
The role of radiotherapy in distant nasopharyngeal carcinoma: a SEER analysis
Highlight box
Key findings
• Radiotherapy (RT) can enhance survival outcomes in patients with distant nasopharyngeal carcinoma (dNPC).
What is known and what is new?
• Cisplatin-based chemotherapy is recommended as the initial treatment modality for dNPC.
• Our study included data from 1,171 patients diagnosed with dNPC in the Surveillance, Epidemiology, and End Results (SEER) database for analysis, highlighting the potential of RT to improve the prognosis of dNPC.
What is the implication, and what should change now?
• The survival benefits associated with local RT appear to exceed those of systemic chemotherapy; however, the potential for significant toxicities in patients must not be overlooked. The question of whether local RT can effectively replace systemic chemotherapy, along with the possibility that minimally invasive surgery may offer greater survival advantages, requires further validation through large-scale clinical trials.
Introduction
Nasopharyngeal carcinoma (NPC) ranks as the seventh most prevalent cancer globally. It exhibits a high sensitivity to both radiotherapy (RT) and chemotherapy (1). For patients with early-stage NPC, RT alone is the first-line treatment, whereas concurrent chemoradiation is advised for those with locoregionally advanced disease (2). The introduction of intensity-modulated radiation therapy (IMRT), with or without adjunctive chemotherapy, has markedly enhanced treatment outcomes for NPC (3). However, RT can lead to substantial toxicities that adversely affect patients’ quality of life, with notable complications such as osteoradionecrosis, muscle fibrosis, and dysphagia resulting from subsequent nerve entrapment injuries (4,5). Approximately 10% of patients present with distant nasopharyngeal carcinoma (dNPC) at the time of initial diagnosis, and an additional 10% experience recurrences or distant metastases following standard therapeutic interventions (2). NPC associated with recurrent or metastatic disease exhibit a mortality rate exceeding 90%. According to the National Comprehensive Cancer Network (NCCN) guidelines for the management of head and neck cancers (6), cisplatin-based chemotherapy is recommended as the initial treatment modality for dNPC. Furthermore, RT should be administered exclusively to patients who have completed systemic chemotherapy for the primary lesion.
For numerous tumors, local therapies like RT have been integrated with systemic chemotherapy to enhance treatment outcomes, including survival rates (7-9). Although data show that reducing tumor burden with RT at metastatic and primary sites improves survival in various cancers (10,11), there are no large-scale studies supporting the NCCN’s recommendation for active RT or chemoradiation in dNPC patients. The potential benefits of RT were primarily supported by case reports and retrospective studies with limited sample sizes (12,13).
Therefore, we conducted a study using the Surveillance, Epidemiology, and End Results (SEER) database to determine whether dNPC patients who received RT had an improved survival compared with patients who did not. We present this article in accordance with the TRIPOD reporting checklist (available at https://tcr.amegroups.com/article/view/10.21037/tcr-2024-2332/rc).
Methods
Study design and participants
In this retrospective study, data were obtained from the SEER 18 registries cohort of the National Cancer Institute. The SEER Registry was queried utilizing SEER*Stat software version 8.4.4 to identify patients diagnosed with dNPC from 2004 to 2019. The study was conducted in accordance with the Declaration of Helsinki and its subsequent amendments. Patients diagnosed with NPC were identified using the International Classification of Diseases for Oncology, 3rd Edition (ICD-O-3) classification, updated to incorporate the hematopoietic codes from the World Health Organization (WHO) Classification of Tumours of Haematopoietic and Lymphoid Tissues [2008]. Distant stage cases were determined according to the Combined Summary Stage [2004+], which integrates data from the SEER Combined Summary Stage 2000 [2004–2017] and the Derived Summary Stage 2018 [2018+]. The study exclusively included patients who received beam radiation therapy, either alone or in conjunction with other forms of RT. Cases lacking data on the administration status of RT (recommended but unknown if administered) were excluded from the analysis.
Procedures and outcomes
The clinical variables considered for each patient in this analysis encompassed age, sex, race, cancer stage, year of diagnosis, surgical intervention, radiation therapy, chemotherapy, survival duration in months, and survival status. The dataset comprised patients diagnosed with dNPC aged 15 years and older. Given that age is treated as a categorical variable in the SEER database, and in light of the age distribution associated with a high incidence of NPC, we categorized age into two groups: individuals younger than 65 years and those aged 65 years and older. In the stage information, we selected several variables: Derived American Joint Committee on Cancer (AJCC) Stage Group 6 [2004–2015], Derived AJCC Stage Group 7 [2010–2015], the 7th edition stage group recode [2016–2017], and Derived Extent of Disease (EOD) 2018 Stage Group (2018 onwards). The valid data from these columns are categorized into stages II, III, IV, and unknown. Surgery is classified as either “No” or “Yes”, while RT is categorized as “No or unknown” or “Yes”. The SEER database classifies chemotherapy into “No” and “Yes”. It is important to note that no data were collected regarding pathological diagnostic subtypes.
