
Increased expression of RIPOR1 predicts the poor prognosis of colorectal cancer patients
Highlight box
Key findings
• Rho family-interacting cell polarization regulator 1 (RIPOR1) was significantly overexpressed in colorectal cancer (CRC) compared with adjacent normal tissues.
• High RIPOR1 expression was associated with the proliferation of CRC cells.
What is known and what is new?
• RIPOR1 was reported to be differentially expressed in various cancers.
• Our results demonstrated that overexpression of RIPOR1 was associated with poor prognosis and high cell proliferation of CRC.
What is the implication, and what should change now?
• RIPOR1 may be a potential predictive biomarker of CRC involved in proliferation and immune infiltration.
Introduction
The incidence and mortality of colorectal cancer (CRC) have progressively increased (1), accounting for about 10.0% and 9.4%, respectively, of all cancers worldwide (2). CRC is a heterogeneous disease, and its tumorigenesis may be significantly influenced by environmental and genetic variables (3). With the deepening research of molecular biology, molecular targeted therapy and immunotherapy are continuously being applied in clinical practice and have achieved good results (4), but the high morbidity, high mortality, and poor prognosis of CRC continue to cause a significant disease burden (5). Therefore, it is of great significance to search for effective therapeutic targets and prognostic biomarkers of CRC.
Rho guanosine triphosphate hydrolases (GTPases) are a family of small G proteins that control a variety of cellular functions (6). The members of Rho GTPases family include Rac1, RhoA, Cdc42, which can regulate the formation of cytoskeleton, cell polarity, and cell cycle progression (7). Research has reported that Rho GTPase signaling plays a significant role in cancer progression (8), and Rho GTPases may regulate proliferation and apoptosis, cell migration, and promote the occurrence and progression of tumors (9), which is correlated with disease progression in many cancers such as gastric cancer (10) and breast cancer (11). Interfering with the activation of Rho signals and inhibiting the effector function of Rho may contribute to cancer treatment and reduce the side effects of traditional anticancer drugs. Therefore, it has become a new target for tumor drug development with good application prospects and clinical significance. Rho family-interacting cell polarization regulator 1 (RIPOR1), also known as family with sequence similarity 65 member A (FAM65A), is a novel effector for the Rho subfamily of Rho GTPases, which can exert biological effects by linking to GTP-bound Rho proteins and may be involved in Golgi reorientation and efficient directional migration (12). RIPOR1 may be related to many cancers, but its expression and role in CRC are still unknown. Given the abnormal activation of Rho proteins in CRC and their interaction with RIPOR1, we speculated that RIPOR1 may interact with Rho GTPases and activate downstream pathways to play a role in CRC. We aimed to explore the significance of RIPOR1 in CRC, as it could enhance our understanding of the signaling pathways and mechanisms of CRC, potentially revealing new therapeutic approaches. To explore the expression of RIPOR1 in CRC and its correlation with cancer pathogenesis, we analyzed the RIPOR1 expression in CRC based on databases and immunohistochemical (IHC) staining of tissue microarray blocks, and analyzed its clinical significance. In addition, immune infiltration and biological pathways related to RIPOR1 in CRC were also detected, which provide clues and basis for further study on the function and treatment of RIPOR1 in the development of CRC. To investigate RIPOR1’s role in the progression of CRC, in vitro cellular tests were employed. We present this article in accordance with the MDAR reporting checklist (available at https://tcr.amegroups.com/article/view/10.21037/tcr-24-2029/rc).
Methods
Data extraction and expression difference analysis of databases
RNA sequencing (RNA-seq) profiles from The Cancer Genome Atlas (TCGA) database (https://portal.gdc.cancer.gov) and the Genotype-Tissue Expression (GTEx) database (https://gtexportal.org/) were downloaded, obtaining a total of 638 CRC samples and 558 normal samples. The edgeR package of R was used to perform differential analysis on the count data of the gene by using quasi-conditional maximum likelihood (qcML) method, and the P value was calculated based on negative binomial distribution combined with Fisher’s exact test. The difference threshold is |log2fold change (FC)| >1, P<0.05. The RNA microarray GSE41258 (13) and GSE44076 (14) datasets were collected from the Gene Expression Omnibus (GEO) database (https://www.ncbi.nlm.nih.gov/geo/), and RIPOR1 expression was calculated based on the Limma package of R.
