
Use of hepatic blood inflow occlusion and hemihepatic artery retention in liver resection for hepatocellular carcinoma
Introduction
Hepatocellular carcinoma (HCC) is one of the most common malignant tumors in China and one of the most common neoplasms worldwide. Surgical hepatic resection at an early stage remains a common treatment method for liver cancer and is considered the first choice and most effective method. Many studies have shown, that for the treatment of small HCC, surgical resection is superior to other nonsurgical ablation treatments (1). However, it is very complex and has many possible complications and risks. Intraoperative bleeding control is crucial. Hepatic portal occlusion is an effective method of reducing the bleeding risk (2), and there are a variety of available occlusion methods. However, the liver undergoes ischemia-reperfusion injury after occlusion, which results in delayed recovery of hepatic function as well as the risk of liver failure and even death. This occurs in both liver resection surgery and transplantation. During liver resection surgery, blood flow occlusion is generally used to control bleeding. However, a long duration of ischemia after blood flow occlusion is bound to lead to an increased hepatic burden, resulting in slowed postoperative recovery of liver function and even liver failure. Consequently, the identification of an effective method of controlling bleeding of the liver parenchyma to avoid ischemic damage to the residual liver has always been the goal of both domestic and foreign researchers. How to scientifically and reasonably select the optimal hepatic blood flow occlusion method to minimize intraoperative bleeding and, thus, decrease liver injury has become one of the key technical problems in hepatic surgery (3). Occluding the blood flow entering the liver is the most commonly used technique to control liver bleeding (2). Among the available methods, the Pringle method is the simplest and most common method of blood flow occlusion since it can completely block blood flow through the hepatic artery and portal vein without the need for fine dissection of the hepatic portal and can be used with almost any hepatic resection type (4-6). However, since this method blocks all of the liver’s blood supply, it can cause ischemia-reperfusion injury of the liver, which greatly impacts the liver function of patients with concomitant cirrhosis (7).
After ischemia-reperfusion injury, the liver is subjected to a series of pathophysiological changes with extremely complicated mechanisms that would definitely increase the incidence of postoperative complications (8). The pathophysiological processes behind it are complex and well-described in the literature. They include interactions between hepatic Kupffer cells, hepatic sinusoidal endothelium, and the cellular and soluble messenger components of blood inflow, which results in a systemic inflammatory response and local injury (9). At the cellular level, one of the main contributing factors to hepatic injury is oxidative stress mediated by oxygen-free radicals (10). Therefore, it is important to choose an appropriate method of blood flow occlusion. Hepatic portal occlusion with hemihepatic arterial blood supply retention (the modified Pringle method, hereafter referred as the modified method) is a new portal occlusion method that has been tested in our department in recent years. Here we compared it to the traditional Pringle method to explore its clinical value in liver resection in patients with HCC.
Methods
Clinical data
This retrospective observational study included 94 patients who underwent liver resection with blood flow occlusion from January 2006 to November 2013. Patients were treated by the Pringle method (Pringle group) or the modified Pringle method (modified group). The 81 male patients and 13 female patients had a median age of 54.5 years. Of the 94 patients, 73 patients were hepatitis B virus surface antigen (HBsAg)-positive and five were anti-hepatitis C virus antibody (anti-HCV)-positive; 90 patients were classified as Child A, while four were classified as Child B. There were 71 patients with concomitant cirrhosis, including 38 mild, 29 moderate, and four severe cases. Liver cirrhosis grade was classified into mild, moderate, and severe mainly based on the size of the largest hardening nodule on the liver surface observed during surgery (<0.4, 0.4–0.8, and >0.8 cm with significantly shrunken and deformed liver, respectively). There was no significant difference in preoperative liver functional classification between the two groups of patients as evidenced by no significant difference in serum aspartate transaminase (AST), total bilirubin, or albumin (ALB) levels. However, the liver cirrhosis degree of the modified group was significantly greater than that of the Pringle group (P<0.01), and the mean alanine transaminase (ALT) level of the modified group was significantly higher than that of the Pringle group (P<0.05) (Table 1). The diagnosis of HCC was confirmed in all 94 cases by postoperative pathology.
