
Investigating additional malignancy rates and prognostic factors in multiple myeloma patients: a Surveillance, Epidemiology, and End Results (SEER) database retrospective cohort study
Highlight box
Key findings
• Among 60,550 multiple myeloma (MM) patients, 6.07% developed second primary malignancies (SPMs), and 2.75% had primary malignancies (PMs).
• In MM patients with additional malignancies, prostate cancer was the most common solid tumor, whereas non-Hodgkin’s lymphoma (NHL) dominated hematologic malignancies.
• In MM patients with SPMs, key risk factors for poor survival included the following: older age, higher sequence of PMs, specific cancer sites. Protective factors included longer latency (≥4 vs. 0–1 year) and higher tumor numbers. In PMs in patients diagnosed with MM, key risk factors for poor survival included the following: older age, higher sequence of PMs, specific cancer sites. Protective factors included longer latency (≥4 vs. 0–1 year), higher tumor numbers, and specific cancer sites.
What is known and what is new?
• Prior studies on MM combined with additional malignancies have primarily focused on SPMs, with few addressing MM occurring after PMs.
• Our study uniquely analyzes survival factors for both PMs and SPMs in MM patients.
What is the implication, and what should change now?
• This study identified key risk factors for additional malignancies in MM patients, such as advanced age, NHL, and higher sequence numbers. The developed nomograms predict survival outcomes, supporting personalized clinical decisions and improved patient management.
Introduction
Multiple myeloma (MM) is a disease characterized by the clonal proliferation of malignant plasma cells within the bone marrow with an age-adjusted annual incidence rate of about 5 per 100,000 (1,2). Although it is classified as a plasma cell malignancy, a study has shown that autologous hematopoietic stem cell transplantation (auto-HSCT) can prolong the survival of MM patients (3). A recent large-scale real-life study investigating the survival of MM reported a median overall survival (OS) of 34.75 months for the 2010–2014 cohort compared to 54.43 months for the 2015–2020 cohort (4). Notably, patients with MM seem to be at risk of developing a second primary malignancy (SPM). Conversely, some patients with tumors may develop MM. Although the coexistence of MM with other malignancies is rare, the reported incidence rate is significantly high, suggesting that it may be driven by several unknown mechanisms (5). Some reports have shown that the microenvironment, biology, and other conditions contribute to the pathogenesis of malignant tumors (6,7). Several factors in the tumor microenvironment can weaken the antitumoral activity of immune cells (6). Similar to other forms of cancer, MM can suppress and evade the immune system, thereby enhancing its progression. The occurrence of additional malignancies may introduce physical and psychological challenges, as well as increasing medical costs (8). This investigation focused on individuals with MM who had either concurrent secondary tumors or developed MM following the occurrence of other malignancies. The results are expected to deepen our understanding of diagnostic processes, therapeutic interventions, and prognostic outcomes.
The initial Durie-Salmon (D-S) staging system comprised the CRAB criteria (hypercalcemia, renal impairment, anemia, and bone lesions) and paraprotein levels for prognosis assessment. The Revised International Staging System (R-ISS) contained β2-microglobulin and lactate dehydrogenase (LDH), along with high-risk cytogenetics (9). Similar to MAF MM, t(4;14) is frequently accompanied by gain/gamp(1q) and del(1p), which contribute to its poor prognosis. However, none of these scoring systems provides a summary of the frequency and prognosis of complications arising from the malignancy. A subset of patients experience relapse within 18 months, characterized by aberrant molecular dynamics, such as mutations in MMSET, MAF, and MAFb (10). A study on patients from the United States (US) diagnosed with MM from 1973 to 2008 found a reduced risk of solid tumor SPM with a standardized incidence ratio (SIR) of 0.94 and an increased risk of blood-related cancers with a SIR of 1.68 (11). A previous study has demonstrated that the likelihood of SPM, whether solid or hematologic, in patients with MM ranges from 3.6% to 5%. Consequently, in older adults with myeloma, initial treatment with lenalidomide does not appear to significantly elevate the risk of developing SPMs (12).
Most of the available studies on the incidence of secondary malignancies in individuals diagnosed with MM are mainly based on small-samples, and are single-center investigations. A previous study has summarized the additional malignancy rates and prognostic factors in MM patients based on the Surveillance, Epidemiology, and End Results (SEER) database. Therefore, we performed a comprehensive analysis on a large registry to evaluate the frequency and likelihood of SPM rates and risks of death in MM patients, as well as to investigate the contributing risk factors. The SEER program offers detailed population-based data on cancer diagnosis stage and patient outcomes, including survival rates (13). The primary aim of this study was to analyze the SEER database and determine the occurrence of additional malignancies in individuals with MM, as well as identifying the associated survival risk factors. In addition, we constructed two nomograms for predicting the prognosis of patients diagnosed with MM, which are expected to improve clinical decision-making. We present this article in accordance with the TRIPOD reporting checklist (available at https://tcr.amegroups.com/article/view/10.21037/tcr-24-1721/rc).
Methods
Patient selection
Data of patients diagnosed with MM as their primary malignancy (PM) were extracted from the SEER database (version 8.4.0) available at www.seer.cancer.gov. The data retrieval was conducted systematically, covering the period between January 2000 and November 2019 for follow-up purposes. The study consists of two parts. In part 1, patients who were diagnosed with MM and developed SPM were enrolled. In part 2, patients who were initially diagnosed with a PM, and later developed MM as a secondary tumor were enrolled (Figure S1).