Correlation analysis of RIPOR1 expression with clinicopathological characteristics, prognosis, and immune infiltration
Sequencing and clinical data of CRC were from the TCGA, and the correlation between RIPOR1 expression and patients’ clinicopathological characteristics and prognosis was analyzed by using analysis of variance (ANOVA), survival packages in R (R Foundation for Statistical Computing, Vienna, Austria), and Kaplan-Meier survival analysis. The Cell-type Identification by Estimating Relative Subsets of RNA Transcripts (CIBERSORT) algorithm and Spearman correlation analysis were used to assess the relationship between RIPOR1 expression and immune cell infiltration in CRC.
Analysis of upstream regulation of RIPOR1 expression
The microRNA (miRNA)-messenger RNA (mRNA) interaction information was extracted by using the miRanda (http://www.microrna.org/microrna/home.do), miRMap (https://mirmap.ezlab.org/), and TargetScan (http://www.targetscan.org/) databases, in order to predict the miRNA regulatory relationship of the RIPOR1 gene. The correlation analysis on the expression of target miRNAs predicted by all three programs and RIPOR1 in CRC were performed. Based on the Encyclopedia of DNA Elements (ENCODE) database (https://www.encodeproject.org/), transcription factor DNA-binding by chromatin immunoprecipitation followed by sequencing (ChIP-seq) data were used to predict the relationship of transcription factors regulating the RIPOR1 gene, and the relationship between RIPOR1 expression and the level of copy number variations (CNV) and DNA methylation in somatic cells were analyzed.
Protein-protein interaction (PPI) relationships
The Search Tool for the Retrieval of Interacting Genes (STRING) database (https://string-db.org/) was used to design a PPI network of RIPOR1, which was screened with an interaction score (IS) ≥0.4.
Functional enrichment analysis
The Gene Set Enrichment Analysis (GSEA) (https://www.gsea-msigdb.org/gsea/downloads.jsp) provided Gene Ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) gene sets. GSEA was performed by using the C2 set from the MSigDB. The results were sorted by normalized enrichment score (NES).
Tissue samples and microarrays
There were 148 CRC tissues and 20 para-carcinoma normal tissues collected in The First Hospital of Lanzhou University (Lanzhou, Gansu, China) from 2016 to 2019. Para-carcinoma normal tissues were defined as >5 cm away from the lesion, and all patients were not treated with radiotherapy or chemotherapy before surgery. Two pathologists evaluated the slides independently, according to the fifth edition of the World Health Organization classification. Tissue microarray paraffin blocks were created from the obtained tissues. The study was conducted in accordance with the Declaration of Helsinki and its subsequent amendments. The study was approved by the Ethics Committee of The First Hospital of Lanzhou University (No. LDYYLL-2025-63). The requirement for informed consent was exempted, because there was no identifiable personal information in the data we analyzed.
IHC staining
IHC was performed via the streptavidin-peroxidase method on serial sections of tissue microarray blocks for RIPOR1. Briefly, paraffin-embedded CRC or para-carcinoma tissues microarray blocks were cut into 4-µm thick slides. After baking at 65 ℃ for 90 minutes, the slides were dewaxed by immersion in xylene and then rehydrated with reduced concentration ethanol. Antigen retrieval was performed by using the high-pressure method with ethylenediaminetetraacetic acid (EDTA) at pH 8.0 for 7 minutes. Next, these slides were incubated with RIPOR1 primary antibodies (rabbit polyclonal to RIPOR1, ab2641441, Invitrogen, Carlsbad, CA, USA, dilution was set to 1/200) at 4 ℃ for 16 hours. After being washed with phosphate-buffered saline (PBS), the secondary antibodies (horseradish peroxidase-conjugated anti-rabbit/mouse immunoglobulin G, Fuzhou Maixin Biotech Co., Ltd., Fuzhou, China) were added at 37 ℃ for 30 minutes. Subsequently, the signal was developed by using diaminobenzidine (DAB) as substrate. Eventually, sections were re-stained with hematoxylin and dehydrated.
