
Association between blood metal elements and lung cancer: a cross-sectional and Mendelian randomization study
Highlight box
Key findings
• In this study, the association of blood metal elements (cadmium, lead, mercury, selenium, manganese, cobalt, copper, iron, and zinc) with lung cancer was explored based on the National Health and Nutrition Examination Survey (NHANES), and the causal relationship between them was investigated through Mendelian randomization (MR).
• NHANES results indicated that lung cancer was positively related to blood cadmium, and negatively linked to serum iron. MR only revealed a negative but not significant trend of causality between serum iron and lung cancer, and subgroup analysis showed a negative causal link between serum iron and squamous lung cancer.
• Our study revealed a negative relation of blood iron levels to the incidence of lung cancer, thus providing a reference for lung cancer prevention and diagnosis.
What is known and what is new?
• There is increasing proof that blood metal elements are crucial in the progression of lung cancer.
• Our study elucidates the association of blood metal elements with lung cancer and further explores the causal relationship between them using MR.
What is the implication, and what should change now?
• This study proves that serum iron is inversely linked to the incidence of lung cancer and negatively causally associated with squamous lung cancer, suggesting that serum iron may be a potential marker for lung cancer identification and prevention.
Introduction
According to the 2022 statistics of the International Agency for Research on Cancer (IARC), 2.48 million new lung cancer cases constitute 12.4% of newly diagnosed cancer patients (1). Lung cancer, as one of the most prevalent cancers, is the main cause of cancer-related mortalities (2,3). In China, estimates from the Cancer Center reported approximately 1.06 million new cases of lung cancer and 733,000 related deaths in 2022, making it the most prevalent and deadly cancer. Despite improvements in lung cancer prognosis, the efficacy of relevant treatment remains poor, and the 5-year survival rate is merely 25% (2,3). Of the 125,000 lung cancer-related deaths, 101,000 were attributed to smoking and 3,000 were caused by exposure to secondhand smoke, indicating that smoking remains a major cause of lung cancer and death (2). Age has also been proved to significantly bear on the occurrence of lung cancer (4). However, the potential causal association between other risk factors, such as blood metal levels, and lung cancer risk remains unclear.
Multiple studies have substantiated the significant involvement of blood metal levels in the progression of lung cancer. A retrospective study of Bai et al. (5) involving 440 lung cancer cases and 1,320 healthy controls revealed the close association between elevated blood zinc levels with a lower incidence of lung cancer [relative ratio (RR) =0.89, 95% confidence interval (CI): 0.79–0.99]. Similarly, Yan et al. (6) found through Mendelian randomization (MR) that zinc reduces the incidence of lung cancer, whereas copper elevates it. Pietrzak et al. (7) demonstrated through a cross-sectional study that blood cadmium levels below 1.47 µg/L may be linked with an increased overall survival rate in stage IA lung cancer patients. Steenland et al. (8) demonstrated the positive relationship between blood lead levels and lung cancer incidence by analyzing cancer incidence in 29,874 lead-exposed workers from Finland (n=20,752) and the UK (n=9,122). However, other studies have shown that certain trace elements (such as copper, magnesium, and selenium) are not linked with lung cancer (9,10). Discrepancies in these findings may arise from the presence of certain potential confounders, such as differences in lung cancer evaluation methods, limited sample size, and distinct methods of trace element detection. These inconsistencies suggest that relying on a single approach is insufficient to comprehensively evaluate the link between blood metal elements and lung cancer.
The National Health and Nutrition Examination Survey (NHANES) is a comprehensive and ongoing cross-sectional research undertaken by the National Center for Health Statistics (NCHS). This survey collects research data every two years to assess public nutrition and health (11). With its large sample sizes, high-quality data, and nationally representative clinical information, NHANES provides a robust foundation for generating reliable evaluation results.
MR offers a valuable approach to overcome the limitations of traditional observational studies by inferring causal association between exposure factors, such as blood metal levels, and outcomes like lung cancer. MR utilizes single nucleotide polymorphisms (SNPs) identified by genome-wide association studies (GWAS) as instrumental variables (IVs) for the assessment of causality between exposure factors and outcomes (12,13). Widely regarded as a complementary method to randomized controlled trials (RCTs) for investigating causal relationships, MR is grounded in Mendel’s second law of independent assortment. This ensures the random distribution of genetic material in cells during meiosis, minimizing the effects of confounding factors and reverse causation deviation in traditional epidemiology (14). In addition, since the impact of genetic variation lasts longer than that of clinical intervention, the real causal effects assessed through MR are more substantial and realistic than those obtained in clinical observational studies (clinical cross-sectional studies and cohort studies) (15,16).
