
Will phenotype help guide immunotherapy in prostate cancer?
Over the past decade, immunotherapy has become a pivotal treatment option for advanced cancers. Advances in checkpoint inhibitors have revolutionized the treatment of melanoma, renal and lung cancer, leading to decreased tumor sizes, prolonged survival and durable responses (1). However, immunotherapy has had a less pronounced impact in prostate adenocarcinoma.
There have been some successes. Sipuleucel-T received U.S. Food and Drug Administration (FDA) approval in 2010 as the first autologous cellular vaccine for a solid tumor. Indeed, Sipuleucel-T was designed to target the prostatic acid phosphatase protein in metastatic castrate resistant prostate cancer (mCRPC). While, it marked a milestone in prostate immunotherapy, its modest benefits, restrictive selection criteria and high costs limited its broader use in the mCRPC setting (2). However, there have been many failures. Clinical trials evaluating the role of checkpoint inhibitors, such as PD-1/PD-L1 inhibitors, have been rather disappointing alone or in combination with other agents in the mCRPC setting. Recently, KEYNOTE-641 (3), KEYNOTE-991 (4), KEYNOTE-921 (5), KEYLINK-010 (5), and the IMbassador 250 trial (6) did not meet their primary endpoints and were negative. Indeed, currently only pembrolizumab and dostarlimab, two PD-1 targeted monoclonal antibodies, are approved in prostate cancer, as part of a blanket approval for refractory solid tumors, having deficient mismatch repair (dMMR) or high tumor mutational burden (TMB). Unfortunately, these features are estimated to be a rarity in prostate cancer, representing <5% of the afflicted patient population. In fact, PD-L1 per se, is less expressed in prostate cancer than in other solid tumors. Indeed, in their study, Haffner et al. noted that PD-L1 expression was present in 7.7% of ductal adenocarcinoma, 16.7% of prostatic duct adenocarcinoma and 42.9% of prostatic small cell carcinoma (7). However, these failures further accentuate that the current approach of using immunotherapy in prostate cancer is inadequate, and should be fundamentally reshaped.
Several theories hypothesize that the underlying issue may be at the pathophysiological level. Indeed, prostate adenocarcinoma has been theorized to have an immunologically cold tumor microenvironment (TME) (8). The latter is characterized by decreased levels of T-cells, particularly CD8+ T cells, low antigen loads, high immunosuppressive elements and decreased immunogenicity leading to blunted responses (9). Hence, further study in this sector is warranted.
Dallos et al. (10) recently sought to better understand the immune phenotype of localized prostate cancer through next-generation multiplexed immunofluorescence, DNA and RNA sequencing. They analysed tissue from 28 patients treated with degarelix [10 treated at 4 days, 10 at 7 days, and 8 at 14 days before radical prostatectomy (RP)] and 35 matched untreated controls. Samples were provided from two completed clinical trials using neoadjuvant degarelix therapy before RP. One trial administered degarelix 14 days prior to RP (NCT01696877), while the other randomized patients received degarelix either 4 or 7 days before surgery (NCT01542021). The inclusion criteria encompassed patients with intermediate or high-risk localized prostate adenocarcinoma (clinical stage T1c-T3b, N0-1, M0, and Gleason score ≥4+3). All subjects provided written informed consent, and the studies adhered to Institutional Review Board and Good Clinical Practice guidelines. DNA sequencing was conducted using Memorial Sloan Kettering’s IMPACT tumor sequencing test or Columbia’s Comprehensive Cancer Panel, detecting mutations in 505 and 467 cancer genes, respectively. Macrodissected tumor-rich regions were used for RNA extraction. RNA sequencing was conducted using the Illumina NovaSeq 6,000 system, with quality checks performed by the Agilent Bioanalyzer. A critical component of this study was the use of spatial transcriptomics and multiplexed immunofluorescence to map immune and tumor cell interactions at a tissue level. Unlike traditional bulk sequencing, which provides average gene expression across a tumor sample, spatial transcriptomics preserves the physical arrangement of cells, allowing researchers to see where specific immune or tumor-related genes are activated within the tissue.