Tissues were grouped positive if the brown-yellow signals were noted localized in the cytoplasm. The IHC staining results were awarded a mean score by two pathologists independently. The proportion of positive cells (0, less than 1%; 1, 1–25%; 2, 26–50%; 3, 51–75%; 4, greater than 75%) and the staining intensity (0, negative; 1, weak; 2, moderate; 3, strong) were evaluated (15,16). The score for positive proportion and the score for staining intensity were multiplied to determine IHC scores (17). Low expression was considered as the IHC score of ≤6, whereas high expression was considered when the IHC score was >6.
Cell culture and transfection
HCT-116 (SCSP-5076) and HT-29 (TCHu103) cell lines were purchased from the Chinese Academy of Sciences (Beijing, China), and cultured with McCoy’s 5A medium containing 10% fetal bovine serum (FBS) and 1% double antibody (streptomycin 100 µg/mL + penicillin 100 U/mL), the incubator conditions were 37 ℃, 5% CO2. For transient knockdown, three RIPOR1 small-interfering RNAs (siRNAs) were used to transfect HCT-116 and HT-29 cell lines according to the instructions of the reagent (Lipo3000, Invitrogen). For stable overexpression of RIPOR1, Pcmv-RIPOR1 (human)-3xFLAG-NEO (Integrated Biotech Solutions, IBSBIO, Shanghai, China) and pcDNA3.1 empty control plasmid (IBSBIO, China) were used. The siRNAs against RIPOR1 (IBSBIO, China) were as follows:
v si-RIPOR1-1: 5'-CAAUGAAGACGAAGAUGAAGA-3';
v si-RIPOR1-2: 5'-CGGAAUUUCUGUCUAUUAAGG-3';
v si-RIPOR1-3: 5'-GCAAGAUCGAUGAGCUGUAUG-3'.
Complementary DNA (cDNA) synthesis and reverse transcription quantitative real-time polymerase chain reaction (RT-qPCR)
The TRIzol reagent and the cDNA reverse transcription kit were used to extract total RNA and carry out cDNA synthesis. RT-qPCR was performed to verify whether the target gene was knocked down or overexpressed. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) served as an internal reference, and then the relative RIPOR1 expression was calculated. The primers of RIPOR1 were as follows:
v Forward primer: 5'-AAACCATCTTTCTCCCTCTACTCA-3';
v Reverse primer: 5'-GACACTGCCCACAACCACAT-3'.
In vitro cell assay
Cell proliferation was assessed using the Cell Counting Kit-8 (CCK-8) kit (Boster Bio, Wuhan, China), and the transfected cells were collected, made into a cell suspension at a concentration of 5×104 cells/mL, and inoculated into 96-well plates at 100 µL/well. CCK-8 reagent of 10 µL was added to each well of the cells after incubation for 24, 48, and 72 hours. The cells were further incubated for 1 hour and the optical density (OD) was measured at 450 nm.
Statistical analysis
All data were processed with SPSS 23.0 (IBM Corp., Armonk, NY, USA) and GraphPad Prism 8 (GraphPad Software, San Diego, CA, USA) statistical software. RIPOR1 expression and its correlation with clinicopathological characteristics were performed by using the χ2 test. In vitro experiments, Student’s t-test and one-way ANOVA were used to compare the variables. The results were considered statistically significant when P<0.05 (*, P<0.05; **, P<0.01; ***, P<0.001).
Results
Expression of RIPOR1 in databases
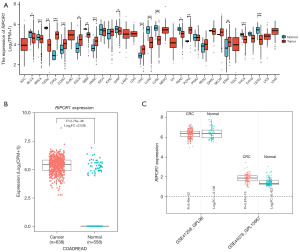
The expression levels of RIPOR1 in different tumor tissues and normal tissues were investigated with the TCGA database and the GTEx database, which showed that RIPOR1 was significantly more highly expressed in a variety of cancer tissues compared with their normal counterparts, such as cholangiocarcinoma (CHOL), colon adenocarcinoma (COAD), esophageal carcinoma (ESCA), liver hepatocellular carcinoma (LIHC), stomach adenocarcinoma (STAD), and so on (Figure 1A). RIPOR1 expression was significantly higher in CRC than in normal tissues (P<0.001) (Figure 1B). GEO database analysis showed RIPOR1 expression in CRC was significantly higher than that in normal tissues in dataset GSE44076 (P<0.001), whereas there was no significant difference in dataset GSE41258 (P=0.09) (Figure 1C).