Data collection
The following clinical data of patients with MM were retrieved: age, sex, race, latency, total number of malignant tumors per patient, sequence number, and site of additional malignancies. Information regarding survival status and survival time was also extracted from the database. The enrolled cohort comprised all patients diagnosed with MM in the SEER database according to primary site and International Classification of Diseases for Oncology, Third Edition (ICD-O-3) histology criteria. Patients with MM as the sole PM were excluded from the analysis.
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study has been granted an exemption from requiring ethics approval by the ethical committee of the First Affiliated Hospital of Zhejiang Chinese Medical University.
Definitions of SPM and PM
We defined SPM as any subsequent malignancy that developed after the diagnosis of MM. Patients previously diagnosed with a malignant tumor other than MM were categorized as PM cases (5,14). Notably, we only included patients with pathological diagnoses. None of the patients had a confirmed distant dissemination from MM or synchronous PM (15).
Statistical analysis
SIR and absolute excess risk (AER) were calculated using SEER*Stat (version 8.4.3) to evaluate the risk of SPMs. Cox regression analysis was conducted to identify factors influencing the development of additional malignancies. The results, presented as odds ratios (ORs) with 95% confidence intervals (CIs), were analyzed in R V. 4.2.0 (R Foundation for Statistical Computing, Vienna, Austria). All statistical analyses were two-sided, with significance set at P<0.05.
Results
Part 1: MM patients with SPMs
Occurrence of different systems and malignancies
A total of 60,550 cases were determined to be primary MM, which occurred from 1992 to 2020. Among them, 3,676 (6.07%) patients developed SPMs. The median age at MM diagnosis was 71.5 years, and the median age at SPMs diagnosis was 75.5 years. For MM patients with SPMs, the median follow-up was 60 months (range, 0–337 months), with an OS rate of 28.63%. Most patients (75.71%) were White. More than half of the patients (61.56%) were male. The characteristics of all patients are shown in Table 1 and Tables S1,S2.
Table 1
| Systems | Count, n (%) | Sex, n (%) | Race, n (%) | Median of latency, years | Median age at MM diagnosis, years | Median age at SPM diagnosis, years | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Male | Female | White | Black | Other | ||||||
| Urinary system | 855 (25.00) | 781 (91.35) | 74 (9.48) | 633 (74.04) | 165 (19.3) | 57 (6.67) | 2 | 68 | 71 | |
| Digestive system | 679 (19.85) | 412 (60.68) | 267 (39.32) | 483 (71.13) | 132 (19.44) | 64 (9.43) | 3 | 70 | 74 | |
| Hematology | 531 (15.53) | 329 (61.96) | 202 (38.04) | 439 (82.67) | 57 (10.73) | 35 (6.59) | 2 | 68 | 72 | |
| Respiratory system | 453 (13.25) | 276 (60.93) | 187 (41.28) | 333 (73.51) | 87 (19.21) | 33 (7.28) | 2 | 70 | 72 | |
| Genital system | 429 (12.54) | 12 (2.8) | 417 (97.2) | 304 (70.86) | 90 (20.98) | 35 (8.16) | 3 | 66 | 70 | |
| Nervous system | 38 (1.11) | 24 (63.16) | 14 (36.84) | 32 (84.21) | 3 (7.89) | 3 (7.89) | 2 | 70.5 | 73 | |
MM, multiple myeloma; SPM, second primary malignancy.
Table 1 presents a summary of the frequency of SPMs in patients with MM based on their gender, race, system affected, median latency period, median age at MM diagnosis, and median age at SPM diagnosis. In our study, urological malignancies were the most common SPM in MM patients, accounting for 855 cases (23.26%). This was followed by digestive system malignancies (679 cases, 18.47%) and hematologic malignancies (531 cases, 14.45%).
All solid tumors (85.55%) are listed in the Table S1, with prostate cancer (PCa) ranked as the most common among the SPMs, followed by lung/bronchus cancer and breast cancer. Hematologic malignancies accounted for 14.45%, and included non-Hodgkin’s lymphoma (NHL), acute myeloid leukemia (AML), chronic lymphocytic leukemia (CLL), acute lymphocytic leukemia (ALL), chronic myeloid leukemia (CML), and so on (Table S2). The patient characteristics are displayed in Figure 1 for reference.
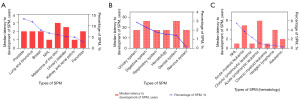
Site-specific risks associated with MM as the PM
Analysis showed that the risk of breast (SIR =0.11; 95% CI: 0.05–0.22), corpus and uterus (SIR =0.12; 95% CI: 0.01–0.42), rectum and rectosigmoid (SIR =0.14; 95% CI: 0.03–0.41), and colon and rectum (SIR =0.16; 95% CI: 0.1–0.24) cancers was decreased, whereas the risk of bones and joints (SIR =19.79; 95% CI: 14.49–26.4), nasopharynx (SIR =2.01; 95% CI: 0.41–5.88), and eye and orbit (SIR =1.72; 95% CI: 0.04–9.6) SPMs was increased. However, the risk of hematologic SPMs was significantly decreased. Moreover, the risk of aleukemic (SIR =31.69; 95% CI: 27.79–35.98), other leukemia (SIR =21.72; 95% CI: 19.18–24.5), ALL (SIR =10.9; 95% CI: 6.66–16.83) was increased (Table 2, Figure 2).
