
Role of TRKC and NT-3 proteins in the progression and prognosis of colorectal cancer
Highlight box
Key findings
• The tropomyosin receptor kinase C (TRKC) and neurotrophic factor-3 (NT-3) proteins are closely associated with the occurrence, metastasis, infiltration, and tumor marker levels of colorectal cancer (CRC), and can be used as independent predictors of prognosis in patients with CRC. The high expression of TRKC in patients with CRC was correlated with a poor prognosis, while the high expression of NT-3 was correlated with a good prognosis.
What is known, and what is new?
• CRC is a highly prevalent malignant tumor worldwide, with the fourth highest incidence rate and the second highest mortality rate. TRKC is a member of the tyrosine kinase receptor (TRK) family of neurotrophin receptors, and is implicated in the growth and survival of human cancer tissues. NT-3 has the highest affinity for TRKC and is its only ligand. Neurotrophic factors and their corresponding receptors have been shown to induce a variety of pleiotropic responses in malignant cells, including enhancing tumor invasiveness and chemotaxis. However, few studies have examined the link between TRKC and NT-3 proteins and CRC.
• This study examined the correlation between the expression levels of TRKC and NT-3 proteins, and the clinicopathological features and prognosis of CRC patients.
What is the implication, and what should change now?
• TRKC and NT-3 may become new molecular markers through the introduction of this study. We will perform cell and animal experiments to explore the specific regulatory mechanisms by which TRKC and NT-3 protein expression affects CRC.
Introduction
Colorectal cancer (CRC) is a common digestive system tumor. In recent years, CRC has had the fourth highest incidence and second highest mortality rate among tumors worldwide (1). Further, in recent years, the incidence of CRC in patients aged <55 years has been increasing at a rate of 1–2% per year, and CRC is now the leading cause of cancer-related deaths in men aged <50 years and the second leading cause of cancer-related deaths in women (1).
In addition to traditional tumor node metastasis (TNM) staging, histologic classification, grading, and the histologic evaluation of lymphatic, perineural, and venous infiltrates, the value of tumor markers (including mismatch repair assays and immuno-scoring) is increasingly recognized (2-6). Clinical trials have shown that customized treatment for the molecular and pathological characteristics of tumors can improve the overall survival (OS) rate (7). Thus, exploring new molecular markers, and studying the correlation between these markers, and the clinical pathological characteristics and prognosis of CRC could lead to developments in the diagnosis and treatment of CRC.
Tyrosine kinase receptor (TRK) was originally identified from colon cancer-derived oncogenes. TRK is a single-transmembrane receptor protein that contains an extracellular ligand-binding domain, a transmembrane domain, and an intracellular domain with kinase activity; and is capable of fusing with another tyrosine kinase domain (8-10). TRK proteins have been identified as members of tropomyosin family that fuse to the tyrosine kinase domain (11). Tropomyosin receptor kinases A, B, and C (TRKA, TRKB, and TRKC) belong to the cell surface receptor tyrosine kinase family that is encoded by neurotrophic receptor tyrosine kinases 1, 2, and 3 (NTRK 1, NTRK 2, and NTRK 3) genes (12). TKR is a central regulator of signaling pathways that control differentiation, proliferation, motility, and invasion (13). TRKC is a high-affinity transmembrane receptor encoded by NTRK 3, which binds to neurotrophic factor-3 (NT-3), while TRKC is a receptor for NT-3 and is rarely activated by other ligands (8,9,11,14).
NT-3 (15) is a soluble small molecule protein of the neurotrophic factor family that regulates the proliferation and regeneration of various nerve cells, and enhances the proliferation of various tumor cells (16,17). Many studies have shown that NT-3 and its receptor TRKC are overexpressed in a variety of cancers, including pancreatic cancer (18,19), colon carcinoma (20), lung cancer (21), and salivary adenoid cystic carcinoma (22). TRKC plays an important role in inducing tumor invasion and malignant cell chemotaxis, regulating angiogenesis, inducing tumor growth, preventing cell apoptosis, and promoting metastasis (14,23-26). The specific activation of the TRKC receptor by the ligand NT-3 enhances the viability of tumor-initiating cells (27). The expression of NT-3 and its receptor TRKC have been shown to be enhanced in lung tumor spheres (21).
NT-3 silencing inhibits the migration and anchorage-independent growth of lung cancer cells, while NT-3 overexpression promotes migration and anchorage-independent growth, and facilitates tumor sphere formation via the upregulation of the expression of cancer stem cell markers (21). Therefore, TRKC and its ligand NT-3 are thought to play a significant role in a variety of cancers (13). Currently, mRNA expression of TRKB and TRKC has been found to be higher in CRC tissues than in noncancerous tissues, and it has been elucidated that TRKB and TRKC promote tumor progression and metastasis by increasing the ability of cells to grow or to invade, and that they inhibit apoptosis in CRC (28). Moreover, TRKC can lead to dysregulation of cellular growth, alteration of cellular behaviors and functions, and enhanced metastasis in CRC; TRKC controls tumorigenicity and metastasis in CRC (23).
In this study, we examined the expression of the TRKC and NT-3 proteins in CRC cancer tissues and adjacent normal tissues, and analyzed the differences between them. We sought to investigate the relationship between their expression in the CRC tissues and the clinicopathological features and prognosis of patients, and to evaluate the prognosis of CRC patients. We present this article in accordance with the REMARK reporting checklist (available at https://tcr.amegroups.com/article/view/10.21037/tcr-2025-519/rc).