The primary exposure of interest in this study is the metal elements naturally present in the environment. Given the known toxicological properties of these metal elements and their potential to induce oxidative stress and DNA damage, it is biologically plausible that variations in blood metal levels could influence lung cancer development. This study comprehensively examines the association of blood metal elements with lung cancer by integrating a large-scale observational cross-sectional investigation of NHANES data from 1999 to 2018 with a bidirectional two-sample MR analysis. Under certain assumptions, MR may estimate causal effects by minimizing confounding and reverse causality commonly faced in observational studies. We present this article in accordance with the STROBE and STROBE-MR reporting checklists (available at https://tcr.amegroups.com/article/view/10.21037/tcr-24-1430/rc).
Methods
Study design and overview
The study consisted of two parts. First, data from the NHANES were analyzed for elucidating the link of blood metal elements (cadmium, lead, mercury, selenium, manganese, cobalt, copper, zinc, and iron) to lung cancer. Second, a bidirectional two-sample MR analysis was conducted with GWAS pooled statistics to examine potential causal association between blood metal elements and lung cancer as well as its subtypes [small cell lung cancer (SCLC), non-small cell lung cancer (NSCLC), and lung squamous cell carcinoma (LUSC) and lung adenocarcinoma (LUAD) within NSCLC]. The key elements of our study design are detailed in Table S1.
NHANES data sources and study populations
The NHANES is a cross-sectional research of ongoing investigations led by the Centers for Disease Control and Prevention (CDC; https://wwwn.cdc.gov/Nchs/Nhanes/) (17). The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013).
Given the differences in body mass index (BMI) assessments between children, adolescents, and adults, as well as the restriction of lung cancer evaluations in NHANES in only to adults, this study included participants aged 18 years or older who had undergone blood metal assessments. The metals assessed included cadmium, lead, mercury, selenium, manganese, cobalt, copper, zinc, and iron. Participants were excluded according to the following criteria: (I) absence of blood metal element testing; (II) age under 18 years; (III) with unspecified lung cancer; (IV) with missing data on covariates (age, gender, race/ethnicity, education, marriage, and smoking, etc.); (V) pregnant female patients. After applying these inclusion and exclusion criteria, 48,132 participants were finally included.
Definition and assessment of lung cancer in NHANES
Based on methodologies outlined in previously published NHANES studies (11,18), lung cancer diagnosis in this study was determined primarily based on two self-reported questions in medical conditions in NHANES: (I) “Have you ever been told by a doctor or other health professional that you had cancer or a malignancy of any sort?” and (II) “How old were you when you were first informed of lung cancer?”.
Covariates in NHANES
Our study incorporated a variety of covariates, such as participants’ demographic characteristics and lifestyle behaviors. The demographic data included age, gender (male and female), race/ethnicity (Mexican American, non-Hispanic Black, non-Hispanic White, other Hispanic, and other races), education (less than high school, high school or equivalent, and college or above), marital status (married and unmarried), poverty income ratio (PIR) (≤1, >1 to <4 and ≥4), annual household income (AHI) (<$20,000 and ≥$20,000), and survey period (11.01–04.30 and 05.01–10.31). Lifestyle behaviors included BMI (≤25, >25 to <30, and ≥30 kg/m2) and smoking status (never smoking, former smoking, and current smoking).
NHANES blood metal elements
Blood samples were gathered via venipuncture at the NHANES mobile examination center using ethylene diamine tetraacetic acid (EDTA)-coated tubes. The samples were centrifuged on-site, stored at −30 ℃, and then sent to the CDC facility in California for cryopreservation prior to testing. Cadmium, lead, mercury, selenium, manganese, cobalt, copper, and zinc, and selenium in blood were detected via inductive coupled plasma mass spectrometry (ICP-MS). Detailed data declarations are accessible at https://wwwn.cdc.gov/Nchs/Data/Nhanes/Public/2017/DataFiles/PBCD_J.htm. To simultaneously detect various forms of iron, including free iron, bound iron, and other iron compounds, an altered high-performance liquid chromatography with a modified AAII-25 colorimetric method with an Alpkem TFA analyzer (Alpkem, Clackamas, OR, USA) was employed. This approach enhanced the accuracy and sensitivity in quantification (19). For detailed methodologies on blood metal detection and measurement, please refer to the laboratory manual (http://www.cdc.gov/nchs/data/nhanes/nhanes_11_12/PbCd_G_met_blood%20metals.pdf). To ensure the reliability of study results, blood metal elements were extracted and analyzed for data spanning at least two survey cycles. Specific details are illustrated in Table S2.
