes in LARC but not in ESRC (27).
The predictive nomogram effectively stratified patients into low-risk and high-risk groups, with Kaplan-Meier analysis revealing significant disparities in OS and CSS between these cohorts. Following PSM in the training group, neither chemotherapy nor radiotherapy conferred survival benefits for ESRC patients, particularly in the low-risk group, where both treatments correlated with poorer OS and CSS. While radiotherapy is integral to the management of CRC, its efficacy is often limited by low tumor radiosensitivity and toxicity to adjacent normal tissues (28). In line with previous studies, our results showed that radiotherapy provided no benefit for ESRC patients across all subgroups. However, a study suggested that ESRC patients who underwent local resection may benefit from (neo) adjuvant therapy due to the risk of lymph node metastasis (29). Contrarily, our results indicated that radiotherapy did not enhance OS or CSS in those receiving local resection, possibly due to the low incidence of perineural invasion and the favorable tumor grades in the cohort. Chemoradiotherapy is the standard of care for LARC in conjunction with radical resection (11). Recently, the benefits of chemotherapy have been observed in ESRC (28,30). Yet, our analysis revealed no significant improvement in OS or CSS for any subgroup receiving chemotherapy. This aligns with recent studies indicating that adjuvant chemotherapy does not confer substantial advantages in early-stage rectal adenocarcinoma (31). A possible reason is that ESRC has low rates of recurrence and distant metastasis (19). Previous research has indicated that radical resection yields better survival outcomes than local resection (29). Our study found no significant differences in OS or CSS within the low-risk group, particularly among younger patients. This may be attributed to the reduced rates of recurrence and metastasis in our selected low-risk cohort. Moreover, the limited sample size of patients undergoing local resection and neoadjuvant therapy underscores the critical need for prospective clinical trials with large sample to validate these findings.
Otherwise, there are a growing number of studies showing the positive effect of neoadjuvant radiotherapy and chemotherapy in rectal cancer, especially in locally advanced disease. Spatola et al. conducted a study of 784 patients with rectal cancer at 13 research centers, and found that neoadjuvant therapy was feasible for most patients in the comprehensive treatment of rectal cancer, which can increase the probability of undergoing sphincter preservation surgery and reduce the toxicity during treatment significantly (32). In addition, Lo et al. found that neoadjuvant therapy based on integrated intensified treatment was proved to be feasible and well-tolerated in patients with LARC, showing high pCR rates (22.2%) and disease-free survival at a median follow-up of 30 months (33). In contrast, studies related to ESRC have been few and mixed. It is undeniable that our results have been elaborated from a perspective of the predictive model. Therefore, more large-scale prospective studies are needed to confirm our conclusions in the environment using multidisciplinary assessment.
A comprehensive understanding of OS and CSS is crucial for alleviating anxiety and enhancing the quality of life in patients, particularly those with initially poor prognoses. The development of OS and CSS nomograms enables clinicians to evaluate mortality risk and design suitable follow-up and monitoring strategies. This methodology provides critical insights into the evolving nature of postoperative survival, empowering both patients and clinicians to make informed decisions regarding therapeutic options.
However, this study had some limitations. First, the SEER database is deficient in key biomarker data, such as microsatellite instability and deficient mismatch repair status, both of which are essential prognostic indicators. Additionally, it offered only basic therapeutic records, lacking detailed information on surgical techniques, chemotherapy regimens, radiation doses, patient health status, and socio-economic factors that may influence survival outcomes. These gaps constrained the depth of our analysis, and future research should aim to incorporate these variables to better elucidate their impact. Furthermore, the retrospective design introduced potential selection bias; thus, prospective cohort studies or randomized controlled trials are necessary to validate our findings and mitigate bias.
Conclusions
Our study provided a comprehensive analysis of prognostic factors influencing OS and CSS in patients with ESRC, utilizing data from the SEER database. We have developed and validated prediction nomograms for both OS and CSS, evaluating the impact of various treatment options across distinct subgroups. While our model exhibited promising performance in forecasting survival outcomes for ESRC patients, further validation through multi-center studies is essential to confirm its applicability in clinical situation.
Acknowledgments
None.