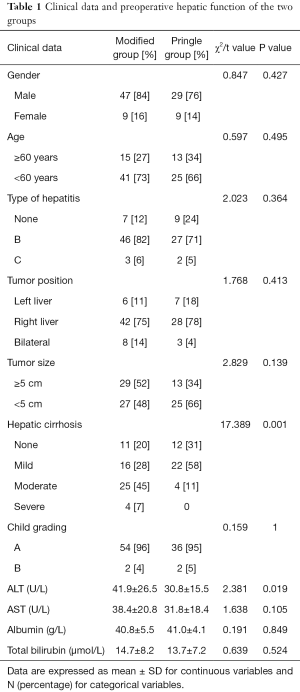
Full table
Surgical method
In the modified group, the hepatoduodenal ligament was dissected, the left or right hepatic artery was isolated, and the remaining hepatoduodenal ligament tissue was wrapped with a catheter. Both ends of the catheter were then passed through a section of a silicone tube, which was then pushed to the hilum using a hemostat. The catheter was then tightened to block the corresponding hepatic blood inflow. The duration of each block was limited to 30 min with an interval of at least 5 min before the next block. The blocking was performed as many as three times, with the longest total time of 48 min (blocking three times) and the shortest time of 4 min (blocking once) (Figure 1).
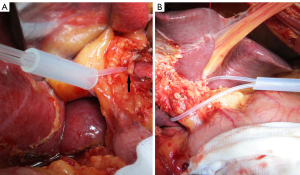
In the Pringle group, the hepatoduodenal ligament was clamped with a catheter and hemostat once or repeatedly to block the hepatic blood supply, with the blocking time limited to less than 15 min each time and an interval of at least 5 min before the next block. The blocking was performed as many as four times in individual patients, with the longest total blocking time of 52.8 min (blocking four times) and the shortest time of 5.3 min (blocking once) (Figure 1).
Observation parameters
The major observation parameters were: duration and mean time of occlusion; amount of blood loss and transfusion during surgery; operation time; operation method; postoperative hospital stay; and ALT, AST, total bilirubin, and ALB measured at 1, 3, 5, and 7 days after surgery. Postoperative complications included pleural effusion, ascites, biliary fistula, massive hemorrhage, and the incidence of postoperative death and reoperation.
Statistical analysis
Data are expressed as mean ± SD for continuous variables and percentages for categorical variables. Student’s t-test was used to compare normally distributed variables between the two groups, while the chi square test was used to compare categorical variables. Differences with values of P<0.05 were considered statistically significant. SPSS 17.0 (SPSS Inc., Chicago, IL, USA) software was used for the statistical data analysis.
Results
Comparison of surgical methods and intraoperative data
As shown in Tables 2,3, during surgery, the two patient groups showed no significant difference in the volume of intraoperative blood loss and transfusion (including infusion of plasma or red blood cells), tumor size, operation time, operation mode, duration of occlusion, number of occlusions, and incidence of occlusion >20 min (Tables 2,3).
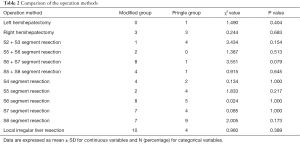
Full table
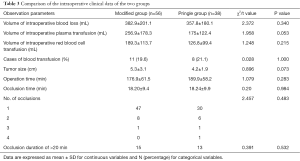
Full table
Postoperative changes of hepatic function
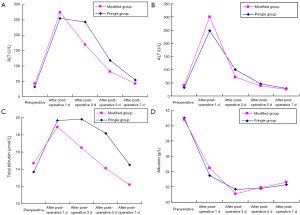
Comparison of liver function in the two groups of patients 1 day after surgery revealed that ALT, AST, total bilirubin, and ALB levels did not differ significantly. However, by this time, the total bilirubin level of the modified group was already within the normal range, while, with the exception of albumin, all other parameters deviated a great deal from the normal value.