The baseline characteristics were considerably different between the groups: the untreated group included a higher percentage of Blacks, 35% vs. 18%, and of Hispanics, 45% vs. 8.3%, whereas lymph node involvement was lower, 14% vs. 50%. The rate of prostate-specific antigen (PSA) recurrence was 50% in patients treated with degarelix compared to 23% in the untreated ones. Genetic analysis revealed evidence for mutations, including TP53, 23%; PIK3CA, 14%; and homologous recombination repair mutations, 24%, of which BRCA2 and ATM are examples. However, the intrinsic nature of this pathological study considerably diminishes the importance of these imbalances.
There were a few major findings from the study. There was an apparent upregulation of immune-related genes at 4 days post-androgen deprivation therapy (ADT) and downregulation of androgen-regulated genes at all points post-ADT. Gene Ontology demonstrated a differential enrichment and activation of immune pathways, favoring the ADT group, 65% vs. 28%. In detail, such enhanced pathways included those related to innate immune responses, leukocyte activation/degranulation, and immune effector processes. No difference between groups was observed with respect to proliferation or apoptosis, suggesting that ADT acts preferentially on immune infiltration rather than tumor kill. Moreover, in the experimental group, an increased expression of immune-related-transcripts was found. This finding was associated with an increase in T cell activity (CD4 and CD8), along with macrophage markers (CD68 and CD163), immune checkpoints (CTLA-4 and PD-L1), and antigen presenting pathways. There was increased expression of both major histocompatibility complex (MHC) class I and II genes. In addition, the anti-phagocytic signal CD47 trended to lower expression post-ADT. The 18-gene tumor inflammation score was higher post-ADT, peaking at 14 days, indicating heightened T cell-inflamed phenotypes.
The CIBERSORT algorithm was used to estimate tumor immune cell type abundance. The increases in post-ADT of both CD3+ and CD3+ CTLA-4+ T cells were significant, with a trend toward higher macrophage infiltration. Increased expression of CD74 on tumor cells, as well as highly significant decreases in CD47 were observed. ADT also changed the spatial relationships among these immune cells in relation to tumor cells. While CD3+ T cells did not show closer proximity to tumor cells after ADT, CD3+CTLA-4+ T cells were closer to tumor cells. In addition, both T cells and macrophages were closer to each other in the post-ADT TME. Specifically, by the CODEX platform, a marked increase in CD4+ and CD8+ T cells was observed, including activated CD8+ granzyme B+ T cells. However, regulatory T cells, responsible for production of immunosuppressive cytokines, also showed upregulation. In addition, macrophage infiltration was higher post-ADT, with a relative increase in M1-like tumor-associated macrophages (TAMs), although there had been no significant change in M2-like TAMs. Besides, tumor cells upregulated both MHC I and II-expressing HLA-A+ and HLA-DR+, which was concordant with previous transcriptomic data.
Interestingly enough, prostate cancer TME is thought to resist checkpoint inhibitor therapies by recruiting suppressive immune cell populations, producing pro-tumorigenic cytokines, and directly inhibiting the immune system through tumor cell activity (8). Immunomodulation to render this TME more responsive should be considered. The IMbassador 250 trial, assessing the role of enzalutamide vs. enzalutamide + atezolizumab, while negative, found that the combination therapy appeared more effective in participants with higher levels of PD-L1 expression and CD8 T cells (6). Hence, targeting the period after initiation of ADT may promote favorable immunomodulation and may serve as an easier way to overcome a cold TME. Hence, a multi-pronged approach relying on timing, the use of multiple agents simultaneously targeting different immune pathways, and pre-selection of patients more likely to respond to immunotherapy through biomarkers, may together help improve immunotherapy in prostate cancer.