GWAS data sources utilized in MR analysis
GWAS data on lung cancer and its subtypes (SCLC, NSCLC, and LUSC and LUAD within NSCLC) from Europeans were sourced from the International Lung Cancer Consortium (ILCCO, https://ilcco.iarc.fr/) and the Finnish Genome Research Project (FinnGen, https://www.finngen.fi/fi). GWAS data of lung cancer closely related to the Asian population were acquired from BioBank Japan (BBJ, https://biobankjp.org/en/index.html) (20). However, no GWAS data on lung cancer subtypes were available for the Asian population. GWAS data on blood cadmium levels for the Asian population were retrieved from the PheWeb phenome-wide association study (PheWeb, https://pheweb.jp/downloads) (21), Corresponding GWAS data for the European population were not available. GWAS data on serum iron for Europeans were from the Genetics of Iron Status (GIS) (22). For the Asian population, GWAS data were acquired from the UK Biobank (UKB, http://www.nealelab.is/uk-biobank). Specific information on all GWAS data is provided in Table S3.
Selection of SNPs for MR
To identify genetic variants that may mediate the causal link of exposure factors (cadmium and iron) to outcomes (lung cancer and its subtypes), the genome-wide significance level was P<5e−08 for identifying SNPs significantly related to the exposure factors. If no SNPs met this threshold, the significance level was relaxed to P<1e−05 for further selection. Linkage disequilibrium parameters (r2<0.01, 10,000 kb) were set to ostracize Linkage disequilibrium SNPs, and the F-statistic (F>10) was calculated. Considering the influence of lung cancer risk factors, potential confounding factors were excluded, including gender, age, race/ethnicity, BMI, PIR, survey period, education, household income, smoking, etc. Ensuring that the same covariate set was applied in both samples helps to minimize bias due to confounding and maintains the comparability of the IV estimates across the two datasets.
Statistical analysis
The link of blood metal elements to lung cancer in the NHANES analysis was examined through adjusted logistic regression. Three models were evaluated: Model 1: with no covariate adjustment; Model 2: adjusted for gender, age, race/ethnicity, education, marital status, PIR, and AHI; Model 3: adjusted for all covariates in Model 2, smoking status and BMI. Quantitative variables were handled in their original scales, where blood metal concentrations were typically measured in micrograms per liter (µg/L). The results were presented as odds ratio (OR) and 95% CI. Due to the intricate probabilistic clustering setting of NHANES, weights were also factored into the statistical analysis.
Genetic variants were included in the MR analysis based on their relation to blood metal levels. SNP weights were determined by the strength of their correlation with the exposure in the GWAS data. In the bidirectional two-sample MR analysis, inverse variance weighted (IVW) was used for examining the causality between cadmium and iron levels and lung cancer as well as its subtypes (SCLC, NSCLC, and LUSC and LUAD within NSCLC). IVW validation was enabled through MR Egger, weighted median, and simple mode. Cochrane’s Q, Mendelian randomization-Egger (MR-Egger), MR-pleiotropy residual sum and outlier (MR-PRESSO), and leave-one-out (LOO) analyses were employed for sensitivity testing. The heterogeneity among IVs was assessed via Cochrane’s Q to ensure their consistency in influencing the exposure. P>0.05 indicated no heterogeneity. MR-Egger was used to detect systematic biases in IVs, particularly biases in directional assumptions, and to provide more robust causal inferences (23). MR-PRESSO helped to detect and correct for pleiotropy and outliers, eliminating their influence on MR results (24). Pleiotropy was evaluated via MR-Egger and MR-PRESSO, with P>0.05 indicating no potential pleiotropy of IVs. In addition, LOO analysis was conducted for assessing the impact of each IV on MR estimates and verifying whether any IV has an excessive influence on the results. In summary, these sensitivity analyses boosted the robustness and reliability of our MR results, ensuring that causal associations were not confounded by potential biases.
Every statistical analysis was enabled via R (version 4.1.3), with specific packages including ‘TwoSampleMR’ (version 0.6.2) for MR. Every statistical test was two-sided, and P<0.05 signified statistical significance.
Results
Demographic characteristics of the NHANES study
There were 48,132 patients finally included after screening in accordance with the eligibility criteria, and the screening flowchart is shown in Figure 1. Among the 48,132 eligible participants, only 114 were diagnosed with lung cancer, representing 0.24% of the total. The basic characteristics of the lung cancer participants and the control group are illustrated in Table 1. Overall, statistically significant variations exist in age, race/ethnicity, marital status, family PIR, and smoking status among participants with lung cancer (P<0.05). Lung cancer patients tended to be older (median =71), and lung cancer was concentrated in groups of non-Hispanic White (82.11%), married (90.76%), family PIR >1 to <4 (74.65%), and former smoking (72.85%).