Footnote
Reporting Checklist: The authors have completed the TRIPOD reporting checklist. Available at https://tcr.amegroups.com/article/view/10.21037/tcr-24-1888/rc
Peer Review File: Available at https://tcr.amegroups.com/article/view/10.21037/tcr-24-1888/prf
Funding: This study was funded by
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tcr.amegroups.com/article/view/10.21037/tcr-24-1888/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Bray F, Laversanne M, Sung H, et al. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2024;74:229-63. [Crossref] [PubMed]
- Randhawa SE, Tenner L. Survivorship in Early-Stage Rectal Cancer Patients Who Have Received Combined Modality Therapy. Clin Colorectal Cancer 2023;22:375-82. [Crossref] [PubMed]
- Siegel RL, Wagle NS, Cercek A, et al. Colorectal cancer statistics, 2023. CA Cancer J Clin 2023;73:233-54. [Crossref] [PubMed]
- Tan S, Gao Q, Cui Y, et al. Oncologic outcomes of watch-and-wait strategy or surgery for low to intermediate rectal cancer in clinical complete remission after adjuvant chemotherapy: a systematic review and meta-analysis. Int J Colorectal Dis 2023;38:246. [Crossref] [PubMed]
- Hirai Y, Toyoshima N, Saito Y. Endoscopic Resection for Colorectal Tumors. Digestion 2025;106:115-21. [PubMed]
- Su J, Liu Q, Zhou D, et al. The status of low anterior resection syndrome: data from a single-center in China. BMC Surg 2023;23:110. [Crossref] [PubMed]
- Kwik C, El-Khoury T, Pathma-Nathan N, et al. Endoscopic and trans-anal local excision vs. radical resection in the treatment of early rectal cancer: A systematic review and network meta-analysis. Int J Colorectal Dis 2023;39:13. [Crossref] [PubMed]
- Wang Y, Yang Y, Liu QQ, et al. Compare clinical efficacy and safety of neoadjuvant therapy and neoadjuvant chemoradiotherapy for locally advanced rectal cancer: Meta-analysis. World J Gastrointest Surg 2024;16:1845-56. [Crossref] [PubMed]
- Horesh N, Emile SH, Freund MR, et al. Local excision vs. proctectomy in patients with ypT0-1 rectal cancer following neoadjuvant therapy: a propensity score matched analysis of the National Cancer Database. Tech Coloproctol 2024;28:128. [Crossref] [PubMed]
- Peltrini R, Castiglioni S, Imperatore N, et al. Short- and long-term outcomes in ypT2 rectal cancer patients after neoadjuvant therapy and local excision: a multicentre observational study. Tech Coloproctol 2023;27:53-61. [Crossref] [PubMed]
- Benson AB, Venook AP, Adam M, et al. NCCN Guidelines® Insights: Rectal Cancer, Version 3.2024. J Natl Compr Canc Netw 2024;22:366-75. [Crossref] [PubMed]
- Schrag D, Shi Q, Weiser MR, et al. Preoperative Treatment of Locally Advanced Rectal Cancer. N Engl J Med 2023;389:322-34. [Crossref] [PubMed]
- Shen D, Wang P, Xie Y, et al. Clinical spectrum of rectal cancer identifies hallmarks of early-onset patients and next-generation treatment strategies. Cancer Med 2023;12:3433-41. [Crossref] [PubMed]
- Carbone F, Spinelli A, Ciardiello D, et al. Prognosis of early-onset versus late-onset sporadic colorectal cancer: Systematic review and meta-analysis. Eur J Cancer 2025;215:115172. [Crossref] [PubMed]
- Chen H, Hu D, Su W, et al. Endoscopic resection vs. endoscopic resection plus chemoradiation for T1 stage colorectal cancer: a real-world retrospective cohort study. Transl Cancer Res 2024;13:989-98. [Crossref] [PubMed]
- Seow W, Dudi-Venkata NN, Bedrikovetski S, et al. Outcomes of open vs laparoscopic vs robotic vs transanal total mesorectal excision (TME) for rectal cancer: a network meta-analysis. Tech Coloproctol 2023;27:345-60. [Crossref] [PubMed]