Relationship of RIPOR1 expression with clinicopathologic features and prognosis
Based on the information of TCGA, statistically significant differences in the expression of RIPOR1 were found in different CRC pathological stages (F=3.18, P=0.02) (Figure 2A). The disease-free survival (DFS) and overall survival (OS) were significantly worse (P=0.005 and P=0.002, respectively) in high RIPOR1 expression CRC patients when compared with the low RIPOR1 expression CRC patients, according to Kaplan-Meier analysis (Figure 2B,2C). The results of one-way Cox regression analysis showed that RIPOR1 (P=0.009), age (P<0.001), pathological stage (P<0.001), tumor-node-metastasis (TNM) stage (P<0.001), and lymph node metastasis (P<0.001) were risk factors for CRC patients (Figure 2D). The χ2 test indicated that RIPOR1 expression was significantly correlated with ethnicity (P<0.001); however, due to the large number of unknown, no expression differences were found between different ethnicities. In addition, there was no significant correlation with age, gender, pathological stage, and TNM stage of CRC patients (Table 1).
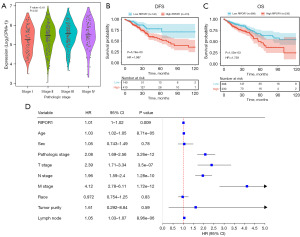
Table 1
| Characteristics | High expression (n=311) | Low expression (n=311) | χ2 | P value |
|---|---|---|---|---|
| Age (years) | 2.571 | 0.28 | ||
| <60 | 97 | 79 | ||
| ≥60 | 213 | 231 | ||
| Unknown | 1 | 1 | ||
| Sex | 0.784 | 0.68 | ||
| Female | 150 | 139 | ||
| Male | 160 | 171 | ||
| Unknown | 1 | 1 | ||
| Pathologic stage | 5.103 | 0.28 | ||
| Stage I | 44 | 61 | ||
| Stage II | 113 | 117 | ||
| Stage III | 100 | 80 | ||
| Stage IV | 45 | 45 | ||
| Unknown | 9 | 8 | ||
| T stage | 7.400 | 0.19 | ||
| T1 | 8 | 12 | ||
| T2 | 43 | 62 | ||
| T3 | 218 | 205 | ||
| T4 | 40 | 30 | ||
| Ti | 0 | 1 | ||
| Unknown | 2 | 1 | ||
| N stage | 6.747 | 0.08 | ||
| N0 | 163 | 189 | ||
| N1 | 76 | 74 | ||
| N2 | 69 | 46 | ||
| Unknown | 3 | 2 | ||
| M stage | 0.804 | 0.67 | ||
| M0 | 226 | 234 | ||
| M1 | 44 | 43 | ||
| Unknown | 41 | 34 | ||
| Race | 19.109 | <0.001 | ||
| Asian | 5 | 7 | ||
| Black | 33 | 32 | ||
| Indian | 1 | 0 | ||
| Unknown | 101 | 150 | ||
| White | 171 | 122 |
RIPOR1, Rho family-interacting cell polarization regulator 1.
Correlation of RIPOR1 expression with immune cell infiltration
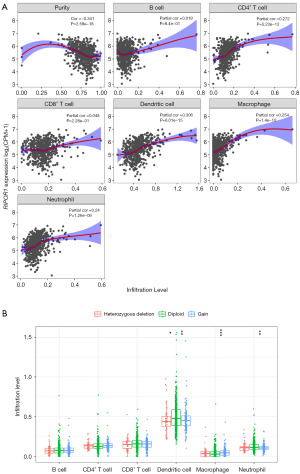
There was a significant positive correlation between RIPOR1 expression and CD4+ T cell, dendritic cell, macrophage, and neutrophil infiltration in CRC (r=0.24–0.306, P<0.001) (Figure 3A). Significant changes of dendritic cell, macrophage, and neutrophil infiltration were observed under different RIPOR1 CNV, suggesting that RIPOR1 variation may affect immune cell infiltration in the CRC microenvironment (Figure 3B).