Table 1
| Character | Non-Lung cancer (N=48,018) | Lung cancer (N=114) | P |
|---|---|---|---|
| Age (years) | 50.00 [35.00, 65.00] | 71.00 [64.25, 78.75] | <0.001 |
| Gender | 0.051 | ||
| Male | 23,621 (48.50) | 67 (50.57) | |
| Female | 24,397 (51.50) | 47 (49.43) | |
| Race/ethnicity | <0.001 | ||
| Mexican American | 8,373 (8.12) | 1 (0.22) | |
| Non-Hispanic Black | 3,986 (5.62) | 4 (1.5) | |
| Non-Hispanic White | 21,357 (68.61) | 73 (82.11) | |
| Other Hispanic | 9,856 (10.76) | 25 (11.31) | |
| Other race-including multi-racial | 4,446 (6.89) | 11 (4.85) | |
| Education | 0.24 | ||
| Less than high school | 12,995 (17.25) | 34 (28.98) | |
| High school or equivalent | 11,123 (24.06) | 32 (28.63) | |
| College or above | 23,900 (58.69) | 48 (42.39) | |
| Marital status | 0.002 | ||
| Married | 36,174 (74.83) | 101 (90.76) | |
| Unmarried | 11,844 (25.17) | 13 (9.24) | |
| Family PIR | 0.002 | ||
| ≤1 | 8,935 (13.98) | 17 (11.19) | |
| >1 to <4 | 23,547 (49.58) | 71 (74.65) | |
| ≥4 | 11,363 (36.44) | 13 (14.17) | |
| BMI (kg/m2) | 0.14 | ||
| ≤25 | 13,990 (31.11) | 43 (37.44) | |
| >25 to <30 | 15,968 (33.33) | 35 (30.44) | |
| ≥30 | 17,234 (35.55) | 35 (32.12) | |
| Smoking status | <0.001 | ||
| Never smoking | 25,983 (53.79) | 13 (11.05) | |
| Former smoking | 11,927 (24.66) | 83 (72.85) | |
| Current smoking | 10,108 (21.56) | 18 (16.11) | |
| Survey period | 0.09 | ||
| 11.01–04.30 | 22,517 (42.01) | 44 (38.48) | |
| 05.01–10.31 | 25,501 (57.99) | 70 (61.52) | |
| AHI | 0.10 | ||
| <$20,000 | 9,771 (14.62) | 30 (19.58) | |
| ≥$20,000 | 36,484 (85.38) | 77 (80.42) |
Data are presented as median [IQR] or unweighted counts (weighted %). AHI, annual household income; BMI, body mass index; IQR, interquartile range; NHANES, National Health and Nutrition Examination Survey; PIR, poverty income ratio.
Distribution of blood metal elements
Data distributions for the nine blood metal elements are shown in Table S4. The respective missing rates of blood metal elements ranged from 1.71% to 66.98%, and the data detection rates ranged from 85.09% to 100.00%. To guarantee procedural accuracy, only data above the detection limit were analyzed, so binomial survey-weighted logistic regression was utilized for analysis.
Logistic regression was used to explore the association between blood metal elements and lung cancer
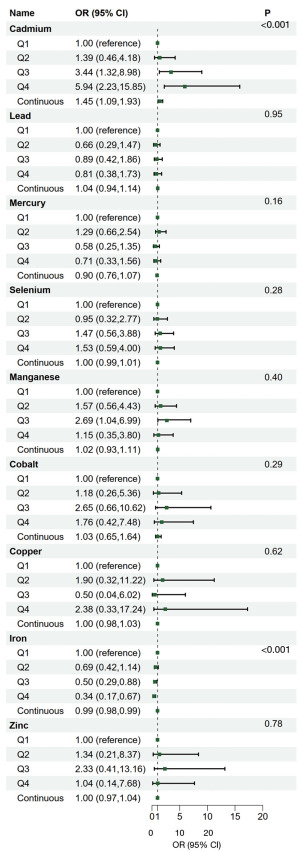
The results of the binomial survey weighted logistic regression between the nine blood metal elements and lung cancer are illustrated in Table S5 and Figure 2. After adjustment for multiple covariates for model 3, the OR (95% CI) for blood cadmium compared to the lowest quartile (Q1) of concentrations in the second (Q2), third (Q3) and fourth (Q4) quartiles of lung cancer participants, were 1.39 (0.46, 4.18), 3.44 (1.32, 8.98), and 5.94 (2.23, 15.85), respectively, with P<0.001. In addition, the OR (95% CI) for lung cancer was 1.45 (1.09, 1.93) per 1 standard deviation increase in blood cadmium concentration, which was consistent with the cadmium quartiles. The OR (95% CI) for blood iron compared to Q1 of concentrations in Q2, Q3 and Q4, were 0.69 (0.42, 1.14), 0.50 (0.29, 0.88), and 0.34 (0.17, 0.67), respectively, with P<0.001. In addition, the OR (95% CI) for lung cancer was 0.99 (0.98, 0.99) per 1 standard deviation increase in blood iron concentration, which was consistent with the iron quartiles.
The non-linear relationships between cadmium, iron and lung cancer was explored via restricted cubic splines (RCS). The RCS results illustrated a nonlinear link of cadmium to lung cancer (P-non-linear <0.05), while the relationship between iron and lung cancer remained linear (P-non-linear >0.05), as presented in Figure 3.

A positive nonlinear association between cadmium and lung cancer was demonstrated, in contrast to the negative linear association noted between iron and lung cancer.