Comparison of liver function in the two groups of patients 3 days after surgery revealed that the ALB level still showed no significant difference, while the ALT, AST, and total bilirubin levels were significantly different. By this time point, these three parameters had decreased significantly in the modified group, while the total bilirubin of the Pringle group had still not recovered to within the normal range.
At 5 days after surgery, the recovery of liver function in the two groups still showed a significant difference. The ALT, AST, and total bilirubin of the modified group were all significantly improved compared to those of the Pringle group. In addition to total bilirubin, the AST of the modified group also returned to within the normal range. At 5 days after surgery, the two groups of patients still demonstrated significant differences in ALT, AST, and total bilirubin levels.
Seven days after surgery, significant differences in ALT, AST, total bilirubin, and ALB levels were observed between the modified and Pringle groups. The mean ALT value of the modified group had basically returned to normal.
More direct comparisons of the liver function of the two groups of patients are shown in Figure 2.
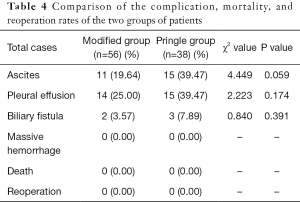
Postoperative complication, mortality, and reoperation rates in the two patient groups
Complication rates varied between the two patient groups. In the Pringle group, there were 15 cases of ascites, 15 of pleural effusion, and three of biliary fistula, while in the modified group, there were 11 cases of ascites, 14 of pleural effusion, and two of biliary fistula. No significant differences were observed between the two groups. No major complications, such as massive hemorrhage or reoperation, were observed, and there were no reported deaths (Table 4). There was no significant difference in the average length of postoperative hospital stay between the two groups.
Full table
Discussion
China is a country with a high incidence of liver cancer, particularly in patients with concomitant cirrhosis (11), which makes their liver particularly sensitive to ischemia. Since the entire liver is in a state of ischemia and hypoxia during Pringle method occlusion, a prolonged occlusion time would lead to severely damage liver function; thus, the duration of blocking of the hepatic blood supply should be controlled within a certain period of time, especially for those patients with concomitant cirrhosis. Due to this time limitation, multiple occlusions might be performed to enable complicated resection surgery. During the releasing period, the visceral blood flow can reflux via the portal vein to avoid excessive intestinal congestion and swelling. However, the disadvantage of the releasing interval is that the dissected liver tissue can lose much blood, extending the operation time. Especially in patients with severely damaged liver function, repeated long occlusions of liver blood supply will result in further damage to the residual liver (12), which will adversely affect the postoperative recovery of liver function and even lead to liver failure. In our hospital, the duration of each single occlusion using the Pringle method was limited to 15 min. The modified method preserved the hepatic artery on the healthy side, thus ensuring the continued supply of nutrition through the arterial blood supply of the healthy side of the liver and avoiding ischemia-reperfusion injury. The results of our previous study regarding liver ischemia-reperfusion damage in rats showed that the modified method was superior to the Pringle method in terms of changes in liver function and micropathological structure of the liver tissue as well as apoptosis (13). The margin of the liver can be clearly defined during the operation and the time of hepatic blood flow occlusion can be relatively prolonged to allow sufficient time for the surgeon to treat the liver section. The hepatic artery normally supplies 25–30% of the blood supply to the liver, while the portal vein supplies the other 70–75%. However, since the blood pressure of the hepatic artery is higher than that of the portal vein, its oxygen content is also higher, and a large proportion of the oxygen supply to the liver is supplied by the hepatic artery, while less oxygen is supplied through the portal vein. When inducing the occlusion using the modified method, since the portal vein is blocked and the hepatic artery at the healthy side is preserved, there is a natural increase in the blood supply through the hepatic artery that provides more blood and an increased oxygen supply to the liver, thus reducing the ischemia-reperfusion injury to the healthy part of the liver, especially for patients with cirrhosis by allowing a relatively abundant oxygen supply. The disadvantage of the modified method is that it requires dissection of the hepatoduodenal ligament and isolation of the contralateral hepatic artery that, considering the complex structure of the hepatic hilum, could easily lead to bleeding and prolonged operation time, blocking of the portal vein, and portal vein congestion. However, relatively speaking, since only the hepatic artery need be dissected and the hepatic artery is the least bifurcated, the operation is much easier compared to hemihepatic blood flow occlusion and avoids the risk of injury to the portal vein and bile duct during the separation process. The longest single occlusion time in the modified group was 28 min.