Methods
Study subjects
Immunohistochemistry clinical samples were obtained from 141 patients undergoing radical CRC surgery at Dazhou Central Hospital from January 2017 to December 2018. The end of surgery was used as the start of the follow-up period, which ended in August 2020. The clinical endpoint was the death of a patient from CRC or the end of the follow-up period. The median follow-up time was 1,625 days.
To be eligible for inclusion in the study, the patients had to meet the following inclusion criteria: (I) have a primary diagnosis of CRC; (II) have postoperative pathological results confirming CRC, and no cancer cell infiltration in the normal tissue; (III) have complete clinicopathological data; and (IV) have not undergone preoperative surgery, chemoradiotherapy, immunotherapy, or other anti-tumor therapy. Patients were excluded from the study if they met any of the following exclusion criteria: (I) died due to other causes; (II) had other tumors at the same time.
The study was conducted in accordance with the Declaration of Helsinki (revised 2013). The study was approved by the Ethics Board of Dazhou Central Hospital (Ethics Board registration number: 010) and informed consent was taken from all the patients.
Experimental methods
Selected wax blocks were serially sliced at 3 microns, baked at 60 centigrade for 1 hour, Xylene dewaxing, alcohol debenzening, pH=6.0 citrate antigen repair solution high-pressure repair antigen, blocking the endogenous peroxidase, by 3%H2O2 at room temperature for 15 min, blocking serum at room temperature for 30 min, diluting primary antibody to 1:250, incubating at 4 ℃ overnight, rewarming for 20 minutes, incubating the reaction enhancement solution and secondary antibody for 30 min at room temperature, DAB (3,3’-Diaminobenzidine) staining for 5 min, counterstaining with hematoxylin for 3 min, hydrochloric acid alcohol to turn red, Ammonia reverse blue, after dehydration with anhydrous alcohol, placed in xylene immersion, finally cover the piece. Phosphate buffered saline was used instead of the primary antibody as the negative control.
Immunohistochemical section scoring
The total immunohistochemistry score was calculated by multiplying the number of positively expressed cells with the coloration intensity of the positively expressed cells, and a total score of <3 was recorded as negative expression, while a total score of ≥3 was recorded as positive expression. The positive expressing cells were observed under a high-power field (×100) and scored as follows: 0 points: <5% positive cells; 1 point: 5–10% positive cells; 2 points: 11–50% positive cells; 3 points: 51–80% positive cells; and 4 points: >80% positive cells. The coloration intensity of the positively expressed cells was scored as follows: 0 points: no positive expression coloration of the cytoplasm; 1 point: pale-yellow coloration; 2 points: brownish-yellow coloration; and 3 points: sepia coloration. Each section was independently scored by two experienced pathologists (9).
Statistical methods
The statistical analysis was performed using SPSS26.0 and GraphPad Prism9 software. The measurement data are expressed as the mean ± standard deviation. The Shapiro-Wilk test was used to examine the distribution of the data. The t-test was used to compare the normally distributed data between two groups. The Mann-Whitney test was used to compare the non-normally distributed data between two groups. The Chi-square test was used to analyze the relationship between the TRKC and NT-3 expression levels and clinicopathological features. The Kaplan-Meier method was used to analyze the relationship between the TRKC and NT-3 expression levels and CRC prognosis. The Cox proportional hazards regression model was used to analyze the factors affecting prognosis. A P value <0.05 was considered statistically significant (22).
Results
General
Ultimately, 141 specimens were collected, 84 from males and 57 from females. The following clinicopathological information was also collected: age, gender, smoking, degree of differentiation, tumor location, tumor size, depth of invasion, lymph node metastasis, distant metastasis, TNM stage, and carcinoembryonic antigen (CEA), carbohydrate antigen 125, and carbohydrate antigen 19-9 (CA199) expression levels.
TRKC expression profile
Immunohistochemistry was used to detect the expression of TRKC in the cancer tissues and adjacent normal tissues of the CRC patients. The results showed that the expression level of TRKC was significantly higher in the CRC cancer tissues than the adjacent normal tissues, and the difference was statistically significant (P<0.0001) (Figure 1). The left image in Figure 1A shows the immunohistochemistry results for TRKC expression in CRC adjacent normal tissue, while the right image shows the immunohistochemistry results for TRKC expression in cancer tissue.
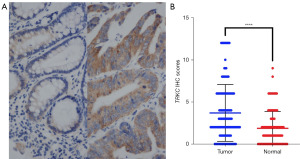
Association between TRKC expression and the clinicopathological features of the patients
The Chi-square test was used to analyze the relationship between TRKC expression and the clinicopathological characteristics of patients, and it was found that the expression of TRKC was correlated with tumor location (P<0.05), lymph node metastasis (P<0.05), TNM stage (P<0.01), serum CEA level (P<0.01) and serum CA199 level (P<0.05) (Table 1).