Causal relationship between cadmium and iron in MR and lung cancer
Since a positive link of cadmium to lung cancer was noted in the aforementioned logistic regression analysis, a negative association between serum iron and lung cancer was also observed. Therefore, our MR analysis demonstrated a causal association between blood cadmium or iron and lung cancer. To guarantee the validity of our MR analysis, the genetic variant-exposure associations were derived from robust GWAS studies with large sample sizes, ensuring consistency across the exposure and outcome datasets. In the Asian population, no causal relationship was observed between blood cadmium or iron and lung cancer, as shown in Figure 4A. Reverse MR results also showed no causal association between lung cancer and cadmium or iron, as shown in Figure 4B. In the European population, no causal relationship was found between iron and lung cancer, but a significant causal association was proved between iron and LUSC, a subtype of lung cancer (ORivw =0.77, 95% CI: 0.65–0.92, P=0.004; ORweighted median =0.75, 95% CI: 0.61–0.92, P=0.006), indicating an inverse association between them, as shown in Figure 5A. The reverse MR results indicated no causal association between lung cancer or its subtypes and iron levels, as shown in Figure 5B. Moreover, given that different GWAS datasets were used for the exposure and outcome analyses, the likelihood of significant overlap was minimal or negligible.


Sensitivity analysis of MR
Cochrane’s Q and MR-Egger test results revealed no significant horizontal pleiotropic and heterogeneity (all P>0.05), as shown in Tables S6,S7. The MR-PRESSO global test results indicated no significant pleiotropy effects or outlier SNPs (all P>0.05), as shown in Tables S6,S7. The symmetry of the funnel plot suggested a similar conclusion, as illustrated in Figure S1. LOO analysis further confirmed that causality was not related to any SNP, as shown in Figure S2. Therefore, the MR results are stable and reliable.
Discussion
This study elucidates the relationship between lung cancer and blood metal elements by incorporating the American representative NHANES observational cross-sectional study from 1999 to 2018 and a bidirectional two-sample MR analysis. The NHANES data showed a positive relation of cadmium to lung cancer and a negative association of iron with lung cancer. RCS analysis demonstrated a nonlinear relationship between cadmium and lung cancer and a linear link between iron and lung cancer. Bidirectional two-sample MR analysis confirmed causality between cadmium, iron and lung cancer. Causal association was not noted between blood cadmium or iron and lung cancer in Asians. This is due to the absence of GWAS data on lung cancer subtypes in the Asian population, preventing further confirmation of any causal association between blood cadmium or iron levels and the lung cancer subtypes. An obvious inverse link was proved between iron levels and LUAD in the European population.
Cadmium is a Group I carcinogen classified by the IARC (25), and its high toxicity and slow metabolism in the human body have caused “itai-itai disease” in Japan (26). The current NHANES study demonstrated the positive relationship between cadmium and lung cancer, but similar results were not obtained through MR analysis. Numerous epidemiological studies have demonstrated a strong link cadmium and lung carcinogenesis (27-29). Lener et al. (28) reported a fourfold OR of Q4 versus Q1 for blood cadmium levels (OR =4.41, 95% CI: 2.01–9.67, P<0.01), with this association mainly prevalent in former smokers (OR =16.8, 95% CI: 3.96–71.2, P<0.01). A prospective clinical study by Pietrzak et al. (7) found that blood cadmium levels below 1.47 µg/L may be related to ameliorated survival in stage IA lung cancer patients. In addition, Lee et al. (29) discovered that environmental exposure to cadmium led to elevated risk of lung cancer and poor prognosis. Currently, the mechanisms of cadmium in influencing lung cancer involve various pathways, including oxidative stress induction, inhibition of DNA damage repair, aberrant DNA methylation, suppression of apoptosis, as well as modulation of gene expression (30-33). Hossain et al. (33) demonstrated that prolonged exposure to low doses of cadmium caused hypomethylation of the long interspersed nuclear element-1 (LINE-1) gene, which gives rise to chromosome breaks, deletions, amplifications, translocations, and heterozygous deletions, ultimately promoting tumor progression. Furthermore, Cao et al. (31) noted that cadmium induces apoptosis in bronchial epithelial cells of humans by increasing intracellular reactive oxygen species, causing cellular oxidative stress, and activating the JNK, ERK, and P38MAPK pathways, as well as mitochondria-mediated apoptosis.