Table 2
| Variables | Observed | Expected | O/E | CI lower | CI upper | Excess risk |
|---|---|---|---|---|---|---|
| Solid malignancies | ||||||
| Bones and joints | 46 | 2.32 | 19.79 | 14.49 | 26.4 | 2.21 |
| Nasopharynx | 3 | 1.49 | 2.01 | 0.41 | 5.88 | 0.08 |
| Eye and orbit | 1 | 0.58 | 1.72 | 0.04 | 9.6 | 0.02 |
| Hypopharynx | 1 | 0.9 | 1.12 | 0.03 | 6.22 | 0.01 |
| Breast | 8 | 70.46 | 0.11 | 0.05 | 0.22 | –3.16 |
| Anus, anal canal, and anorectum | 1 | 1.55 | 0.65 | 0.02 | 3.6 | –0.03 |
| Brain and other nervous system | 17 | 26.62 | 0.64 | 0.37 | 1.02 | –0.49 |
| Small intestine | 2 | 3.16 | 0.63 | 0.08 | 2.28 | –0.06 |
| Hodgkin lymphoma | 1 | 2.07 | 0.48 | 0.01 | 2.69 | –0.05 |
| Liver and intrahepatic bile duct | 21 | 50.14 | 0.42 | 0.26 | 0.64 | –1.48 |
| Kidney and renal pelvis | 13 | 31.01 | 0.42 | 0.22 | 0.72 | –0.91 |
| Liver | 15 | 38.5 | 0.39 | 0.22 | 0.64 | –1.19 |
| Stomach | 10 | 30.28 | 0.33 | 0.16 | 0.61 | –1.03 |
| Gum and other mouth | 1 | 3.02 | 0.33 | 0.01 | 1.84 | –0.1 |
| Male genital system | 32 | 103.4 | 0.31 | 0.21 | 0.44 | –3.62 |
| Prostate | 32 | 102.24 | 0.31 | 0.21 | 0.44 | –3.56 |
| Oral cavity and pharynx | 6 | 20.22 | 0.3 | 0.11 | 0.65 | –0.72 |
| Urinary system | 20 | 73.11 | 0.27 | 0.17 | 0.42 | –2.69 |
| Other oral cavity and pharynx | 1 | 3.79 | 0.26 | 0.01 | 1.47 | –0.14 |
| Digestive system | 85 | 349.69 | 0.24 | 0.19 | 0.3 | –13.4 |
| Corpus uteri | 2 | 8.44 | 0.24 | 0.03 | 0.86 | –0.33 |
| Ovary | 6 | 24.84 | 0.24 | 0.09 | 0.53 | –0.95 |
| Pancreas | 20 | 89.22 | 0.22 | 0.14 | 0.35 | –3.51 |
| Esophagus | 7 | 34.42 | 0.2 | 0.08 | 0.42 | –1.39 |
| Lung and bronchus | 74 | 372.83 | 0.2 | 0.16 | 0.25 | –15.13 |
| Respiratory system | 74 | 384.88 | 0.19 | 0.15 | 0.24 | –15.74 |
| Cervix uteri | 1 | 5.38 | 0.19 | 0 | 1.04 | –0.22 |
| Female genital system | 9 | 51.05 | 0.18 | 0.08 | 0.33 | –2.13 |
| Urinary bladder | 7 | 40.09 | 0.17 | 0.07 | 0.36 | –1.68 |
| Chronic lymphocytic leukemia | 2 | 11.69 | 0.17 | 0.02 | 0.62 | –0.49 |
| Colon and rectum | 20 | 126.9 | 0.16 | 0.1 | 0.24 | –5.41 |
| Colon excluding rectum | 17 | 105.54 | 0.16 | 0.09 | 0.26 | –4.48 |
| Rectum and rectosigmoid | 3 | 21.36 | 0.14 | 0.03 | 0.41 | –0.93 |
| Corpus and uterus | 2 | 17.18 | 0.12 | 0.01 | 0.42 | –0.77 |
| Hematologic malignancies | ||||||
| Aleukemic | 238 | 7.51 | 31.69 | 27.79 | 35.98 | 11.67 |
| Other leukemia | 264 | 12.16 | 21.72 | 19.18 | 24.5 | 12.75 |
| Acute lymphocytic leukemia | 20 | 1.84 | 10.9 | 6.66 | 16.83 | 0.92 |
| Leukemia | 348 | 52.66 | 6.61 | 5.93 | 7.34 | 14.96 |
| Other acute leukemia | 26 | 4.65 | 5.6 | 3.66 | 8.2 | 1.08 |
| Other lymphocytic leukemia | 3 | 1.08 | 2.79 | 0.58 | 8.15 | 0.1 |
| Other myeloid/monocytic leukemia | 5 | 1.94 | 2.58 | 0.84 | 6.03 | 0.16 |
| Acute myeloid leukemia | 50 | 20.82 | 2.4 | 1.78 | 3.17 | 1.48 |
| Myeloid and monocytic leukemia | 59 | 25.9 | 2.28 | 1.73 | 2.94 | 1.68 |
| Lymphocytic leukemia | 25 | 14.6 | 1.71 | 1.11 | 2.53 | 0.53 |
| Chronic myeloid leukemia | 4 | 2.89 | 1.39 | 0.38 | 3.55 | 0.06 |
| Lymphoma | 77 | 52.02 | 1.48 | 1.17 | 1.85 | 1.27 |
| Non-Hodgkin’s lymphoma | 76 | 49.95 | 1.52 | 1.2 | 1.9 | 1.32 |
CI, confidence interval; MM, multiple myeloma; O/E, observed/expected.