It is worth noting that the portal structure is complicated, and with concomitant cirrhosis and portal hypertension as well as many blood vessel branches, extra patience is necessary when separating the hepatic artery to prevent intraoperative bleeding. There was no significant difference between the two groups in terms of mean intraoperative bleeding volume or blood transfusion requirement as well as plasma versus red blood cell transfusion. However, it should be noted that these two values were slightly higher in the modified group than in the Pringle group, which could be due to the relatively more severe liver cirrhosis of patients of the modified group compared with the Pringle group. There was no obvious difference between the two groups in overall operation time, suggesting that the need to dissect the hepatic artery of one side did not affect the overall operation time.
To observe the postoperative liver function recovery, ALT, AST, albumin, and bilirubin were monitored. Between the selected two groups of patients, those in the modified group demonstrated significantly higher preoperative ALT as well as more severe cirrhosis extent. At day 1 after surgery, the two groups did not show large differences; however, by 3 days after surgery, the modified group demonstrated quicker hepatic functional recovery, which was first demonstrated by the normalization of bilirubin levels. On days 5 and 7, liver function recovery of the modified group was significantly faster than that of the Pringle group, with significantly lowered ALT and AST. In addition, the AST level returned to normal by 5 days after surgery and the ALT level was restored to normal 7 days after surgery. Such results indicate that the retention of the hepatic artery on one side ensured the oxygen supply to the residual liver tissue (14). The albumin levels of the two groups showed no significant difference, with both being lower than the normal value but within the controllable range. These results suggest that, compared to the Pringle method, the modified method enables quicker postoperative recovery of liver injury, showing significantly better efficacy, especially in patients with concomitant cirrhosis.
The incidence of postoperative complications including ascites, pleural effusion, and biliary fistula was not significantly different between two groups. There were no reports of severe complications, such as major hemorrhage, reoperation, or liver function failure. Along with the recent improvement of liver resection techniques, more proficient application of hepatic portal occlusion, and peri-operative treatment, the incidence of postoperative complications and mortality also decreased gradually (15-17). Intraoperative blood loss and blood transfusion can also increase the incidence of postoperative complications and mortality (18), especially in patients with cirrhosis (19).
Patients in the modified group exhibited a higher degree of preoperative liver cirrhosis, which may increase the incidence of postoperative complications. Further studies of larger samples are needed to make definite conclusions whether this is a consequence of the modified method. With the gradual recovery of liver injury and physical condition, ascites and pleural effusion were self-absorbed and relieved, while the biliary fistula naturally healed. There was no significant difference between the two groups in the length of postoperative hospital stay, indicating that reasonable choice of blood flow occlusion methods in patients with liver cirrhosis is of positive significance for postoperative rehabilitation. We also showed that liver ischemia-reperfusion injury could increase the risk of postoperative tumor recurrence (7). Further, whether the modified method reduces the recurrence of liver cancer requires further evaluation in clinical practice.
Conclusions
Surgical resection of HCC using the hemihepatic artery retention occlusion method can achieve the same hemostatic effect as the Pringle method but reduces ischemia-reperfusion injury to the liver, favoring postoperative liver function recovery in patients with HCC and cirrhosis, providing an ideal blood supply–controlling approach worthy of future use.
Several issues remain to be resolved. First, the classification of liver cirrhosis in this study used morphological and not histological criteria. Second, future well-designed randomized controlled trials are needed to confirm our findings.
Acknowledgments
We thank Medjaden Bioscience Limited for assistance in the preparation of this manuscript.