Table 1
| Pathological characteristics | Positive expression (n=71), n (%) | Negative expression (n=70), n (%) | P |
|---|---|---|---|
| Gender | 0.20 | ||
| Male | 46 (64.79) | 38 (54.29) | |
| Female | 25 (35.21) | 32 (45.71) | |
| Age (years) | 0.45 | ||
| <60 | 24 (33.80) | 28 (40.00) | |
| ≥60 | 47 (66.20) | 42 (60.00) | |
| Degree of differentiation | 0.45 | ||
| High differentiation | 3 (4.23) | 4 (5.71) | |
| Medium differentiation | 66 (92.96) | 61 (87.14) | |
| Low differentiation | 2 (2.82) | 5 (7.14) | |
| Tumor location | 0.04 | ||
| Right hemicolon | 10 (14.08) | 20 (28.57) | |
| Left hemicolon | 61 (85.92) | 50 (71.43) | |
| Smoking | 0.26 | ||
| No | 34 (47.89) | 27 (38.57) | |
| Yes | 37 (52.11) | 43 (61.43) | |
| Tumor size (cm) | 0.53 | ||
| <5 | 43 (47.89) | 46 (38.57) | |
| ≥5 | 28 (52.11) | 24 (61.43) | |
| Infiltration depth | 0.34 | ||
| Muscle layer and within | 20 (47.89) | 25 (38.57) | |
| Outside the muscle layer | 51 (52.11) | 45 (61.43) | |
| Lymph node metastasis | 0.01 | ||
| No | 42 (47.89) | 55 (38.57) | |
| Yes | 29 (52.11) | 15 (61.43) | |
| Distant metastasis | 0.15 | ||
| No | 65 (47.89) | 68 (38.57) | |
| Yes | 6 (52.11) | 2 (61.43) | |
| TNM | 0.008 | ||
| I + II | 41 (47.89) | 55 (38.57) | |
| III + IV | 30 (52.11) | 15 (61.43) | |
| CEA (μg/L) | 0.002 | ||
| <5 | 29 (47.89) | 47 (38.57) | |
| ≥5 | 42 (52.11) | 23 (61.43) | |
| CA199 (U/mL) | 0.01 | ||
| <27 | 43 (47.89) | 56 (38.57) | |
| ≥27 | 28 (52.11) | 14 (61.43) | |
| CA125 (U/mL) | 0.77 | ||
| <35 | 65 (47.89) | 65 (38.57) | |
| ≥35 | 6 (52.11) | 5 (61.43) |
CRC, colorectal cancer; CEA, carcinoembryonic antigen; CA199, carbohydrate antigen 19-9; CA125, carbohydrate antigen 125; TNM, tumor, node, and metastasis; TRKC, tropomyosin receptor kinase C.
The relationship between TRKC expression and patient prognosis
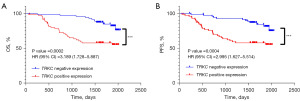
In the Kaplan-Meier survival analysis based on the follow-up information of the CRC patients, the OS time and progression-free survival (PFS) time in the TRKC-positive expression group were lower than those in the TRKC-negative expression group (P<0.001) (Figure 2).
NT-3 expression profile
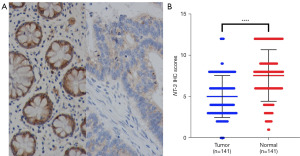
The expression of NT-3 in the CRC tissues and adjacent normal tissues was detected by immunohistochemistry, and the results showed that the expression level of NT-3 was significantly lower in the CRC tissues than the adjacent normal tissues, and the difference was statistically significant (P<0.0001) (Figure 3). The left panel of Figure 3A shows the immunohistochemical results of NT-3 expression in the normal tissues adjacent to the CRC tissues, and the right panel shows the immunohistochemical results of NT-3 expression in the cancer tissues.
Association between NT-3 expression and the clinicopathological features of patients
The Chi-square test was used to analyze the relationship between NT-3 expression and the clinicopathological characteristics of the patients. The results showed that the expression of NT-3 was correlated with lymph node metastasis (P<0.05), distant metastasis (P<0.05), TNM stage (P<0.05) and serum CEA level (P<0.05) (Table 2).
Table 2
| Pathological characteristics | Positive expression (n=91), n (%) | Negative expression (n=50), n (%) | P |
|---|---|---|---|
| Gender | 0.66 | ||
| Male | 53 (58.24) | 31 (62.00) | |
| Female | 38 (41.76) | 19 (38.00) | |
| Age (years) | 0.87 | ||
| <60 | 34 (37.36) | 18 (36.00) | |
| ≥60 | 57 (62.64) | 32 (64.00) | |
| Degree of differentiation | 0.85 | ||
| High differentiation | 5 (5.49) | 2 (4.00) | |
| Medium differentiation | 81 (89.01) | 46 (92.00) | |
| Low differentiation | 5 (5.49) | 2 (4.00) | |
| Tumor location | 0.56 | ||
| Right hemicolon | 18 (19.78) | 12 (24.00) | |
| Left hemicolon | 73 (80.22) | 38 (76.00) | |
| Smoking | 0.82 | ||
| No | 40 (43.96) | 21 (42.00) | |
| Yes | 51 (56.04) | 29 (58.00) | |
| Tumor size (cm) | 0.87 | ||
| <5 | 57 (62.64) | 32 (64.00) | |
| ≥5 | 34 (37.36) | 18 (36.00) | |
| Infiltration depth | 0.06 | ||
| Muscle layer and within | 34 (37.36) | 11 (22.00) | |
| Outside the muscle layer | 57 (62.64) | 39 (78.00) | |
| Lymph node metastasis | 0.04 | ||
| No | 68 (74.73) | 29 (58.00) | |
| Yes | 23 (25.27) | 21 (42.00) | |
| Distant metastasis | 0.02 | ||
| No | 89 (97.80) | 44 (88.00) | |
| Yes | 2 (2.20) | 6 (12.00) | |
| TNM | 0.02 | ||
| I + II | 68 (74.73) | 28 (56.00) | |
| III + IV | 23 (25.27) | 22 (44.00) | |
| CEA (μg/L) | 0.03 | ||
| <5 | 43 (47.25) | 33 (66.00) | |
| ≥5 | 48 (52.75) | 17 (34.00) | |
| CA199 (U/mL) | 0.73 | ||
| <27 | 63 (69.23) | 36 (72.00) | |
| ≥27 | 28 (30.77) | 14 (28.00) | |
| CA125 (U/mL) | 0.17 | ||
| <35 | 86 (94.51) | 44 (88.00) | |
| ≥35 | 5 (5.49) | 6 (12.00) |
CEA, carcinoembryonic antigen; CA199, carbohydrate antigen 19-9; CA125, carbohydrate antigen 125; CRC, colorectal cancer; NT-3, neurotrophic factor-3; TNM, tumor, node, and metastasis.