The association of dietary iron, serum iron, with lung cancer remains unclear, with no definitive conclusions reached (34-36). A prospective cohort investigation revealed that increased iron consumption was linked to a reduced likelihood of lung cancer [hazard ratio (HR) =0.58, 95% CI: 0.37–0.92, P=0.02] (9). Luan et al. (37) found through clinical studies that serum iron enhances the efficacy of immunosuppressants by improving innate immunity and cytokine release in advanced metastatic cancer, suggesting that serum iron could be utilized as a biomarker to predict the response to immunosuppressants. However, high iron intake raises the incidence of lung cancer. For instance, Sukiennicki et al. (38) discovered that elevated serum iron concentrations were related to an increased incidence of lung cancer compared with lower serum iron concentrations. Zhou et al. (39), through a case-control investigation, illustrated that iron consumption showed a positive link to lung cancer, particularly among current smokers, when combined with smoking history and other potential risk factors. Clinical studies addressing the inconsistency may be limited due to inherent factors, such as small sample size, evaluation at a single point in time, focus on the state of a unit, and the potential impact of dietary iron intake on serum iron levels (34,36). The present study, based on the NHANES database to reduce some of these inherent limitations, found an adverse association of serum iron with lung cancer, suggesting serum iron status as a potential target for the therapy of this cancer.
MR studies have established a causal association between iron and LUAD, which may be attributed to the fact that ferroptosis, an iron-dependent cell death form, is essential in inhibiting the proliferation of lung cancer cells (40,41). Ferroptosis is suppressed in pulmonary cancer, and the proliferation and invasive migration of lung cancer cells are hindered by the activation (42,43). Huang et al. (41) demonstrated that Baihuajiejie injection activates ferroptosis through the Bax/Bcl2/VDAC2/3 pathway, significantly reducing the survival rate of LUAD cells and inhibiting tumor progression. Fu et al. (44) found that circSCUBE3 inhibits the development of LUAD by activating ferroptosis through the CREB/GPX4/GSH axis. In summary, the induction of ferroptosis through various pathways represents a promising therapeutic strategy for LUAD (45). However, MR studies have not established a causal association of ferroptosis with lung cancer, and more research is necessary for elucidating the causality between ferroptosis and lung cancer and its subtypes.
There are notable strengths in this study: (I) it is based on the large-scale observational cross-sectional research from NHANES 1999–2018, which provides a robust representation of Americans; (II) the bidirectional two-sample MR approach uses genetic variants like IVs to minimize reverse causation, eliminate confounding influences, and enhance the robustness of the results; (III) the integration of NHANES data with bidirectional two-sample MR analysis enables the examination of associations and causality between blood metal elements and lung cancer, enhancing the reliability of the conclusions. Nevertheless, this study, like other NHANES and MR studies, does have significant limitations: (I) lung cancer diagnosis in NHANES was based on self-reports from patients, lacking additional diagnostic information (e.g., medical records, cancer registries, or cancer subtype information). This may introduce recall bias and cause limitations to perform subgroup analysis, potentially affecting the accuracy of the findings; (II) although multiple covariates were adjusted in the NHANES observational study, the influence of unmeasured or residual covariates on the results cannot be fully excluded; (III) while the NHANES observational study reveals associations between exposures and outcomes, it cannot establish causality; (IV) the validity of bidirectional MR results may be compromised if the assumptions of association, independence, and no direct effect are not met; (V) individual-level information in GWAS data is limited, and subgroup analyses by age and gender are not possible; (VI) due to the lack of certain GWAS data, such as blood cadmium levels in the European population and lung cancer subtype data in the Asian population, this study only compares MR results for iron and lung cancer between the Asian and European populations, showing no significant differences. However, comparisons of other MR results between the two populations are not possible. Future studies should include the missing GWAS data, re-conduct MR analysis, and further explore potential differences in MR results between the Asian and European populations in greater detail. In conclusion, observational studies, which provide detailed information on short-term exposures or complex multifactorial relationships, complement the bidirectional MR results that are not affected by reverse causality or confounding factors, thereby enhancing the scientific rigor and validity of this study.
Conclusions
The findings of this investigation suggest that iron has preventive effects on lung cancer. RCS analysis revealed a linear association between iron and lung cancer. Although the MR findings did not prove a causal link of iron to lung cancer, an inverse association was noted between iron and LUAD, which was further confirmed by sensitivity analysis. The association between serum iron and lung cancer through additional testing should be further examined.
Acknowledgments
None.