- Dang H, Verhoeven DA, Boonstra JJ, et al. Management after non-curative endoscopic resection of T1 rectal cancer. Best Pract Res Clin Gastroenterol 2024;68:101895. [Crossref] [PubMed]
- Zeng X, Zhang R, Jiang W, et al. Local Excision Versus Radical Resection for Grade 2 Rectal Neuroendocrine Tumors: A Multicenter Propensity Score-Matched Analysis. Dis Colon Rectum 2024;67:911-9. [Crossref] [PubMed]
- Binda C, Secco M, Tuccillo L, et al. Early Rectal Cancer and Local Excision: A Narrative Review. J Clin Med 2024;13:2292. [Crossref] [PubMed]
- Sun YC, Zhao ZD, Yao N, et al. Risk prediction of second primary malignancies in patients after rectal cancer: analysis based on SEER Program. BMC Gastroenterol 2023;23:354. [Crossref] [PubMed]
- Guo F, Sun Z, Wang Z, et al. Nomogram for predicting prolonged postoperative ileus after laparoscopic low anterior resection for rectal cancer. World J Surg Oncol 2023;21:380. [Crossref] [PubMed]
- Tang C, Xu J, Lin M, et al. Risk Factors for Distant Metastasis in T3 T4 Rectal Cancer. Clin Med Insights Oncol 2024;18:11795549241227423. [Crossref] [PubMed]
- Choi JS, Kim MJ, Shin R, et al. Risk Factor Analysis of Lymph Node Metastasis for Rectal Neuroendocrine Tumors: Who Needs a Radical Resection in Rectal Neuroendocrine Tumors Sized 1-2 cm? Ann Surg Oncol 2024;31:2414-24. [Crossref] [PubMed]
- Gefen R, Emile SH, Garoufalia Z, et al. Local vs radical resection of stage I-III rectal cancer in very elderly patients: an exact matched analysis of the National Cancer Database. J Gastrointest Surg 2024;28:1259-64. [Crossref] [PubMed]
- El-Nakeep S, Madala S, Chidharla A, et al. Radical versus Local Surgical Excision for Early Rectal Cancer: A Systematic Review and Meta-Analysis. Arch Intern Med Res 2024;7:1-11. [Crossref] [PubMed]
- Calmels M, Labiad C, Lelong B, et al. Local excision after neoadjuvant chemoradiotherapy for mid and low rectal cancer: a multicentric French study from the GRECCAR group. Colorectal Dis 2023;25:1973-80. [Crossref] [PubMed]
- Verheij FS, Omer DM, Williams H, et al. Long-Term Results of Organ Preservation in Patients With Rectal Adenocarcinoma Treated With Total Neoadjuvant Therapy: The Randomized Phase II OPRA Trial. J Clin Oncol 2024;42:500-6. [Crossref] [PubMed]
- Sun M, Moquet J, Barnard S, et al. In vitro study of radiosensitivity in colorectal cancer cell lines associated with Lynch syndrome. Front Public Health 2024;12:1369201. [Crossref] [PubMed]
- Donohue K, Rossi A, Deek MP, et al. Local Excision for Early-Stage Rectal Adenocarcinomas. Cancer J 2024;30:245-50. [Crossref] [PubMed]
- Sun Myint A, Rao C, Barbet N, et al. The safety and efficacy of total mesorectal excision (TME) surgery following dose-escalation: Surgical outcomes from the organ preservation in early rectal adenocarcinoma (OPERA) trial, a European multicentre phase 3 randomised trial (NCT02505750). Colorectal Dis 2023;25:2160-9. [Crossref] [PubMed]
- Liao H, Zeng T, Xie X, et al. The benefit of adjuvant chemotherapy in pathological T1-3N0M0 rectal mucinous adenocarcinoma: no improvement survival outcomes based on long-term survival analysis of large population data. J Gastrointest Oncol 2024;15:1568-79. [Crossref] [PubMed]
- Spatola C, Privitera G, Milazzotto R, et al. Trends in combined radio-chemotherapy for locally advanced rectal cancer: a survey among radiation oncology centers of Sicily region on behalf of AIRO. Radiol Med 2019;124:671-81. [Crossref] [PubMed]
- Lo Greco MC, La Rocca M, Marano G, et al. Integrated Intensified Chemoradiation in the Setting of Total Neoadjuvant Therapy (TNT) in Patients with Locally Advanced Rectal Cancer: A Retrospective Single-Arm Study on Feasibility and Efficacy. Cancers (Basel) 2023;15:921. [Crossref] [PubMed]