The relationship between NT-3 expression and patient prognosis
A Kaplan-Meier survival analysis was performed based on the follow-up information of the CRC patients, and it was found that the OS time and PFS time of the patients in the NT-3-positive expression group were higher than those in the NT-3-negative expression group (P<0.01) (Figure 4).

A Cox proportional hazards regression model for CRC patients
The clinicopathologic characteristics of the CRC patients, and the expression of TRKC and NT-3 detected by immunohistochemistry were collected and analyzed univariately using Cox proportional hazards regression models (Table 3). The results showed that gender [hazard ratio (HR) =0.441, P<0.05], age (HR =2.350, P<0.05), smoking (HR =2.067, P<0.05), lymph node metastasis (HR =2.439, P<0.01), distant metastasis (HR =4.650, P<0.01), TNM stage (HR =2.639, P<0.01), serum CA199 (HR =2.738, P<0.01), TRKC expression (HR =3.316, P<0.0001), and NT-3 expression (HR =0.428, P<0.01) were associated with prognosis. The statistically significant indicators in the univariate analysis were then incorporated into a multivariate analysis model (Table 4), and the results showed that TRKC expression (HR =2.679, P<0.01) and NT-3 expression (HR =0.433, P<0.05) were independent predictors of prognosis in the CRC patients.
Table 3
| Clinicopathologic features | HR (95% CI) | P |
|---|---|---|
| Age | 2.350 (1.124–4.913) | 0.02 |
| Gender | 0.441 (0.217–0.897) | 0.02 |
| Smoking | 2.067 (1.116–3.830) | 0.02 |
| Tumor location | 0.767 (0.385–1.527) | 0.45 |
| Degree of differentiation | 1.480 (0.568–3.854) | 0.42 |
| Tumor size | 1.053 (0.565–1.962) | 0.87 |
| Infiltration depth | 2.089 (0.967–4.514) | 0.06 |
| Lymph node metastasis | 2.439 (1.329–4.475) | 0.004 |
| Distant metastasis | 4.650 (1.932–11.191) | 0.001 |
| TNM stage | 2.639 (1.439–4.841) | 0.002 |
| CEA | 1.353 (0.738–2.480) | 0.33 |
| CA199 | 2.738 (1.336–5.609) | 0.006 |
| CA125 | 0.997 (0.308–3.228) | 0.995 |
| TRKC expression | 3.316 (1.694–6.490) | <0.001 |
| NT-3 expression | 0.428 (0.233–0.785) | 0.006 |
CEA, carcinoembryonic antigen; CA199, carbohydrate antigen 19-9; CA125, carbohydrate antigen 125; CI, confidence interval; CRC, colorectal cancer; HR, hazard ratio; NT-3, neurotrophic factor-3; TNM, tumor, node, and metastasis; TRKC, tropomyosin receptor kinase C.
Table 4
| Clinicopathologic features | HR (95% CI) | P |
|---|---|---|
| Gender | 0.698 (0.253–1.929) | 0.49 |
| Age | 1.989 (0.929–4.256) | 0.08 |
| Smoking | 1.349 (0.561–3.243) | 0.50 |
| Lymph node metastasis | 1.226 (0.139–10.779) | 0.85 |
| Distant metastasis | 1.490 (0.492–4.510) | 0.48 |
| TNM stage | 1.351 (0.135–13.506) | 0.80 |
| CA199 | 1.407 (0.710–2.788) | 0.33 |
| NT-3 expression | 0.433 (0.223–0.838) | 0.01 |
| TRKC expression | 2.679 (1.266–5.672) | 0.003 |
CRC, colorectal cancer; CI, confidence interval; CA199, carbohydrate antigen 19-9; HR, hazard ratio; NT-3, neurotrophic factor-3; TNM, tumor, node, and metastasis; TRKC, tropomyosin receptor kinase C.