Footnote
Reporting Checklist: The authors have completed the STROBE and STROBE-MR reporting checklists. Available at https://tcr.amegroups.com/article/view/10.21037/tcr-24-1430/rc
Peer Review File: Available at https://tcr.amegroups.com/article/view/10.21037/tcr-24-1430/prf
Funding: This work was supported by
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tcr.amegroups.com/article/view/10.21037/tcr-24-1430/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Bray F, Laversanne M, Sung H, et al. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2024;74:229-63. [Crossref] [PubMed]
- Siegel RL, Giaquinto AN, Jemal A. Cancer statistics, 2024. CA Cancer J Clin 2024;74:12-49. [Crossref] [PubMed]
- Zheng RS, Chen R, Han BF, et al. Cancer incidence and mortality in China, 2022. Zhonghua Zhong Liu Za Zhi 2024;46:221-31. [PubMed]
- Ma Z, Zhu C, Wang H, et al. Association between biological aging and lung cancer risk: Cohort study and Mendelian randomization analysis. iScience 2023;26:106018. [Crossref] [PubMed]
- Bai Y, Wang G, Fu W, et al. Circulating essential metals and lung cancer: Risk assessment and potential molecular effects. Environ Int 2019;127:685-93. [Crossref] [PubMed]
- Yan H, Jin X, Yin L, et al. Investigating Causal Associations of Circulating Micronutrients Concentrations with the Risk of Lung Cancer: A Mendelian Randomization Study. Nutrients 2022;14:4569. [Crossref] [PubMed]
- Pietrzak S, Wójcik J, Baszuk P, et al. Influence of the Levels of Arsenic, Cadmium, Mercury and Lead on Overall Survival in Lung Cancer. Biomolecules 2021;11:1160. [Crossref] [PubMed]
- Steenland K, Barry V, Anttila A, et al. Cancer incidence among workers with blood lead measurements in two countries. Occup Environ Med 2019;76:603-10. [Crossref] [PubMed]
- Muka T, Kraja B, Ruiter R, et al. Dietary mineral intake and lung cancer risk: the Rotterdam Study. Eur J Nutr 2017;56:1637-46. [Crossref] [PubMed]
- Mohammadzadeh M, Bahrami A, Ghafouri-Taleghani F, et al. Dietary iron and the risk of lung cancer. Int J Vitam Nutr Res 2024;94:264-74. [Crossref] [PubMed]
- Huang Q, Wan J, Nan W, et al. Association between manganese exposure in heavy metals mixtures and the prevalence of sarcopenia in US adults from NHANES 2011-2018. J Hazard Mater 2024;464:133005. [Crossref] [PubMed]
- Skrivankova VW, Richmond RC, Woolf BAR, et al. Strengthening the Reporting of Observational Studies in Epidemiology Using Mendelian Randomization: The STROBE-MR Statement. JAMA 2021;326:1614-21. [Crossref] [PubMed]
- Yuan M, He J, Hu X, et al. Hypertension and NAFLD risk: Insights from the NHANES 2017-2018 and Mendelian randomization analyses. Chin Med J (Engl) 2024;137:457-64. [Crossref] [PubMed]
- Pu B, Gu P, Zheng C, et al. Self-reported and genetically predicted effects of coffee intake on rheumatoid arthritis: Epidemiological studies and Mendelian randomization analysis. Front Nutr 2022;9:926190. [Crossref] [PubMed]
- Burgess S, Butterworth A, Malarstig A, et al. Use of Mendelian randomisation to assess potential benefit of clinical intervention. BMJ 2012;345:e7325. [Crossref] [PubMed]
- Skrivankova VW, Richmond RC, Woolf BAR, et al. Strengthening the reporting of observational studies in epidemiology using mendelian randomisation (STROBE-MR): explanation and elaboration. BMJ 2021;375:n2233. [Crossref] [PubMed]
- Zhao E, Cheng Y, Yu C, et al. The systemic immune-inflammation index was non-linear associated with all-cause mortality in individuals with nonalcoholic fatty liver disease. Ann Med 2023;55:2197652. [Crossref] [PubMed]
- Li M, Liu Q, Shi M, et al. Association between remnant cholesterol and the risk of 4 site-specific cancers: evidence from a cross-sectional and Mendelian randomization study. Lipids Health Dis 2024;23:256. [Crossref] [PubMed]
- Tan L, Zhou Q, Liu J, et al. Association of iron status with non-alcoholic fatty liver disease and liver fibrosis in US adults: a cross-sectional study from NHANES 2017-2018. Food Funct 2023;14:5653-62. [Crossref] [PubMed]
- Sakaue S, Kanai M, Tanigawa Y, et al. A cross-population atlas of genetic associations for 220 human phenotypes. Nat Genet 2021;53:1415-24. [Crossref] [PubMed]
- Yang W, Li L, Feng X, et al. Genome-wide association and Mendelian randomization study of blood copper levels and 213 deep phenotypes in humans. Commun Biol 2022;5:405. [Crossref] [PubMed]