Funding: None.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2016.10.50). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). Ethics committee approval (ID 2016PS068K) was obtained from the Institutional Ethics Committee of Shengjing Hospital of China Medical University prior to the commencement of the study. Each patient provided informed consent.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Dong W, Zhang T, Wang ZG, et al. Clinical outcome of small hepatocellular carcinoma after different treatments: a meta-analysis. World J Gastroenterol 2014;20:10174-82. [Crossref] [PubMed]
- Huntington JT, Royall NA, Schmidt CR. Minimizing blood loss during hepatectomy: a literature review. J Surg Oncol 2014;109:81-8. [Crossref] [PubMed]
- Karamarković A, Doklestić K. Pre-resectional inflow vascular control: extrafascial dissection of Glissonean pedicle in liver resections. Hepatobiliary Surg Nutr 2014;3:227-37. [PubMed]
- Pringle JH. V. Notes on the Arrest of Hepatic Hemorrhage Due to Trauma. Ann Surg 1908;48:541-9. [Crossref] [PubMed]
- Hoekstra LT, van Trigt JD, Reiniers MJ, et al. Vascular occlusion or not during liver resection: the continuing story. Dig Surg 2012;29:35-42. [Crossref] [PubMed]
- Boyko VV, Pisetska ME, Tyshchenko OM, et al. Role of ischemic preconditioning in hepatic ischemia-reperfusion injury. Hepatobiliary Surg Nutr 2014;3:179-84. [PubMed]
- Orci LA, Lacotte S, Oldani G, et al. The role of hepatic ischemia-reperfusion injury and liver parenchymal quality on cancer recurrence. Dig Dis Sci 2014;59:2058-68. [Crossref] [PubMed]
- Saidi RF, Kenari SK. Liver ischemia/reperfusion injury: an overview. J Invest Surg 2014;27:366-79. [Crossref] [PubMed]
- Lander HM. An essential role for free radicals and derived species in signal transduction. FASEB J 1997;11:118-24. [PubMed]
- Yu BP. Cellular defenses against damage from reactive oxygen species. Physiol Rev 1994;74:139-62. [PubMed]
- Tanaka M, Katayama F, Kato H, et al. Hepatitis B and C virus infection and hepatocellular carcinoma in China: a review of epidemiology and control measures. J Epidemiol 2011;21:401-16. [Crossref] [PubMed]
- Sanjay P, Ong I, Bartlett A, et al. Meta-analysis of intermittent Pringle manoeuvre versus no Pringle manoeuvre in elective liver surgery. ANZ J Surg 2013;83:719-23. [PubMed]
- Jin S, Dai CL. Attenuation of reperfusion-induced hepatocyte apoptosis is associated with reversed bcl-2/bax ratio in hemi-hepatic artery-preserved portal occlusion. J Surg Res 2012;174:298-304. [Crossref] [PubMed]
- Jin S, Dai CL. Hepatic blood inflow occlusion without hemihepatic artery control in treatment of hepatocellular carcinoma. World J Gastroenterol 2010;16:5895-900. [Crossref] [PubMed]
- Fan ST, Mau Lo C, Poon RT, et al. Continuous improvement of survival outcomes of resection of hepatocellular carcinoma: a 20-year experience. Ann Surg 2011;253:745-58. [Crossref] [PubMed]
- Ni CY, Yang Y, Chang YQ, et al. Fast-track surgery improves postoperative recovery in patients undergoing partial hepatectomy for primary liver cancer: A prospective randomized controlled trial. Eur J Surg Oncol 2013;39:542-7. [Crossref] [PubMed]
- Kingham TP, Correa-Gallego C, D'Angelica MI, et al. Hepatic parenchymal preservation surgery: decreasing morbidity and mortality rates in 4,152 resections for malignancy. J Am Coll Surg 2015;220:471-9. [Crossref] [PubMed]
- Shiba H, Ishida Y, Fujiwara Y, et al. Practice to minimize the use of blood products improve outcome after hepatic resection for hepatocellular carcinoma. Hepatogastroenterology 2013;60:1681-3. [PubMed]
- Yang T, Zhang J, Lu JH, et al. Risk factors influencing postoperative outcomes of major hepatic resection of hepatocellular carcinoma for patients with underlying liver diseases. World J Surg 2011;35:2073-82. [Crossref] [PubMed]