Risk factors and nomograms for developing SPMs in MM patients
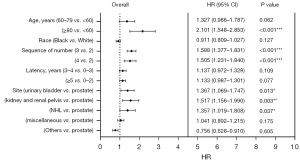
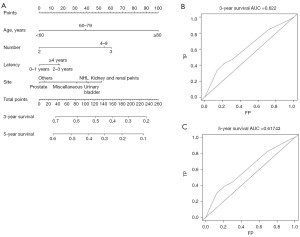
Seven risk factors, including age at MM diagnosis, sex, race, the number and sequence of SPM, total number of malignant tumors per patient, site of SPM and latency for MM patients to obtain SPM were identified (Table 3, Figure 3). Parameters found to be significant in univariate analysis were included in multivariate analysis. In the latter analysis, several independent risk factors for patient survival were identified, including age at MM diagnosis [60–79 vs. <60 years, hazard ratio (HR) =1.327, P<0.001; ≥80 vs. <60 years, HR =2.101, P<0.001], sequence number of PMs (second of two or more primaries vs. first, HR =2.006, P<0.001; third or more vs. first, HR =5.483, P<0.001), and cancer site (thyroid vs. prostate). Furthermore, the number of tumors (4–9 vs. 2 malignant tumors, HR =0.650, P=0.01), specific cancer sites (NHL vs. prostate, HR =0.651, P=0.06; pancreas vs. prostate, HR =0.487, P=0.047; sigmoid colon vs. prostate, HR =0.359, P=0.008), and latency period (2–3 vs. 0–1 year, HR =0.610, P<0.001; ≥4 vs. 0–1 year, HR =0.306, P<0.001) were identified as prognostic factors for survival. The constructed nomogram displayed outstanding performance in predicting OS of SPM, as demonstrated by receiver operating characteristic (ROC) analysis. The area under the curve (AUC) for 3- and 5-year OS prediction was 0.944 and 0.94377, respectively, indicating the nomogram’s strong predictive ability for OS. The ROC curve further confirmed the robustness of our predictive model in estimating SPM OS, underscoring its reliability and efficacy (Figure 4).
Table 3
| Variables | Univariate | Multivariate | |||
|---|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | ||
| Age at MM diagnosis (years) | |||||
| 60–79 vs. <60 | 1.736 (1.696–1.776) | <0.001*** | 1.327 (0.986–1.787) | 0.006** | |
| ≥80 vs. <60 | 3.884 (3.779–3.991) | <0.001*** | 2.101 (1.548–2.853) | <0.001*** | |
| Sex | |||||
| Female vs. male | 1.014 (0.996–1.032) | 0.13 | – | – | |
| Race | |||||
| Black vs. White | 1.303 (1.274–1.332) | <0.001*** | 0.911 (0.809–1.027) | 0.78 | |
| Other vs. White | 1.136 (1.092–1.183) | <0.001*** | 1.240 (0.991–1.551) | 0.06 | |
| Sequence number† | |||||
| 2 vs.1 | 0.333 (0.262–0.303) | <0.001*** | 2.006 (1.558–2.581) | <0.001*** | |
| 3 vs.1 | 0.342 (0.163–0.717) | 0.005** | 5.483 (2.339–12.852) | <0.001*** | |
| 3 vs. 2 | 0.289 (0.255–0.327) | <0.001*** | 0.980 (0.847–1.133) | 0.78 | |
| Total number of malignant tumors for patient‡ | |||||
| 4 vs. 2 | 0.236 (0.171–0.325) | <0.001*** | 0.650 (0.466–0.907) | 0.01* | |
| Site | |||||
| Lung and bronchus vs. prostate | 0.660 (0.502–0.867) | 0.003** | 0.767 (0.583–1.011) | 0.06 | |
| Breast vs. prostate | 0.947 (0.755–1.187) | 0.64 | – | – | |
| Miscellaneous vs. prostate | 0.703 (0.521–0.948) | 0.02* | 0.775 (0.574–1.0474) | 0.10 | |
| Melanoma of the skin vs. prostate | 1.001 (0.787–1.274) | 0.99 | – | – | |
| Urinary bladder vs. prostate | 0.858 (0.632–1.167) | 0.33 | – | – | |
| Kidney and renal pelvis vs. prostate | 1.237 (0.910–1.681) | 0.18 | – | – | |
| AML vs. prostate | 1.237 (0.312––0.765) | 0.002** | 0.682 (0.434–1.073) | 0.10 | |
| NHL vs. prostate | 0.488 (0.561–1.030) | 0.08 | 0.651 (0.479–0.885) | 0.006* | |
| Pancreas vs. prostate | 0.372 (0.183–0.754) | 0.006** | 0.487 (0.240–0.989) | 0.047* | |
| CLL vs. prostate | 1.853 (1.255–2.736) | 0.002** | 1.349 (0.907–2.006) | 0.14 | |
| Thyroid vs. prostate | 1.753 (1.284–2.394) | <0.001*** | 1.514 (1.105–2.074) | 0.01* | |
| Sigmoid colon vs. prostate | 0.339 (0.159–0.719) | 0.005** | 0.359 (0.169–0.764) | 0.008** | |
| Others vs. prostate | 0.756 (0.628–0.910) | 0.003** | 0.971 (0.827–1.142) | 0.73 | |
| Latency | |||||
| 2–3 years vs. 0–1 year | 0.604 (0.511–0.713) | <0.001*** | 0.610 (0.515–0.723) | <0.001*** | |
| ≥4 years vs. 0–1 year | 0.289 (0.251–0.332) | <0.001*** | 0.306 (0.264–0.354) | <0.001*** | |
†, 1= first of two or more primaries, 2= second of two or more primaries, 3= third of two or more primaries; ‡, 2= 2 malignant tumors, 4= 4–9 malignant tumors (3= 3 malignant tumors). *, P<0.05; **, P<0.01; ***, P<0.001. AML, acute myeloid leukemia; CI, confidence interval; CLL, chronic lymphocytic leukemia; HR, hazard ratio; MM, multiple myeloma; NHL, non-Hodgkin’s lymphoma; SPM, second primary malignancy.