This study focused on patients with distant diseases. Some of these patients might not have received more individualised treatment since the NCCN guidelines recommend the same treatment for patients with locally advanced disease (IIIB–IVA).
The primary outcome of the study was overall survival (OS), defined as the length of time from the date of first diagnosis until the date of death from any cause, or the last follow-up if the patient was still alive at the end of the study period.
Statistical analysis
In categorical variables, Pearson’s Chi-squared or Fisher’s exact tests were used to compare baseline demographic characteristics between the groups treated with or without RT. The study focused on survival months and status, analyzed using complete-case data. Univariate analyses employed the Kaplan-Meier method with log-rank test and the Cox proportional hazards (PH) model. Cox PH modeling included age, sex, race, year of diagnosis, surgery status, RT status, and chemotherapy status. We then use the ‘step’ function to select variables based on the Akaike Information Criterion (AIC) values to arrive at the optimal model.
The log form of survival time, event indicator, and cumulative hazard H0(T) were all used as predictors for multiple imputation. A total of eight imputed datasets were generated. Analyses by Cox regression were conducted on each imputed dataset, and results were then pooled by Rubin’s Rules.
Finally, the year in diagnosis variable was eliminated after AIC value screening, and other variables were used in developing the final nomogram. And the nomogram model’s accuracy was assessed through internal and external validation using SEER training and validation datasets.
P values <0.05 (two-sided) were considered statistically significant. All statistical analyses were performed using R environment for statistical computing and graphics (version. 4.4.0).
Results
Patient characteristics
A total of 1,171 patients who received treatment for dNPC were identified from the SEER database. These patients were categorized into two distinct cohorts: those who received RT (case group, n=944) and those who did not receive RT (control group, n=227) (refer to Figure 1). A comprehensive summary of all baseline characteristics is provided in Table 1. Patients with grade IV dNPC constituted 81.64% of the total sample, although 2.65% of patients had an unknown tumor grade. Chemotherapy was the most common treatment modality, administered to 84.71% of patients, while only 5.64% of patients underwent surgical intervention.
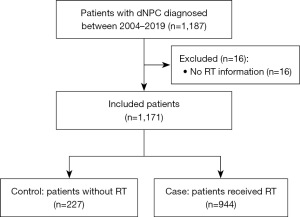
Table 1
| Variables | Radiotherapy | Statistics | P value# | ||
|---|---|---|---|---|---|
| Overall (N=1,171) | No (N=227, 19%) | Yes (N=944, 81%) | |||
| Age | 36.92 | <0.001 | |||
| <65 years | 869 (74.21) | 132 (58.15) | 737 (78.07) | ||
| ≥65 years | 302 (25.79) | 95 (41.85) | 207 (21.93) | ||
| Sex | 1.78 | 0.18 | |||
| Male | 886 (75.66) | 180 (79.30) | 706 (74.79) | ||
| Female | 285 (24.34) | 47 (20.70) | 238 (25.21) | ||
| Race | – | 0.28 | |||
| White | 417 (35.61) | 90 (39.65) | 327 (34.64) | ||
| Black | 119 (10.16) | 26 (11.45) | 93 (9.85) | ||
| Other | 630 (53.80) | 111 (48.90) | 519 (54.98) | ||
| Unknown | 5 (0.43) | 0 | 5 (0.53) | ||
| Year of diagnosis | 10.67 | 0.001 | |||
| 2004–2012 | 586 (50.04) | 91 (40.09) | 495 (52.44) | ||
| 2013–2019 | 585 (49.96) | 136 (59.91) | 449 (47.56) | ||
| Stage | – | <0.001 | |||
| Grade II | 73 (6.23) | 6 (2.64) | 67 (7.10) | ||
| Grade III | 111 (9.48) | 3 (1.32) | 108 (11.44) | ||
| Grade IV | 956 (81.64) | 210 (92.51) | 746 (79.03) | ||
| Unknown | 31 (2.65) | 8 (3.52) | 23 (2.44) | ||
| Surgery | 0.54 | 0.46 | |||
| No | 1,105 (94.36) | 217 (95.59) | 888 (94.07) | ||
| Yes | 66 (5.64) | 10 (4.41) | 56 (5.93) | ||
| Chemotherapy | 289.32 | <0.001 | |||
| No | 179 (15.29) | 118 (51.98) | 61 (6.46) | ||
| Yes | 992 (84.71) | 109 (48.02) | 883 (93.54) | ||
Data are presented as n (%). #, Pearson’s Chi-squared test; Fisher’s exact test for count data with simulated P value (based on 2,000 replicates).