Moreover, intervention post-ADT but before castration resistance may be a better target for immunotherapy. Data from mice models suggested that following castration, changes in tumor necrosis factor (TNF) and C-C motif chemokine ligand 2 (CCL2) signaling can lead to basal stem cell stimulation. CCL2 inhibits C-C chemokine receptor type 2 (CCR2), which acts as a chemoattractant for macrophages (8). Hence increases in pro-tumorigenic macrophages, inhibition of phagocytosis, and decreased CD8 T cells can cause tumor immunosuppression promoting recurrence (11). Furthermore, the androgen receptor (AR) can negatively regulate CD8 T cells in mCRPC, thus decreasing response to checkpoint inhibitors like anti-PD-1/PD-L1 agents (12). Though early, a phase I trial of trial of short term ADT with bicalutamide and an anti-CTLA-4 agent, tremelimumab, in PSA recurrent patients demonstrated feasibility and warranted further exploration.
Overall, in their study, Dallos et al. demonstrated that ADT induces significant immune changes in the prostate TME, including increased T cell activation and macrophage infiltration, as well as altered tumor cell immune signaling. These findings suggest that ADT might influence both innate and adaptive immune responses within the TME (Figure 1). ADT rapidly induces transcriptomic and protein-level changes in the prostate TME, enhancing immune activation and altering immune cell composition. This trial also highlights the functional significance of CD47 downregulation in ADT-treated tumors. CD47 acts as a “don’t-eat-me” signal that helps cancer cells evade immune clearance by macrophages. Its reduction following ADT suggests that these tumors may become more vulnerable to macrophage-mediated destruction. Given that CD47-targeting therapies, such as anti-CD47 monoclonal antibodies, are currently in clinical (13), this finding presents a potential therapeutic opportunity. Indeed, combining ADT with CD47 blockade may enhance immune-mediated tumor clearance, offering a novel approach to prostate cancer treatment. Overall, these findings suggest potential for combination therapies, with radiotherapy or chemotherapy alongside immunotherapy, priming immune-related pathways in prostate cancer.
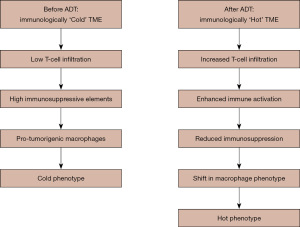
Furthermore, strides in testing new immunotherapy agents, other than anti-PD-1/PD-L1 agents, targeting more common immune pathways specific to prostate cancer may prove beneficial. Finally, ADT was found to induce a progressively more immunologically active environment in the prostate TME within the first days to weeks, characterized by increased immune gene expression, higher tumor inflammation scores, and greater immune cell infiltration. Additionally, this study suggests that initiating immune checkpoint Inhibitors (ICIs) shortly after ADT is started could capitalize on the temporary immune activation triggered by ADT, transforming an otherwise cold prostate cancer TME into a more immunogenic state. This approach may help overcome the historical failures of ICIs in mCRPC. The implications of this should be verified in the clinical setting, by considering ADT timing and immunotherapy in the non-castrate-resistant setting in future trials. Future detailed next-generation studies delving into phenotypical plasticity and impact on therapeutic resistance in the mCRPC setting may also further help guide clinical research (14).
Acknowledgments
None.
Footnote
Provenance and Peer Review: This article was commissioned by the editorial office, Translational Cancer Research. The article has undergone external peer review.
Peer Review File: Available at https://tcr.amegroups.com/article/view/10.21037/tcr-2024-2477/prf
Funding: None.