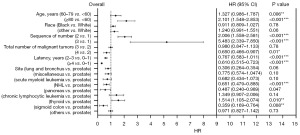
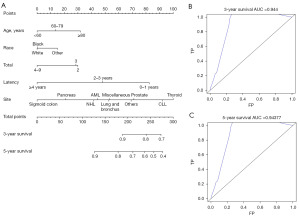
Part 2: PMs in patients diagnosed with MM
Occurrence of different systems and malignancies
From 1992 to 2020, there were 60,550 confirmed cases of primary MM, among whom 1,663 patients (2.75%) experienced PM. The median age at diagnosis for MM was 81 years, whereas that of PM was 70 years. The median follow-up duration was 26 months (ranging from 0 to 245 months), with an OS rate of 31.11%. Most patients (82.85%) were of White ethnicity, and males accounted for 64.50% of the cases. A summary of patient demographics is presented in Tables S3,S4, and the corresponding graphical representation is shown in Figure 5.
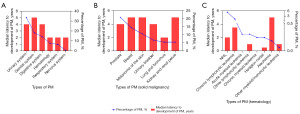
All solid and hematologic malignancies are presented in Tables S3,S4, with PCa (20.88%) ranking highest among PMs, followed by breast cancer (14.50%) and melanoma of the skin (10.71%). Hematologic malignancies included NHL (5.11%) and CLL (1.38%) (Table S2).
Risk factors and nomograms for PM in MM patients
The performance of the constructed nomogram in predicting the OS of PM patients was determined using the ROC and AUC. Seven risk factors were identified. Multivariate analysis of PMs in patients diagnosed with MM identified several independent risk factors for patient survival. These included age at MM diagnosis (≥80 vs. <60 years, HR =2.101, P<0.001), the sequence number of primary tumors (third of two or more primaries vs. second of two or more primaries, HR =1.588, P<0.001; fourth or more primaries vs. second of two or more primaries, HR =1.505, P<0.001), and tumor site (urinary bladder vs. prostate, HR =1.367, P=0.01; kidney and renal pelvis vs. prostate, HR =1.517, P=0.003; NHL vs. prostate, HR =1.357, P=0.04) (Table 4, Figure 6), and were included in the nomogram. The nomogram predicted the 3-year OS and 5-year OS with AUC values of 0.622 and 0.61743, respectively, suggesting that the nomogram was effective. The results of the ROC analysis further supported the reliability of our nomogram in predicting the OS of PM patients (Figure 7).
Table 4
| Variables | Univariate | Multivariate | |||
|---|---|---|---|---|---|
| HR (95% CI) | P | HR (95% CI) | P | ||
| Age at MM diagnosis (years) | |||||
| 60–79 vs. <60 | 1.394 (1.037–1.873) | 0.03* | 1.327 (0.986–1.787) | 0.06 | |
| ≥80 vs. <60 | 2.200 (1.624–2.979) | <0.001*** | 2.101 (1.548–2.853) | <0.001*** | |
| Sex | |||||
| Female vs. male | 1.019 (0.900–1.153) | 0.77 | – | – | |
| Race | |||||
| Black vs. White | 0.882 (0.783–0.994) | 0.04* | 0.911 (0.809–1.027) | 0.13 | |
| Other vs. White | 0.996 (0.678–1.462) | 0.98 | – | – | |
| Sequence of number† | |||||
| 3 vs.2 | 1.544 (1.345–1.771) | <0.001*** | 1.588 (1.377–1.831) | <0.001*** | |
| 4 vs.2 | 1.565 (1.287–1.901) | <0.001*** | 1.505 (1.231–1.840) | <0.001*** | |
| Total number of malignant tumors for patient‡ | |||||
| 3 vs. 2 | 0.919 (0.730–1.157) | 0.47 | – | – | |
| 4 vs. 2 | 0.967 (0.687–1.361) | 0.85 | – | – | |
| Site | |||||
| Breast vs. prostate | 0.660 (0.502–0.867) | 0.90 | – | – | |
| Melanoma vs. prostate | 0.947 (0.755–1.187) | 0.78 | – | – | |
| Urinary bladder vs. prostate | 0.703 (0.521–0.948) | <0.001*** | 1.367 (1.069–1.747) | 0.01* | |
| Lung and bronchus vs. prostate | 1.001 (0.787–1.274) | 0.11 | – | – | |
| Kidney and renal pelvis vs. prostate | 0.858 (0.632–1.167) | <0.001*** | 1.517 (1.156–1.990) | 0.003** | |
| NHL vs. prostate | 0.858 (0.910–1.681) | 0.03* | 1.357 (1.019–1.808) | 0.04* | |
| Miscellaneous vs. prostate | 1.237 (0.312–0.765) | 0.04* | 1.041 (0.892–1.215) | 0.18 | |
| Thyroid vs. prostate | 0.488 (0.561–1.030) | 0.47 | – | – | |
| Sigmoid colon vs. prostate | 0.372 (0.183–0.754) | 0.18 | – | – | |
| Rectum vs. prostate | 1.852 (1.255–2.736) | 0.41 | – | – | |
| Corpus uteri vs. prostate | 1.753 (1.284–2.394) | 0.22 | – | – | |
| Cecum vs. prostate | 0.339 (0.159–0.719) | 0.42 | – | – | |
| Other vs. prostate | 0.756 (0.628–0.910) | 0.003** | 0.756 (0.628–0.910) | 0.61 | |
| Latency | |||||
| 2–3 years vs. 0–1 year | 0.604 (0.511–0.713) | <0.001*** | 1.137 (0.972–1.329) | 0.11 | |
| ≥4 years vs. 0–1 year | 0.289 (0.251–0.332) | <0.001*** | 1.133 (0.987–1.301) | 0.08 | |
†, 2= second of two or more primaries, 3= third of two or more primaries, 4= fourth of two or more primaries; ‡, 2= 2 malignant tumors, 3= 3 malignant tumors, 4= 4–9 malignant tumors. *, P<0.05; **, P<0.01; ***, P<0.001. MM, multiple myeloma; PM, primary malignancy; HR, hazard ratio; CI, confidence interval; NHL, non-Hodgkin’s lymphoma.