- Benyamin B, Esko T, Ried JS, et al. Novel loci affecting iron homeostasis and their effects in individuals at risk for hemochromatosis. Nat Commun 2014;5:4926. [Crossref] [PubMed]
- Burgess S, Thompson SG. Interpreting findings from Mendelian randomization using the MR-Egger method. Eur J Epidemiol 2017;32:377-89. [Crossref] [PubMed]
- Ong JS, MacGregor S. Implementing MR-PRESSO and GCTA-GSMR for pleiotropy assessment in Mendelian randomization studies from a practitioner's perspective. Genet Epidemiol 2019;43:609-16. [Crossref] [PubMed]
- Yang C, Wang Z. The Epitranscriptomic Mechanism of Metal Toxicity and Carcinogenesis. Int J Mol Sci 2022;23:11830. [Crossref] [PubMed]
- Baba H, Tsuneyama K, Yazaki M, et al. The liver in itai-itai disease (chronic cadmium poisoning): pathological features and metallothionein expression. Mod Pathol 2013;26:1228-34. [Crossref] [PubMed]
- Nawrot TS, Martens DS, Hara A, et al. Association of total cancer and lung cancer with environmental exposure to cadmium: the meta-analytical evidence. Cancer Causes Control 2015;26:1281-8. [Crossref] [PubMed]
- Lener MR, Reszka E, Marciniak W, et al. Blood cadmium levels as a marker for early lung cancer detection. J Trace Elem Med Biol 2021;64:126682. [Crossref] [PubMed]
- Lee NW, Wang HY, Du CL, et al. Air-polluted environmental heavy metal exposure increase lung cancer incidence and mortality: A population-based longitudinal cohort study. Sci Total Environ 2022;810:152186. [Crossref] [PubMed]
- Joseph P. Mechanisms of cadmium carcinogenesis. Toxicol Appl Pharmacol 2009;238:272-9. [Crossref] [PubMed]
- Cao X, Fu M, Bi R, et al. Cadmium induced BEAS-2B cells apoptosis and mitochondria damage via MAPK signaling pathway. Chemosphere 2021;263:128346. [Crossref] [PubMed]
- Tan HW, Liang ZL, Yao Y, et al. Lasting DNA Damage and Aberrant DNA Repair Gene Expression Profile Are Associated with Post-Chronic Cadmium Exposure in Human Bronchial Epithelial Cells. Cells 2019;8:842. [Crossref] [PubMed]
- Hossain MB, Vahter M, Concha G, et al. Low-level environmental cadmium exposure is associated with DNA hypomethylation in Argentinean women. Environ Health Perspect 2012;120:879-84. [Crossref] [PubMed]
- Chen HF, Wu LX, Li XF, et al. A meta-analysis of association between serum iron levels and lung cancer risk. Cell Mol Biol (Noisy-le-grand) 2018;64:33-7. [Crossref] [PubMed]
- Yildirim A, Meral M, Kaynar H, et al. Relationship between serum levels of some acute-phase proteins and stage of disease and performance status in patients with lung cancer. Med Sci Monit 2007;13:CR195-200. [PubMed]
- Wang Q, Cui Q, Gao JP, et al. Role of iron biomarkers and iron intakes in lung cancer risk: A systematic review and meta-analysis. J Trace Elem Med Biol 2022;74:127060. [Crossref] [PubMed]
- Luan F, Wang J, Liu L, et al. Serum iron element: A novel biomarker for predicting PD-1 immunotherapy efficacy. Int Immunopharmacol 2024;131:111823. [Crossref] [PubMed]
- Sukiennicki GM, Marciniak W, Muszyńska M, et al. Iron levels, genes involved in iron metabolism and antioxidative processes and lung cancer incidence. PLoS One 2019;14:e0208610. [Crossref] [PubMed]
- Zhou W, Park S, Liu G, et al. Dietary iron, zinc, and calcium and the risk of lung cancer. Epidemiology 2005;16:772-9. [Crossref] [PubMed]
- Gao M, Lai K, Deng Y, et al. Eriocitrin inhibits epithelial-mesenchymal transformation (EMT) in lung adenocarcinoma cells via triggering ferroptosis. Aging (Albany NY) 2023;15:10089-104. [Crossref] [PubMed]
- Huang F, Pang J, Xu L, et al. Hedyotis diffusa injection induces ferroptosis via the Bax/Bcl2/VDAC2/3 axis in lung adenocarcinoma. Phytomedicine 2022;104:154319. [Crossref] [PubMed]
- Chen M, Jiang Y, Sun Y. KDM4A-mediated histone demethylation of SLC7A11 inhibits cell ferroptosis in osteosarcoma. Biochem Biophys Res Commun 2021;550:77-83. [Crossref] [PubMed]
- Liu Y, Fan X, Zhao Z, et al. LncRNA SLC7A11-AS1 Contributes to Lung Cancer Progression Through Facilitating TRAIP Expression by Inhibiting miR-4775. Onco Targets Ther 2020;13:6295-302. [Crossref] [PubMed]
- Fu H, Zhao Q. CircSCUBE3 promoted ferroptosis to inhibit lung adenocarcinoma progression. Cell Mol Biol (Noisy-le-grand) 2024;70:161-8. [Crossref] [PubMed]
- Wang W, Fu F, Huang Z, et al. Inhalable Biomimetic Protein Corona-Mediated Nanoreactor for Self-Amplified Lung Adenocarcinoma Ferroptosis Therapy. ACS Nano 2022;16:8370-87. [Crossref] [PubMed]