Prediction of upstream regulation of RIPOR1
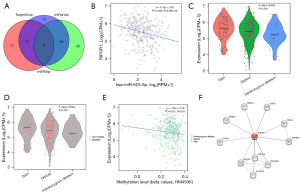
There were 132 miRNA target genes identified by miRMap, 78 miRNA target genes identified by miRanda, and 40 miRNA target genes identified by Targetscan. The miRNA regulatory database predictions were intersected to screen out four potential upstream miRNA target genes of RIPOR1: hsa-miR-625-5p, hsa-miR-1233-3p, hsa-miR-1275, and hsa-miR-1914-3p (Figure 4A). Correlation analysis revealed a significant negative correlation between RIPOR1 and hsa-miR-625-5p in CRC (r=−0.244, P<0.001) (Figure 4B), identifying hsa-miR-625-5p as a potential upstream miRNA of RIPOR1 in CRC. The expression of RIPOR1 may be associated with a variety of transcription factors such as ATF2, BACH1, BHLHE40, BRCA1, CCNT2, and CEBPB, and several members have been shown to produce effect in the regulation of iron death. Figure 4C,4D shows RIPOR1 expression in various types of CNVs, as well as the occurrence of single-nucleotide variants (SNVs) or Indels mutations. The expression of RIPOR1 in CRC showed a significant negative correlation with the DNA methylation level (Figure 4E).
Protein interaction relationships
The STRING database was used for RIPOR1 protein network interaction analysis, and the top 10 key proteins were selected using the degree value as a screening criterion (Figure 4F).
Gene enrichment analysis
GSEA showed that the high expression of RIPOR1 was associated with a variety of pathways, such as a positive association with the pathways of elastin fiber formation, elastin fiber-associated molecular responses, and collagen chain trimerization (Figure 5). The RIPOR1-related genes included NLGN2, PACS1, GLI2, RAB3IL1, and CAVIN1, among others.
RIPOR1 was highly expressed in CRC and associated with poor prognosis
IHC revealed that RIPOR1 was expressed in the cytoplasm and nucleus in the positive control group of liver tissues, but diffusely and strongly expressed in the cytoplasm in CRC, and weakly or not expressed in para-carcinoma normal tissues (Figure 6A). The expression of RIPOR1 in CRC (84.46%, 125/148) was higher than that in para-carcinoma normal tissues (35.00%, 7/20), and the difference was statistically significant (P<0.001) (Figure 6B). Statistical analysis showed that RIPOR1 expression was significantly correlated with the lymph node metastasis (P=0.03), whereas there was no significant difference with age, sex, differentiation, T stage, distant metastasis, Dukes’ classification, anatomic site, tumor size, and the Ki-67 proliferation index (Table 2). Kaplan-Meier analysis was used to plot survival curves, and the log-rank results showed that high RIPOR1 expression (P=0.03, Figure 6C), differentiation (P=0.02), lymph node metastasis (P=0.002), and Dukes’ classification (P=0.03) were poor prognostic factors for CRC patients. Multivariate Cox analysis revealed that RIPOR1 expression (P=0.044), differentiation (P=0.01), and lymph node metastasis (P=0.02) were independent factors affecting the prognosis of CRC patients (Figure 6D).