Discussion
CRC is a modern disease and has the highest incidence in developed countries (29). The incidence of CRC is likely to increase as the world becomes more affluent, and more people adopt Western diets and lifestyles (29). The pathogenesis of CRC involves genetic alterations that disrupt DNA repair mechanisms, leading to the formation of abnormal crypts in the colon that further lead to adenomatous polyps or serrated polyps, and ultimately colorectal tumors (30).
Screening is the mainstay of prevention and early detection of CRC; however, due to the widespread belief that cancer is associated with aging, only individuals aged 50–60 years and older are eligible for regular screening, and in younger patients, characteristic symptoms, such as blood in the stool and abdominal pain, are often overlooked (31).
In an epidemiologic study, males and advancing age have been consistently shown to have a strong association with disease incidence (2). One analysis provided strong evidence that men are at greater risk of developing advanced colorectal tumors in all age groups, in comparison to women (32). Prognostic factors of CRC mainly include treatment-related prognostic factors and tumor-related prognostic factors (33). The prognosis of patients with CRC depends on the treatment, and the quality of surgery and pathology can be assessed by the number of lymph nodes removed (33). The assessment of specimen integrity in rectal cancer surgery is a valuable surgical quality control method with proven prognostic results (33,34).
TNM staging at diagnosis is the most important prognostic factor. It provides valuable prognostic information and guides therapeutic decisions, but it cannot predict the response to and outcome of treatment in individual patients (35). To overcome this limitation, the molecular characterization of tumors has been recommended, and many potential molecular prognostic markers have been identified, such as: Kirsten rat sarcoma viral oncogene homolog (k-ras), myelocytomatosis oncogene (c-myc), B-cell lymphoma-2 (bcl-2) and transforming growth factor (TGF) (36). All guidelines recommend that in addition to performing a complete blood count at the time of diagnosis, the laboratory check the CEA level (37). Elevated baseline CEA concentrations are associated with a poor prognosis, and concentrations that do not return to normal after surgery may indicate residual disease (2).
Smoking is an established risk factor for colorectal adenomas, as well as for CRC morbidity and mortality, which suggests that smoking may also affect the prognosis of patients with CRC (38). Various factors have been identified that affect CRC prognosis, and many of these molecular markers have also been recognized to affect CRC prognosis, but fewer studies have been conducted on TRKC and NT-3 proteins and the prognosis of CRC. This study sought to investigate whether the expression of TRKC and NT-3 was correlated with the clinicopathologic characteristics, such as age, gender, smoking, and TNM stage, of CRC patients, and whether they can serve as an independent predictor of CRC prognosis.
The TRK family of receptor tyrosine kinases encoded by the NTRK gene are initially synthesized as precursor proteins; the post-translational glycosylation of the extracellular structural domains of these precursors produces the mature protein products TRKA (140 kDa), TRKB (145 kDa), and TRKC (145 kDa) (39-41). The TRK receptor family plays an important role in neuronal growth and development; however, aberrant signaling by TRK proteins has been associated with a variety of malignancies (39). Fusions of NTRK proteins have been identified in a variety of solid tumors, including lung tumors, gastrointestinal tumors, thyroid tumors, primary brain tumors, sarcomas, and acute myeloid leukemia (42-44). Somatic NTRK mutations have also been identified in a variety of tumor types, including CRC (40,41). The abnormal expression of TRK and the enhanced expression of various neurotrophic factors have also been found to be associated with the development of human prostate cancer and pancreatic ductal adenocarcinoma (42). There is also growing evidence that TRK oncogene rearrangements are common in non-neuronal tumors, such as colon and papillary thyroid carcinomas, and that the receptor signaling encoded by the proto-oncogene TRK regulates growth, differentiation, and apoptosis in neuron-originated tumors, such as neuroblastoma and medulloblastoma (43).
TRKC, a member of the TRK family, is associated with human cancer tissue growth and survival (44). TRKC is also a potent oncoprotein expressed in tumors of multiple cell line origins, and it functions as an active protein tyrosine kinase via NT-3 (44). NT-3 binds to all three TRK receptors but has the highest affinity for TRKC, and is its only ligand (45). Neurotrophic factors and their corresponding receptors have been shown to induce multiple pleiotropic responses in malignant cells, including enhanced tumor invasiveness and chemotaxis (44). In addition, neurotrophic factors and their receptors are important in regulating angiogenic and pro-mitotic signals that promote tumor growth, and prevent apoptosis, cell spreading, and metastasis (44,46-49).
The ligand of the TRK family is neurotrophin; and TRKC binds to NT-3 (50). TRK/NT-3 co-expression has been observed in many CRC specimens (28,51,52). Further, research has shown that TRKC promotes tumor progression and metastasis by increasing the cell growth or invasion capacity, and inhibits apoptosis in CRC (23,28). A previous study has reported that TRKC expression is more strongly upregulated in CRC cancer tissues than normal tissues, and TRKC can lead to the dysregulation of cell growth, alter cellular behavior and function, and control tumorigenicity and enhance metastasis in CRC (23). In this study, we similarly found that the expression level of the TRKC protein was significantly higher in the CRC cancer tissues than the normal adjacent tissues. We analyzed the relationship between TRKC expression and the clinicopathological characteristics of the patients, and found that TRKC expression was correlated with the tumor site (P<0.05), lymph node metastasis (P<0.05), TNM stage (P<0.01), serum CEA level (P<0.01), and serum CA199 level (P<0.01). The up-regulation of TRKC may play a role in the development of CRC, and the expression of TRKC may contribute to the diagnosis of CRC. However, this study did not examine the relevant conduction pathways.