Discussion
MM is a disorder characterized by abnormal proliferation of clonal plasma cells (16-18). Zhao et al. proposed an innovative multi-scale feature fusion encoding-decoding model architecture specifically designed for the segmentation, early detection, and diagnosis of MM (19). A study utilizing the National Inpatient Sample database indicated an increased rate of spinal stabilization procedures in the surgical management for MM (20). The recent developments in tumor diagnostic techniques and therapeutic approaches have led to the identification of an increasing number of MM patients with two different PMs. Vukelić et al. found that second primary tumors can potentially shorten the survival period by up to 20% (21). Therefore, attention should be paid to the diagnosis, prevention of misdiagnosis, and early diagnosis and treatment if additional malignancies is present (22).
In this study, most patients were diagnosed with a malignancy within 4 years, which is consistent with findings from past studies (5). This suggests that during the 0–4-year period when the first PM occurs, the patients should be followed up for any signs of cancer.
Notably, PCa was the most frequent among the additional malignancies in the cohort of MM. This is because PCa is the second most common cancer among men worldwide (23). Malignant tumors, particularly PCa, have an extended survival period due to in the development of more advanced treatments. Patients undergoing endocrine therapy for PCa, for example, have a median survival time of 7.81 years, surpassing the natural survival rate observed in many other types of malignancies (5,24).
Recent research has demonstrated the impact of familial risk on the development of additional malignancies among MM patients (19). For instance, a study revealed a potential association between RB1 gene deletion and the occurrence of additional malignancies (20). Notably, loss of TP53 in cytogenetics was considered a high-risk factor in PCa and MM, similar to our findings (21,22). In addition, another study found that the interaction between GRP78 and the adhesion protein N-cadherin was influenced by the bone microenvironment, which also modulates the interaction between MM and metastatic PCa (23). Moreover, a rare case report reported the occurrence of PCa metastases to the bone marrow alongside synchronous MM. They highlighted the correlation of insulin-like growth factor 1 (IGF-1), interleukin-6 (IL-6), stromal cell-derived factor 1 (SDF-1), and vascular endothelial growth factor (VEGF) with MM and PCa within the microenvironment (24). The progression and prognosis of PCa are intricately linked to C-myc signaling, a pathway disrupted in 45–90% of advanced myeloma cells (25). Notably, the NF-κB signaling pathway, which is hyperactivated in MM patients, exhibits enhanced responsiveness to bortezomib treatment. Moreover, NF-κB signaling regulates inflammation and contributes to the development of various cancers, including MM and PCa (25,26). NF-κB activation is also observed in castration-resistant prostate cancer (CRPC) and is influenced by PCAT1, which is upregulated in MM (26,27).
NHL as additional malignancy of MM is the most frequent type of hematology. A study reviewed 6 cases of concurrent B cell lymphoma and MM, along with 2 borderline cases showing both pathological and histological characteristics of B cell lymphoma and MM, which were obtained from the PubMed database (27). Although there have been reports of the clustering of hematologic malignancies within families for several decades, the underlying cause(s) of this rare phenomenon are not completely understood (28,29). Researchers have shown that both MM and NHL are neoplasms derived from plasma cells (30). NHL exhibits several features similar to MM with the exception of clonal plasma cells (31). Previous research has highlighted the importance of examining FOXP expression patterns to identify the malignant mature B-cell characteristics, suggesting a potential role of the vitamin D pathway in plasmablastic subtypes of lymphoma and MM (32).
A high overall percentage of SIR was detected in hematologic SPMs, especially in leukemia. In a previous study (33), a patient with plasmacytoma who developed AML was reported. Researchers have speculated that chemotherapy or radiotherapy may trigger the occurrence of AML, known as therapy-related AML. On the contrary, most solid tumors were categorized as lower observed/expected (O/E) ratio. However, potential association between MM and certain other cancers should be further explored in larger sample sizes. Further longitudinal studies and in-depth analyses are needed to fully explore this relationship. Nevertheless, in our analysis, we found that most patients with myeloma also presented with solid tumors, as opposed to hematologic malignancies. This phenomenon might be attributed to treatment modalities. Some researchers have reported that the risk of SPMs in MM is relatively low and is influenced by several factors. Although lenalidomide in combination with certain treatments may elevate the risk of SPMs, the survival benefits it provides could outweigh this risk. In addition, not all combinations of lenalidomide have been linked to increased rates of SPMs (34,35).
Research shows that the development of an SPM after auto-HSCT for MM leads to worse progression-free survival (PFS) and OS. Moreover, MM is the leading cause of mortality in patients experiencing SPMs after auto-HSCT (36). In this study, age was identified as a primary factor influencing the survival of additional malignancies of MM patients. Older patients had higher mortality rates, similar to a previous report (37). Furthermore, we observed a correlation between the number of malignant tumors and mortality rate. Patients with more aggressive malignancies may have died from their disease before developing subsequent tumors. Therefore, the relationship between the number of malignant tumors and survival rate should be interpreted with caution. Furthermore, our study highlighted impact of latency period for the onset of SPMs on survival prediction. We observed a positive correlation between longer latency periods and higher 3- and 5-year mortality rates. This implies that patients with a shorter interval between the first tumor and the appearance of an SPM might have lower survival rates. It has been shown that second primary thyroid cancer arising within the initial year is often characterized by increased aggressiveness (38). Moreover, our study identified thyroid cancer and lymphocytic leukemia as risk factors for SPMs in MM patients, thereby increasing mortality rates within a span of 3–5 years. These results are expected to improve the clinical management and prognostic evaluation of MM patients. The analysis of urinary bladder malignancies found that kidney and renal pelvis malignancies, and NHL as PMs increased the 3- and 5-year mortality rates. The mechanisms by which additional malignancies increase cancer invasiveness need to be further investigated.