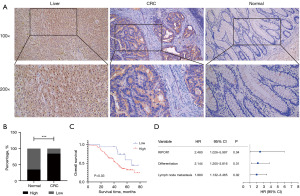
Table 2
| Characteristics | Patients (n=148) | RIPOR1 expression | P value | |
|---|---|---|---|---|
| Low | High | |||
| Age (years) | 0.74 | |||
| ≤60 | 79 | 13 | 66 | |
| >60 | 69 | 10 | 59 | |
| Sex | 0.54 | |||
| Male | 88 | 15 | 73 | |
| Female | 60 | 8 | 52 | |
| Differentiation | 0.32 | |||
| Well and moderate | 109 | 15 | 94 | |
| Poor and mucinous | 39 | 8 | 31 | |
| T stage | 0.38 | |||
| I–II | 15 | 4 | 11 | |
| III–IV | 133 | 19 | 114 | |
| Lymph node metastasis | 0.03 | |||
| No | 65 | 15 | 50 | |
| Yes | 83 | 8 | 75 | |
| Distant metastasis | 0.63 | |||
| No | 109 | 16 | 93 | |
| Yes | 39 | 7 | 32 | |
| Dukes’ classification | 0.08 | |||
| A+B | 53 | 12 | 41 | |
| C+D | 95 | 11 | 84 | |
| Tumor location | 0.11 | |||
| Right-sided | 42 | 9 | 33 | |
| Left-sided | 38 | 2 | 36 | |
| Rectal cancers | 68 | 12 | 56 | |
| Tumor size | 0.52 | |||
| <5 cm | 67 | 9 | 58 | |
| ≥5 cm | 81 | 14 | 67 | |
| Ki-67 | 0.56 | |||
| <50% | 22 | 2 | 20 | |
| ≥50% | 126 | 21 | 105 | |
RIPOR1, Rho family-interacting cell polarization regulator 1.
Overexpression of RIPOR1 affects the proliferation of CRC cell lines
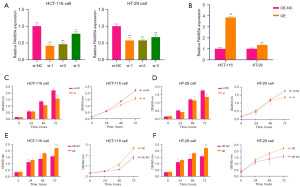
We constructed RIPOR1 knockdown (Figure 7A) and overexpressing cells (Figure 7B) with appropriate controls, and the si-RIPOR1-2 was selected for further experiments. CCK-8 cell proliferation assay in both HCT-116 and HT-29 cells revealed that RIPOR1 knockdown significantly decreased cell viability and proliferation compared to the control group (Figure 7C,7D), whereas the reverse impact was observed in RIPOR1-overexpressing cells (Figure 7E,7F). The results of cell experiments suggested that high RIPOR1 expression was associated with the proliferation ability of CRC cells.
Discussion
RIPOR1 can interact with many effector proteins to exert biological effects, and is expressed abnormally in many tumors (18). However, studies on its expression and mechanism in CRC are insufficient and have caught our attention (19). Our study further confirmed that RIPOR1 was highly expressed in CRC tissues, which was consistent with the TCGA database and GSE44076 dataset. Based on TCGA, RIPOR1 expression levels varied among different pathological stages. However, IHC did not reveal a significant correlation between RIPOR1 expression and pathological staging, which may be attributed to differences in staging methods and insufficient sample size. According to IHC staining, RIPOR1 was expressed diffusely and strongly in the cytoplasm in CRC, and was significantly associated with the lymph node metastasis. This suggested that RIPOR1 may participate in the development of CRC by promoting lymphatic metastasis. Moreover, IHC results showed that RIPOR1 expression, differentiation, and lymph node metastasis were independent factors affecting the prognosis of CRC patients, suggesting that RIPOR1 plays an important role in the development of CRC and may be a potential prognostic predictor for CRC. Cell experiments further confirmed its cancer promoting effect in CRC development and cell proliferation.
The migration and invasion of cancer cells require actin cytoskeletal reorganization, which may allow cells to disperse and metastasize. Rho GTPases, as regulatory factors for actin cytoskeletal signaling, have been shown to be associated with various diseases (20) and cancer invasion (21); interfering with the function of Rho and inhibiting the effector function of Rho may be effective in the treatment of cancer and development of a new avenue of drug discovery (10,22). RIPOR1, as a Rho effector, contains a Rho-GTP-binding homologous region 1 (HR1) domain. Our results confirmed its correlation with lymph node metastasis, which may be related to its downstream effect in promoting cell migration or proliferation. Meanwhile, drugs that disrupt RIPOR1 activity may cause the blockade of downstream pathways.