Survival analyses
The median survival duration was determined to be 33 months, with a range spanning from 0 to 215 months. The outcomes of the multivariate analyses conducted using Cox regression are comprehensively presented in Figure 2. Notably, RT was significantly correlated with enhanced OS [hazard ratio (HR): 0.35, 95% confidence interval (CI): 0.29–0.43, P<0.001]. Furthermore, patients of non-black racial backgrounds and those who underwent chemotherapy exhibited significantly improved OS. Conversely, stage IV disease and undergoing surgery were associated with increased survival risks. It is important to highlight that the number of subgroups used for data comparison varied significantly. The Kaplan-Meier survival curve for patients receiving RT is depicted in Figure 3.
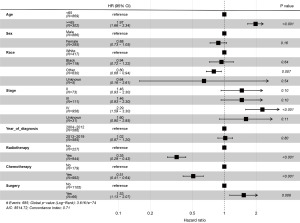

In accordance with the univariate analyses, the multivariate analyses conducted using the Cox proportional hazards model demonstrated that RT was independently associated with a 50% reduction in mortality risk concerning OS (HR: 0.35, 95% CI: 0.29–0.43, P<0.001). Figure 2 presents the findings of the unweighted multivariate analyses of OS.
The results indicate that, after adjusting for covariates, RT significantly reduced the risk of mortality, with the difference being statistically significant (P<0.001). In order to optimize the Cox PH model, we calculated the results of the likelihood ratio test and AIC for each independent variable (Table 2). The variable “year of diagnosis” had the largest P value and the smallest AIC value, P=0.80 and AIC =8,512.8, respectively. In the Cox proportional hazards model developed after the exclusion of this variable, the HR for patients who did not receive RT was 2.83 times that of patients who underwent RT.
Table 2
| Covariable | Number | HR (95% CI) | AIC | LRT | Pr(>Chi) |
|---|---|---|---|---|---|
| Age | 8,569.5 | 56.81 | <0.001 | ||
| <65 years | 869 | Reference | |||
| ≥65 years | 302 | 1.97 (1.66−2.34) | |||
| Sex | 8,514.7 | 2.02 | 0.16 | ||
| Male | 886 | Reference | |||
| Female | 285 | 0.88 (0.73−1.05) | |||
| Race | 8,516.6 | 7.85 | 0.049 | ||
| White | 417 | Reference | |||
| Black | 119 | 0.94 (0.72−1.22) | |||
| Other | 630 | 0.80 (0.68−0.94) | |||
| Unknown | 5 | 0.64 (0.16−2.61) | |||
| Stage | 8,542.7 | 33.99 | <0.001 | ||
| II | 73 | Reference | |||
| III | 111 | 1.46 (0.93−2.30) | |||
| IV | 956 | 2.29 (1.59−3.30) | |||
| Unknown | 31 | 1.60 (0.90−2.85) | |||
| Year of diagnosis | 8,512.8 | 0.07 | 0.80 | ||
| 2004−2012 | 586 | Reference | |||
| 2013−2019 | 585 | 1.02 (0.87−1.20) | |||
| Radiotherapy | 8,605.6 | 92.89 | <0.001 | ||
| No | 227 | Reference | |||
| Yes | 944 | 0.35 (0.29−0.43) | |||
| Chemotherapy | 8,546.3 | 33.56 | <0.001 | ||
| No | 179 | Reference | |||
| Yes | 992 | 0.51 (0.41−0.64) | |||
| Surgery | 8,519.4 | 6.65 | 0.006 | ||
| No | 1105 | Reference | |||
| Yes | 66 | 1.53 (1.13−2.07) |
AIC, Akaike information criterion; CI, confidence interval; dNPC, distant nasopharyngeal carcinoma; HR, hazard ratio; LRT, likelihood ratio test; OS, overall survival.