This study has several limitations that need to be discussed. Notably, the SEER database lacks critical prognostic variables, such as information on the extent and location of bone destruction, as well as laboratory markers such as LDH levels. Despite these omissions, the proposed nomograms were constructed using readily accessible and generalizable variables, making it highly applicable to the SEER database. Moreover, given the retrospective nature of this study, the potential selection bias cannot be ruled out. In future, prospective clinical data should be analyzed to validate the accuracy and validity of our findings.
In this study, we found that the presence of any other tumor prior to MM is a poor prognostic factor, and decreases the OS. The site of additional malignancies, age, sequence number of malignancies, and latency need to be considered in these patients. Of note, certain rare cases of SPMs may exhibit higher O/E values than expected. Nomograms can help clinicians in assessing the diagnosis and prognosis of MM patients with additional malignancies, enabling the optimization of personalized treatment plans.
Conclusions
The findings of this analysis and the constructed nomograms are expected to help clinicians in assessing individual survival outcomes of additional malignancies and implementing personalized clinical decisions. Several factors can decrease the survival rate of MM patients with SPMs including bones and joints in solid tumors, hematopoietic system disorders, occurrence of third or subsequent PMs, and advanced age. During the assessment of MM patients with PMs, various parameters should be considered such as advanced age, presence of NHL, and development of third or subsequent PMs.
Acknowledgments
We thank all the patients for providing medical records and answering our review calls.
Footnote
Reporting Checklist: The authors have completed the TRIPOD reporting checklist. Available at https://tcr.amegroups.com/article/view/10.21037/tcr-24-1721/rc
Peer Review File: Available at https://tcr.amegroups.com/article/view/10.21037/tcr-24-1721/prf
Funding: The present study was supported by
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tcr.amegroups.com/article/view/10.21037/tcr-24-1721/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study has been granted an exemption from requiring ethics approval by the ethical committee of the First Affiliated Hospital of Zhejiang Chinese Medical University.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Medical Masterclass contributors; Firth J. Haematology: multiple myeloma. Clin Med (Lond) 2019;19:58-60.
- Kyle RA, Rajkumar SV. Multiple myeloma. Blood 2008;111:2962-72. [Crossref] [PubMed]
- Cavo M, Gay F, Beksac M, et al. Autologous haematopoietic stem-cell transplantation versus bortezomib-melphalan-prednisone, with or without bortezomib-lenalidomide-dexamethasone consolidation therapy, and lenalidomide maintenance for newly diagnosed multiple myeloma (EMN02/HO95): a multicentre, randomised, open-label, phase 3 study. Lancet Haematol 2020;7:e456-e468. Erratum in: Lancet Haematol 2020;7:e443; Lancet Haematol 2020;7:e785. [Crossref] [PubMed]
- Lopez-Muñoz N, Hernández-Ibarburu G, Alonso R, et al. Large-scale real-life analysis of survival and usage of therapies in multiple myeloma. J Hematol Oncol 2023;16:76. [Crossref] [PubMed]
- Wang J, Lv C, Zhou M, et al. Second Primary Malignancy Risk in Multiple Myeloma from 1975 to 2018. Cancers (Basel) 2022;14:4919. [Crossref] [PubMed]
- Ajani JA, Song S, Hochster HS, et al. Cancer stem cells: the promise and the potential. Semin Oncol 2015;42:S3-17. [Crossref] [PubMed]
- Song F, Qian Y, Peng X, et al. The frontline of immune response in peripheral blood. PLoS One 2017;12:e0182294. [Crossref] [PubMed]
- Grassi L. Psychiatric and psychosocial implications in cancer care: the agenda of psycho-oncology. Epidemiol Psychiatr Sci 2020;29:e89. [Crossref] [PubMed]
- Wallington-Beddoe CT, Mynott RL. Prognostic and predictive biomarker developments in multiple myeloma. J Hematol Oncol 2021;14:151. [Crossref] [PubMed]
- Rees MJ, Kumar S. High-risk multiple myeloma: Redefining genetic, clinical, and functional high-risk disease in the era of molecular medicine and immunotherapy. Am J Hematol 2024;99:1560-75. [Crossref] [PubMed]
- Poh C, Keegan T, Rosenberg AS. Second primary malignancies in multiple myeloma: A review. Blood Rev 2021;46:100757. [Crossref] [PubMed]
- Giri S, Barth P, Costa LJ, et al. Second primary malignancy among older adults with multiple myeloma receiving first-line lenalidomide-based therapy: A population-based analysis. J Geriatr Oncol 2021;12:256-61. [Crossref] [PubMed]
- Che WQ, Li YJ, Tsang CK, et al. How to use the Surveillance, Epidemiology, and End Results (SEER) data: research design and methodology. Mil Med Res 2023;10:50. [Crossref] [PubMed]