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at https://tcr.amegroups.com/article/view/10.21037/tcr-2024-2477/coif). S.T. received honoraria from Tolmar, AbbVie, Knight, Bayer, the Canadian Urological Association, and Janssen; and support for attending meetings from Tolmar, Bayer, and Janssen. He also serves as a Data Safety Monitoring Board or Advisory Board for Knight, Sumitomo, and Pfizer. T.N. received grants or contracts from Bayer, Janssen, Astellas, Tersera, and Sanofi Canada; consulting fees from AbbVie, Astellas, Janssen, Tersera, Tolmar, Bayer, AAA, Pfizer, Knight, AstraZeneca, and Sumitomo Pharma; honoraria from Merck, AbbVie, Janssen, Tolmar, AAA, Knight, Astellas, Tersera, Bayer, Pfizer, and AstraZeneca; and support for attending meetings and/or travel from Janssen, Tolmar, Knight, and Bayer. He also serves as the unpaid Chair of the Quebec GU Radiation Oncology Group and Co-Chair of the Canadian GU Radiation Oncology Group. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Shiravand Y, Khodadadi F, Kashani SMA, et al. Immune Checkpoint Inhibitors in Cancer Therapy. Curr Oncol 2022;29:3044-60. [Crossref] [PubMed]
- Caram MEV, Ross R, Lin P, et al. Factors Associated With Use of Sipuleucel-T to Treat Patients With Advanced Prostate Cancer. JAMA Netw Open 2019;2:e192589. [Crossref] [PubMed]
- Merck Sharp & Dohme LLC. A Phase 3, Randomized, Double-blind Trial of Pembrolizumab (MK-3475) Plus Enzalutamide Versus Placebo Plus Enzalutamide in Participants With Metastatic Castration-Resistant Prostate Cancer (mCRPC) (KEYNOTE-641). clinicaltrials.gov; 2023 Nov [cited 2024 Nov 9]. Report No.: NCT03834493. Available online: https://clinicaltrials.gov/study/NCT03834493
- Merck Sharp & Dohme LLC. A Phase 3, Randomized, Double-blind Trial of Pembrolizumab (MK-3475) Plus Enzalutamide Plus ADT Versus Placebo Plus Enzalutamide Plus ADT in Participants With Metastatic Hormone-Sensitive Prostate Cancer (mHSPC) (KEYNOTE-991). clinicaltrials.gov; 2024 May [cited 2024 Nov 9]. Report No.: NCT04191096. Available online: https://clinicaltrials.gov/study/NCT04191096
- Petrylak DP, Ratta R, Matsubara N, et al. Pembrolizumab plus docetaxel for patients with metastatic castration-resistant prostate cancer (mCRPC): Randomized, double-blind, phase 3 KEYNOTE-921 study. J Clin Oncol 2023;41:19. [Crossref]
- Powles T, Yuen KC, Gillessen S, et al. Atezolizumab with enzalutamide versus enzalutamide alone in metastatic castration-resistant prostate cancer: a randomized phase 3 trial. Nat Med 2022;28:144-53. [Crossref] [PubMed]
- Haffner MC, Guner G, Taheri D, et al. Comprehensive Evaluation of Programmed Death-Ligand 1 Expression in Primary and Metastatic Prostate Cancer. Am J Pathol 2018;188:1478-85. [Crossref] [PubMed]
- Stultz J, Fong L. How to turn up the heat on the cold immune microenvironment of metastatic prostate cancer. Prostate Cancer Prostatic Dis 2021;24:697-717. [Crossref] [PubMed]
- Gulley J. Immunotherapy for castration-resistant prostate cancer - UpToDate. [cited 2024 Nov 9]. Available online: https://www.uptodate.com/contents/immunotherapy-for-castration-resistant-prostate-cancer
- Dallos MC, Obradovic AZ, McCann P, et al. Androgen Deprivation Therapy Drives a Distinct Immune Phenotype in Localized Prostate Cancer. Clin Cancer Res 2024;30:5218-30. [Crossref] [PubMed]
- Sha K, Zhang R, Maolake A, et al. Androgen deprivation triggers a cytokine signaling switch to induce immune suppression and prostate cancer recurrence. eLife 2024; [Crossref] [PubMed]
- Maselli FM, Giuliani F, Laface C, et al. Immunotherapy in Prostate Cancer: State of Art and New Therapeutic Perspectives. Curr Oncol 2023;30:5769-94. [Crossref] [PubMed]
- Murata Y, Saito Y, Kotani T, et al. CD47-signal regulatory protein α signaling system and its application to cancer immunotherapy. Cancer Sci 2018;109:2349-57. [Crossref] [PubMed]
- De Velasco MA, Tanaka M, Yamamoto Y, et al. Androgen deprivation induces phenotypic plasticity and promotes resistance to molecular targeted therapy in a PTEN-deficient mouse model of prostate cancer. Carcinogenesis 2014;35:2142-53. [Crossref] [PubMed]