The immune microenvironment plays a crucial role in tumor development (23), and its changes may be the initiating factor of many pathophysiologies with complex mechanisms of action. The expression of RIPOR1 was positively correlated with the infiltration of immune cells such as CD4+ T-cells, dendritic cells, macrophages, and neutrophilic cells in the microenvironment of CRC tumors as analyzed in TCGA database, and it has been demonstrated that CRC is associated with the macrophages promotion of immune escape (24), so therapeutic strategies targeting changes in the immune microenvironment may benefit CRC patients.
MiRNAs are a class of small endogenous single-stranded non-coding RNAs that can regulate numerous biological processes by modulating the expression of the corresponding mRNA targets, and aberrant miRNA expression is frequently observed during the development of different tumors (25). In CRC cells, miRNAs are crucial in regulating apoptosis and therapeutic treatment resistance (26). The miRNA regulatory database prediction results screened potential upstream miRNA target genes, which revealed that RIPOR1 was significantly negatively correlated with hsa-miR-625-5p, indicating that hsa-miR-625-5p may play a role in tumorigenesis of CRC and may be considered able to regulate the expression of RIPOR1, and different miRNA expression levels may be used to elucidate the different biological features of CRC (27), which provides new insights into clinical applications and targeted therapies.
Conclusions
Our study revealed that RIPOR1 was highly expressed and had a significant impact on the occurrence and development of CRC, and its high expression may promote cell proliferation and predict the poor prognosis of CRC. It could be a viable focus for further experimental studies, which may provide a reference for the treatment of CRC patients and prognosis judgment. Larger sample-sized studies and mechanism experiments are needed to further investigate the function and application prospects of RIPOR1 in CRC.
Acknowledgments
None.
Footnote
Reporting Checklist: The authors have completed the MDAR reporting checklist. Available at https://tcr.amegroups.com/article/view/10.21037/tcr-24-2029/rc
Data Sharing Statement: Available at https://tcr.amegroups.com/article/view/10.21037/tcr-24-2029/dss
Peer Review File: Available at https://tcr.amegroups.com/article/view/10.21037/tcr-24-2029/prf
Funding: This work was supported by
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tcr.amegroups.com/article/view/10.21037/tcr-24-2029/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki and its subsequent amendments. The study was approved by the Ethics Committee of The First Hospital of Lanzhou University (No. LDYYLL-2025-63). The requirement for informed consent was waived because there was no identifiable personal information in the data we analyzed.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Morgan E, Arnold M, Gini A, et al. Global burden of colorectal cancer in 2020 and 2040: incidence and mortality estimates from GLOBOCAN. Gut 2023;72:338-44. [Crossref] [PubMed]
- Bray F, Laversanne M, Sung H, et al. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2024;74:229-63. [Crossref] [PubMed]
- Janney A, Powrie F, Mann EH. Host-microbiota maladaptation in colorectal cancer. Nature 2020;585:509-17. [Crossref] [PubMed]
- Zhu G, Pei L, Xia H, et al. Role of oncogenic KRAS in the prognosis, diagnosis and treatment of colorectal cancer. Mol Cancer 2021;20:143. [Crossref] [PubMed]
- Li N, Lu B, Luo C, et al. Incidence, mortality, survival, risk factor and screening of colorectal cancer: A comparison among China, Europe, and northern America. Cancer Lett 2021;522:255-68. [Crossref] [PubMed]