- Chinen Y, Tanba K, Takagi R, et al. Second primary malignancy after rituximab-containing immunochemotherapy for diffuse large B cell lymphoma. Leuk Lymphoma 2020;61:3378-86. [Crossref] [PubMed]
- Suton P, Prpic M, Tarle M, et al. Outcomes for patients with second primary malignancy after primary surgical treatment for early-stage squamous cell carcinoma of the oral cavity. Head Neck 2018;40:2347-52. [Crossref] [PubMed]
- Rajkumar SV, Dimopoulos MA, Palumbo A, et al. International Myeloma Working Group updated criteria for the diagnosis of multiple myeloma. Lancet Oncol 2014;15:e538-48. [Crossref] [PubMed]
- Minnie SA, Hill GR. Immunotherapy of multiple myeloma. J Clin Invest 2020;130:1565-75. [Crossref] [PubMed]
- Luo Y, Chen Y, Ai C, et al. PARP1 promotes tumor proliferation in lenalidomide-resistant multiple myeloma via the downregulation of microRNA-192-5p-AKT signaling. Transl Cancer Res 2024;13:6273-81. [Crossref] [PubMed]
- Zhao X, Chen L, Zhang N, et al. Multiple myeloma segmentation net (MMNet): an encoder-decoder-based deep multiscale feature fusion model for multiple myeloma segmentation in magnetic resonance imaging. Quant Imaging Med Surg 2024;14:7176-99. [Crossref] [PubMed]
- Zehri AH, Calafiore RL, Peterson KA, et al. Surgical management of spinal multiple myeloma: insights from the National Inpatient Sample database. J Spine Surg 2024;10:428-37. [Crossref] [PubMed]
- Vukelić J, Dobrila-Dintinjana R, Marijić B, et al. Clinical course of the disease and treatment outcome in patients with malignant laryngeal tumor: retrospective five-year analysis. Acta Clin Croat 2022;61:311-9. [Crossref] [PubMed]
- Rashed WM, Saad AM, Al-Husseini MJ, et al. Incidence of adrenal gland tumor as a second primary malignancy: SEER-based study. Endocr Connect 2018;7:1040-8. [Crossref] [PubMed]
- Bergengren O, Pekala KR, Matsoukas K, et al. 2022 Update on Prostate Cancer Epidemiology and Risk Factors-A Systematic Review. Eur Urol 2023;84:191-206. [Crossref] [PubMed]
- Yan J, Li C, Zhang X, et al. Degarelix vs. leuprorelin for the treatment of prostate cancer in China: A cost-utility analysis. Front Public Health 2022;10:942800. [Crossref] [PubMed]
- Qian S, Han X, Sha X, et al. Aqueous Extract of Cimicifuga dahurica Reprogramming Macrophage Polarization by Activating TLR4-NF-κB Signaling Pathway. J Inflamm Res 2022;15:1027-46. [Crossref] [PubMed]
- Duan J, Jin M, Yang D, et al. Ubiquitin-specific peptidase 2 inhibits epithelial-mesenchymal transition in clear cell renal cell carcinoma metastasis by downregulating the NF-κB pathway. Bioengineered 2022;13:4455-67. [Crossref] [PubMed]
- Li T, Tan J, Chen L, et al. Case report: simultaneous occurrence of multiple myeloma and non-Hodgkin lymphoma treated by CAR T therapy. Medicine (Baltimore) 2020;99:e19739. [Crossref] [PubMed]
- Wiernik PH, Dutcher JP. Families with non-Hodgkin lymphoma and plasma cell dyscrasias in their pedigrees. J Investig Med 2024;72:26-31. [Crossref] [PubMed]
- Perini GF, Ribeiro GN, Pinto Neto JV, et al. BCL-2 as therapeutic target for hematological malignancies. J Hematol Oncol 2018;11:65. [Crossref] [PubMed]
- Soliman K, Herberth J, Fülöp T, et al. Refractory ascites as a presenting feature of extramedullary plasmacytoma in an end-stage renal disease patient with HIV infection. Clin Nephrol Case Stud 2019;7:7-10. [Crossref] [PubMed]
- Barley K, Harris JA, Diefenbach C, et al. Misdiagnosis of non-hodgkin lymphoma as multiple myeloma. J Clin Oncol 2012;30:e364-7. [Crossref] [PubMed]
- Gascoyne DM, Lyne L, Spearman H, et al. Vitamin D Receptor Expression in Plasmablastic Lymphoma and Myeloma Cells Confers Susceptibility to Vitamin D. Endocrinology 2017;158:503-15. [Crossref] [PubMed]
- Ge S, Zhu G, Yi Y. Extramedullary plasmacytoma of the larynx: Literature review and report of a case who subsequently developed acute myeloid leukemia. Oncol Lett 2018;16:2995-3004. [Crossref] [PubMed]
- Musto P, Anderson KC, Attal M, et al. Second primary malignancies in multiple myeloma: an overview and IMWG consensus. Ann Oncol 2017;28:228-45. [Crossref] [PubMed]
- Landgren O, Mailankody S. Update on second primary malignancies in multiple myeloma: a focused review. Leukemia 2014;28:1423-6. [Crossref] [PubMed]
- Ragon BK, Shah MV, D'Souza A, et al. Impact of second primary malignancy post-autologous transplantation on outcomes of multiple myeloma: a CIBMTR analysis. Blood Adv 2023;7:2746-57. [Crossref] [PubMed]
- Jung JO, Schulz ER, Nienhüser H, et al. Characteristics and Prognostic Factors of Metachronous Second Primary Upper Gastrointestinal Cancer. J Surg Res 2021;258:254-64. [Crossref] [PubMed]
- Hussein M, Mueller L, Issa PP, et al. Latency Trend Analysis as a Guide to Screening Malignancy Survivors for Second Primary Thyroid Cancer. Biomedicines 2022;10:1984. [Crossref] [PubMed]