NT-3 and its receptor TRKC have been shown to play an important role in cancer, and both NT-3 and TRKC have been found to be expressed in various cancers (24,53). However, the expression level of the NT-3 protein was significantly lower in the CRC cancer tissues than the normal adjacent tissues in this study. We analyzed the relationship between NT-3 expression and the clinicopathological characteristics of patients, and found that NT-3 expression was correlated with lymph node metastasis (P<0.05), distant metastasis (P<0.05), TNM stage (P<0.05), and serum CEA level (P<0.05). Thus, it can be inferred that the positive expression of NT-3 in the CRC tissues inhibits tumor growth, proliferation and metastasis, and the upregulation of its expression has some positive significance for the development and prognosis of CRC.
TRKC has different prognostic significance for different cells and different tumors. For example, the high expression of TRKC messenger RNA in primitive neuroectodermal brain tumors has been shown to be a strong independent predictor of good clinical outcomes (54). In medulloblastoma, TRKC expression was found to be associated with a favorable prognosis (55,56). In this study, it was found that the patients in the TRKC-positive expression group had lower OS and PFS times than those in the TRKC-negative expression group (P<0.001). This finding suggests that the high expression of TRKC in CRC is associated with a poor prognosis. The results of the univariate and multivariate analyses of the Cox proportional risk regression model showed the result of TRKC expression (HR =2.679, P<0.01), further confirming that TRKC can be used as an independent predictor of the prognosis of CRC patients. High TRKC expression in CRC patients implies a poor prognosis. This is because the high expression of TRKC results in abnormal signaling and promotes tumor growth, infiltration, and metastasis, thus affecting the prognosis of CRC patients.
It has been shown that NT-3 overexpression promotes the formation of tumor spheres in lung cancer, and that NT-3 and NT-3/TRKC decrease the survival of lung cancer patients (21). A study has suggested that NT-3 expression in neuroblastoma has clinical significance independent of TRKC expression, and that its prognostic significance depends on the status of TRKC expression. NT-3 expression in the presence of its primary receptor, TRKC, has been shown to be correlated with a significantly better PFS time (57). However, this study found that NT-3 expression was lower in the cancer tissues than the normal adjacent tissues of CRC patients, and the OS and DFS times of the patients in the NT-3-positive expression group were higher than those in the NT-3-negative expression group (P<0.01). Further, NT-3 expression (HR =0.433, P<0.05) was examined in the Cox regression model analysis, and the results indicated that NT-3-positive expression favors the prognosis of CRC.
However, this study has some limitations: firstly it is a retrospective study, it has a small number of cases and a short follow-up period. We will next collect more case data to complete further studies, such as exploring the signaling pathways and regulatory mechanisms of TRKC and NT-3 expression in CRC patients.
Conclusions
In summary, this study found that the TRKC and NT-3 proteins are closely associated with CRC occurrence, metastasis, infiltration, and tumor marker levels, and thus can be used as independent predictors of prognosis in CRC patients. The high expression of TRKC in CRC patients is correlated with a poor prognosis in patients, while the high expression of NT-3 is correlated with a good prognosis in patients.
Acknowledgments
None.
Footnote
Reporting Checklist: The authors have completed the REMARK reporting checklist. Available at https://tcr.amegroups.com/article/view/10.21037/tcr-2025-519/rc
Data Sharing Statement: Available at https://tcr.amegroups.com/article/view/10.21037/tcr-2025-519/dss
Peer Review File: Available at https://tcr.amegroups.com/article/view/10.21037/tcr-2025-519/prf
Funding: This study was supported by
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tcr.amegroups.com/article/view/10.21037/tcr-2025-519/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (revised 2013). The study was approved by the Ethics Board of Dazhou Central Hospital (Ethics Board registration number: 010) and informed consent was taken from all the patients.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Siegel RL, Giaquinto AN, Jemal A. Cancer statistics, 2024. CA Cancer J Clin 2024;74:12-49. [Crossref] [PubMed]
- Dekker E, Tanis PJ, Vleugels JLA, et al. Colorectal cancer. Lancet 2019;394:1467-80. [Crossref] [PubMed]
- Lin KJ, Cheung WY, Lai JY, et al. The effect of estrogen vs. combined estrogen-progestogen therapy on the risk of colorectal cancer. Int J Cancer 2012;130:419-30. [Crossref] [PubMed]