- Chardin P, Boquet P, Madaule P, et al. The mammalian G protein rhoC is ADP-ribosylated by Clostridium botulinum exoenzyme C3 and affects actin microfilaments in Vero cells. EMBO J 1989;8:1087-92. [Crossref] [PubMed]
- Bement WM, Goryachev AB, Miller AL, et al. Patterning of the cell cortex by Rho GTPases. Nat Rev Mol Cell Biol 2024;25:290-308. [Crossref] [PubMed]
- Lawson CD, Ridley AJ. Rho GTPase signaling complexes in cell migration and invasion. J Cell Biol 2018;217:447-57. [Crossref] [PubMed]
- Sahai E, Marshall CJ. RHO-GTPases and cancer. Nat Rev Cancer 2002;2:133-42. [Crossref] [PubMed]
- Komatsu M, Ichikawa H, Chiwaki F, et al. ARHGAP-RhoA signaling provokes homotypic adhesion-triggered cell death of metastasized diffuse-type gastric cancer. Oncogene 2022;41:4779-94. [Crossref] [PubMed]
- Yi M, Zhang D, Song B, et al. Increased expression of ECT2 predicts the poor prognosis of breast cancer patients. Exp Hematol Oncol 2022;11:107. [Crossref] [PubMed]
- Mardakheh FK, Self A, Marshall CJ. RHO binding to FAM65A regulates Golgi reorientation during cell migration. J Cell Sci 2016;129:4466-79. [Crossref] [PubMed]
- Sheffer M, Bacolod MD, Zuk O, et al. Association of survival and disease progression with chromosomal instability: a genomic exploration of colorectal cancer. Proc Natl Acad Sci U S A 2009;106:7131-6. [Crossref] [PubMed]
- Solé X, Crous-Bou M, Cordero D, et al. Discovery and validation of new potential biomarkers for early detection of colon cancer. PLoS One 2014;9:e106748. [Crossref] [PubMed]
- Hu T, Liu H, Liang Z, et al. Tumor-intrinsic CD47 signal regulates glycolysis and promotes colorectal cancer cell growth and metastasis. Theranostics 2020;10:4056-72. [Crossref] [PubMed]
- Sun H, Ou B, Zhao S, et al. USP11 promotes growth and metastasis of colorectal cancer via PPP1CA-mediated activation of ERK/MAPK signaling pathway. EBioMedicine 2019;48:236-47. [Crossref] [PubMed]
- Remmele W, Stegner HE. Recommendation for uniform definition of an immunoreactive score (IRS) for immunohistochemical estrogen receptor detection (ER-ICA) in breast cancer tissue. Pathologe 1987;8:138-40. [PubMed]
- Kotawong K, Thitapakorn V, Roytrakul S, et al. Plasma phosphoproteome and differential plasma phosphoproteins with opisthorchis viverrini-related cholangiocarcinoma. Asian Pac J Cancer Prev 2015;16:1011-8. [Crossref] [PubMed]
- Liang W, Mo C, Wei J, et al. FAM65A as a novel prognostic biomarker in human tumors reveal by a pan-cancer analysis. Discov Oncol 2021;12:60. [Crossref] [PubMed]
- Crosas-Molist E, Samain R, Kohlhammer L, et al. Rho GTPase signaling in cancer progression and dissemination. Physiol Rev 2022;102:455-510. [Crossref] [PubMed]
- Calvo F, Sahai E. Cell communication networks in cancer invasion. Curr Opin Cell Biol 2011;23:621-9. [Crossref] [PubMed]
- Guo D, Yang X, Shi L. Rho GTPase Regulators and Effectors in Autism Spectrum Disorders: Animal Models and Insights for Therapeutics. Cells 2020;9:835. [Crossref] [PubMed]
- Shang S, Yang YW, Chen F, et al. TRIB3 reduces CD8(+) T cell infiltration and induces immune evasion by repressing the STAT1-CXCL10 axis in colorectal cancer. Sci Transl Med 2022;14:eabf0992. [Crossref] [PubMed]
- Liu C, Yao Z, Wang J, et al. Macrophage-derived CCL5 facilitates immune escape of colorectal cancer cells via the p65/STAT3-CSN5-PD-L1 pathway. Cell Death Differ 2020;27:1765-81. [Crossref] [PubMed]
- Jafarzadeh M, Soltani BM. MiRNA-Wnt signaling regulatory network in colorectal cancer. J Biochem Mol Toxicol 2021;35:e22883. [Crossref] [PubMed]
- Wang H. MicroRNAs and Apoptosis in Colorectal Cancer. Int J Mol Sci 2020;21:5353. [Crossref] [PubMed]
- Eneh S, Heikkinen S, Hartikainen JM, et al. MicroRNAs Associated With Biological Pathways of Left- and Right-sided Colorectal Cancer. Anticancer Res 2020;40:3713-22. [Crossref] [PubMed]