- Mayer RJ, Van Cutsem E, Falcone A, et al. Randomized trial of TAS-102 for refractory metastatic colorectal cancer. N Engl J Med 2015;372:1909-19. [Crossref] [PubMed]
- Ricciardiello L, Ahnen DJ, Lynch PM. Chemoprevention of hereditary colon cancers: time for new strategies. Nat Rev Gastroenterol Hepatol 2016;13:352-61. [Crossref] [PubMed]
- Pietrantonio F, Vernieri C, Siravegna G, et al. Heterogeneity of Acquired Resistance to Anti-EGFR Monoclonal Antibodies in Patients with Metastatic Colorectal Cancer. Clin Cancer Res 2017;23:2414-22. [Crossref] [PubMed]
- Biller LH, Schrag D. Diagnosis and Treatment of Metastatic Colorectal Cancer: A Review. JAMA 2021;325:669-85. [Crossref] [PubMed]
- Segal RA. Selectivity in neurotrophin signaling: theme and variations. Annu Rev Neurosci 2003;26:299-330. [Crossref] [PubMed]
- Solomon JP, Benayed R, Hechtman JF, et al. Identifying patients with NTRK fusion cancer. Ann Oncol 2019;30:viii16-22. [Crossref]
- Jiang T, Wang G, Liu Y, et al. Development of small-molecule tropomyosin receptor kinase (TRK) inhibitors for NTRK fusion cancers. Acta Pharm Sin B 2021;11:355-72. [Crossref] [PubMed]
- Jin W. Roles of TrkC Signaling in the Regulation of Tumorigenicity and Metastasis of Cancer. Cancers (Basel) 2020;12:147. [Crossref] [PubMed]
- Huang EJ, Reichardt LF. Neurotrophins: roles in neuronal development and function. Annu Rev Neurosci 2001;24:677-736. [Crossref] [PubMed]
- Mehlen P, Tauszig-Delamasure S. Dependence receptors and colorectal cancer. Gut 2014;63:1821-9. [Crossref] [PubMed]
- Brodeur GM, Minturn JE, Ho R, et al. Trk receptor expression and inhibition in neuroblastomas. Clin Cancer Res 2009;15:3244-50. [Crossref] [PubMed]
- Cattaneo E, McKay R. Proliferation and differentiation of neuronal stem cells regulated by nerve growth factor. Nature 1990;347:762-5. [Crossref] [PubMed]
- Louie E, Chen XF, Coomes A, et al. Neurotrophin-3 modulates breast cancer cells and the microenvironment to promote the growth of breast cancer brain metastasis. Oncogene 2013;32:4064-77. [Crossref] [PubMed]
- Truzzi F, Marconi A, Lotti R, et al. Neurotrophins and their receptors stimulate melanoma cell proliferation and migration. J Invest Dermatol 2008;128:2031-40. [Crossref] [PubMed]
- Ohta T, Numata M, Tsukioka Y, et al. Neurotrophin-3 expression in human pancreatic cancers. J Pathol 1997;181:405-12. [Crossref] [PubMed]
- Miknyoczki SJ, Lang D, Huang L, et al. Neurotrophins and Trk receptors in human pancreatic ductal adenocarcinoma: expression patterns and effects on in vitro invasive behavior. Int J Cancer 1999;81:417-27. [Crossref] [PubMed]
- Luo Y, Kaz AM, Kanngurn S, et al. NTRK3 is a potential tumor suppressor gene commonly inactivated by epigenetic mechanisms in colorectal cancer. PLoS Genet 2013;9:e1003552. [Crossref] [PubMed]
- Peng TJ, Chang Wang CC, Tang SJ, et al. Neurotrophin-3 Facilitates Stemness Properties and Associates with Poor Survival in Lung Cancer. Neuroendocrinology 2024;114:921-33. [Crossref] [PubMed]
- Li H, Yang Z, Wang W, et al. NT-3/TrkC Axis Contributes to the Perineural Invasion and the Poor Prognosis in Human Salivary Adenoid Cystic Carcinoma. J Cancer 2019;10:6065-73. [Crossref] [PubMed]
- Kim MS, Suh KW, Hong S, et al. TrkC promotes colorectal cancer growth and metastasis. Oncotarget 2017;8:41319-33. [Crossref] [PubMed]
- Kim MS, Lee WS, Lee H, et al. TrkC, a novel prognostic marker, induces and maintains cell survival and metastatic dissemination of Ewing sarcoma by inhibiting EWSR1-FLI1 degradation. Cell Death Dis 2022;13:836. [Crossref] [PubMed]
- Punnett HH, Tomczak EZ, Pawel BR, et al. ETV6-NTRK3 gene fusion in metastasizing congenital fibrosarcoma. Med Pediatr Oncol 2000;35:137-9. [Crossref] [PubMed]
- Kim MS, Jeong J, Seo J, et al. Dysregulated JAK2 expression by TrkC promotes metastasis potential, and EMT program of metastatic breast cancer. Sci Rep 2016;6:33899. [Crossref] [PubMed]
- Lawn S, Krishna N, Pisklakova A, et al. Neurotrophin signaling via TrkB and TrkC receptors promotes the growth of brain tumor-initiating cells. J Biol Chem 2015;290:3814-24. [Crossref] [PubMed]
- Sasahira T, Ueda N, Kurihara M, et al. Tropomyosin receptor kinases B and C are tumor progressive and metastatic marker in colorectal carcinoma. Hum Pathol 2013;44:1098-106. [Crossref] [PubMed]
- Brody H. Colorectal cancer. Nature 2015;521:S1. [Crossref] [PubMed]
- Spaander MCW, Zauber AG, Syngal S, et al. Young-onset colorectal cancer. Nat Rev Dis Primers 2023;9:21. [Crossref] [PubMed]
- The Lancet Oncology. Colorectal cancer: a disease of the young? Lancet Oncol 2017;18:413. [Crossref]
- Nguyen SP, Bent S, Chen YH, et al. Gender as a risk factor for advanced neoplasia and colorectal cancer: a systematic review and meta-analysis. Clin Gastroenterol Hepatol 2009;7:676-81.e1-3.
- Weitz J, Koch M, Debus J, et al. Colorectal cancer. Lancet 2005;365:153-65. [Crossref] [PubMed]
- Nagtegaal ID, van de Velde CJ, van der Worp E, et al. Macroscopic evaluation of rectal cancer resection specimen: clinical significance of the pathologist in quality control. J Clin Oncol 2002;20:1729-34. [Crossref] [PubMed]
- Brenner H, Kloor M, Pox CP. Colorectal cancer. Lancet 2014;383:1490-502. [Crossref] [PubMed]
- Compton CC. Colorectal carcinoma: diagnostic, prognostic, and molecular features. Mod Pathol 2003;16:376-88. [Crossref] [PubMed]
- Labianca R, Nordlinger B, Beretta GD, et al. Early colon cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2013;24:vi64-72. [Crossref] [PubMed]
- Walter V, Jansen L, Hoffmeister M, et al. Smoking and survival of colorectal cancer patients: systematic review and meta-analysis. Ann Oncol 2014;25:1517-25. [Crossref] [PubMed]
- Lange AM, Lo HW. Inhibiting TRK Proteins in Clinical Cancer Therapy. Cancers (Basel) 2018;10:105. [Crossref] [PubMed]
- Cocco E, Scaltriti M, Drilon A. NTRK fusion-positive cancers and TRK inhibitor therapy. Nat Rev Clin Oncol 2018;15:731-47. [Crossref] [PubMed]
- Geiger TR, Song JY, Rosado A, et al. Functional characterization of human cancer-derived TRKB mutations. PLoS One 2011;6:e16871. [Crossref] [PubMed]
- Miknyoczki SJ, Wan W, Chang H, et al. The neurotrophin-trk receptor axes are critical for the growth and progression of human prostatic carcinoma and pancreatic ductal adenocarcinoma xenografts in nude mice. Clin Cancer Res 2002;8:1924-31. [PubMed]
- Nakagawara A. Trk receptor tyrosine kinases: a bridge between cancer and neural development. Cancer Lett 2001;169:107-14. [Crossref] [PubMed]
- Jin W, Yun C, Kim HS, et al. TrkC binds to the bone morphogenetic protein type II receptor to suppress bone morphogenetic protein signaling. Cancer Res 2007;67:9869-77. [Crossref] [PubMed]
- Amatu A, Sartore-Bianchi A, Bencardino K, et al. Tropomyosin receptor kinase (TRK) biology and the role of NTRK gene fusions in cancer. Ann Oncol 2019;30:viii5-viii15.
- Eggert A, Grotzer MA, Ikegaki N, et al. Expression of neurotrophin receptor TrkA inhibits angiogenesis in neuroblastoma. Med Pediatr Oncol 2000;35:569-72. [Crossref] [PubMed]
- Singer HS, Hansen B, Martinie D, et al. Mitogenesis in glioblastoma multiforme cell lines: a role for NGF and its TrkA receptors. J Neurooncol 1999;45:1-8. [Crossref] [PubMed]
- Astolfi A, Nanni P, Landuzzi L, et al. An anti-apoptotic role for NGF receptors in human rhabdomyosarcoma. Eur J Cancer 2001;37:1719-25. [Crossref] [PubMed]
- Menter DG, Herrmann JL, Marchetti D, et al. Involvement of neurotrophins and growth factors in brain metastasis formation. Invasion Metastasis 1994-1995;14:372-84. [PubMed]
- Lamballe F, Klein R, Barbacid M. trkC, a new member of the trk family of tyrosine protein kinases, is a receptor for neurotrophin-3. Cell 1991;66:967-79. [Crossref] [PubMed]
- Thiele CJ, Li Z, McKee AE. On Trk--the TrkB signal transduction pathway is an increasingly important target in cancer biology. Clin Cancer Res 2009;15:5962-7. [Crossref] [PubMed]
- Jin W, Lee JJ, Kim MS, et al. DNA methylation-dependent regulation of TrkA, TrkB, and TrkC genes in human hepatocellular carcinoma. Biochem Biophys Res Commun 2011;406:89-95. [Crossref] [PubMed]
- Bouzas-Rodriguez J, Cabrera JR, Delloye-Bourgeois C, et al. Neurotrophin-3 production promotes human neuroblastoma cell survival by inhibiting TrkC-induced apoptosis. J Clin Invest 2010;120:850-8. [Crossref] [PubMed]
- Grotzer MA, Janss AJ, Fung K, et al. TrkC expression predicts good clinical outcome in primitive neuroectodermal brain tumors. J Clin Oncol 2000;18:1027-35. [Crossref] [PubMed]
- McGregor LM, McCune BK, Graff JR, et al. Roles of trk family neurotrophin receptors in medullary thyroid carcinoma development and progression. Proc Natl Acad Sci U S A 1999;96:4540-5. [Crossref] [PubMed]
- Segal RA, Goumnerova LC, Kwon YK, et al. Expression of the neurotrophin receptor TrkC is linked to a favorable outcome in medulloblastoma. Proc Natl Acad Sci U S A 1994;91:12867-71. [Crossref] [PubMed]
- Seo E, Kim JS, Ma YE, et al. Differential Clinical Significance of Neurotrophin-3 Expression according to MYCN Amplification and TrkC Expression in Neuroblastoma. J Korean Med Sci 2019;34:e254. [Crossref] [PubMed]