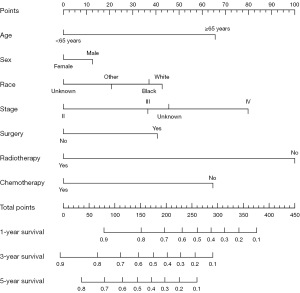
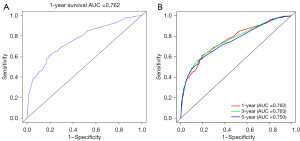
A nomogram was conducted to estimate the probability of survival in dNPC patients (Figure 4). The concordance index (C-index) of this model is 0.71. Based on the Cox model results, receiver operating characteristic (ROC) curves were plotted. The areas under the curves (AUCs) for 1-year survival, 3-year survival, and 5-year survival were 0.762, 0.763, and 0.750, respectively (Figure 5).
Discussion
Hu et al. examined data from 679 metastatic nasopharyngeal carcinoma (mNPC) patients in SEER [1988–2012] and concluded, using inverse probability of treatment weighting (IPTW) correction, that local RT enhances survival rates (14). Our study expands the patient dataset and introduces a nomogram and ROC curves for dNPC treatment. This analysis involved 1,171 patients diagnosed with dNPC at their first presentation, utilizing data from the SEER database between 2004 and 2019. The patients in our analysis were treated in a ‘real-world’ clinical setting, which may differ from the more selectively chosen cohorts typically found in clinical trials or single-institution studies. Our research underscores the importance of RT in the management of dNPC. To our knowledge, this represents the first population-based study to employ a nomogram and ROC curves to evaluate the efficacy of local RT in dNPC, which we believe may provide very meaningful guidance for physicians in the treatment of NPC and future research. Our findings have certain limitations. First, due to the relatively limited resources available in the SEER database, we included patients over an extended time period and incorporated those classified under various tumor-node-metastasis (TNM) staging criteria to ensure a sufficient sample size for analysis. In our data analysis, we noted that, compared with TNM staging, the corresponding Roman Numeral Staging exhibits far less heterogeneity among the participants. Therefore, we used Roman Numeral Staging for the construction of the nomogram, which may have potential impact on the evaluation of results. Second, pathological classification information for patients was not available for subgroup analysis. Third, the cohort of patients who underwent surgical intervention was relatively small, and no additional investigations were performed. According to the analysis results, RT can benefit the survival outcomes of patients with dNPC. However, it is more controversial that local RT has no therapeutic effect on distant lesions in patients with newly diagnosed dNPC and cannot be used as a standalone treatment (15,16). Therefore, it often needs to be combined with systemic therapies such as chemotherapy (17). Furthermore, patients with dNPC require extensive RT, and the side effects cannot be ignored (18). Notably, the SEER database did not record information on treatment-related complications. According to current knowledge, repeat RT after recurrence or distant metastasis significantly increases the likelihood of severe treatment complications, such as carotid artery rupture. Therefore, a more comprehensive assessment should include data on relevant treatment complications.
Surgery combined with RT may be a better option for patients with dNPC. The study included data on patients initially diagnosed with dNPC between 2004 and 2019, revealing a low incidence of surgical intervention. This is related to the indications for nasopharyngeal cancer surgery recommended in the guidelines. Currently, surgical treatment for NPC is mainly suitable for patients with early-stage disease, recurrence, and local progression; it is not recommended for dNPC patients to undergo surgical treatment. As we know, the surgical management of NPC has been limited due to factors such as the challenging anatomical location, proximity to the brain, and the carotid artery. However, with the further improvement and popularization of endoscopic surgery for the nasal skull base, the surgical indications for NPC are gradually expanding. At present, although advancements in intensity-modulated RT technology have greatly reduced the side effects for patients, issues such as neck sclerosis, chronic vascular injury, and related long-term complications remain patient (19). More and more medical experts recommend surgery for patients with early or recurrent NPC to reduce the incidence of long-term complications from RT and the risk of fatal complications from subsequent RT. In patients with NPC, the risk of carotid artery rupture increases significantly with re-RT. Studies have shown that the incidence of carotid artery rupture in patients receiving re-RT is 2.6%, with most cases being fatal (20,21). Therefore, further studies on the efficacy and complications of clinical surgery and RT are needed in the future.
Conclusions
Our findings suggest that RT can enhance survival in patients with dNPC. In contrast, chemotherapy alone seems to have minimal effect on long-term survival for those not receiving RT. We are also concerned about the low rate of surgery in these patients and believe that advancements in minimally invasive techniques could benefit more individuals. Therefore, larger-scale prospective studies are needed to confirm these findings.
Acknowledgments
The authors would like to thank The Second Affiliated Hospital of Guangxi Medical University and The Third Affiliated Hospital of Guangxi Medical University for their logistical support, facilitating data analysis, and offering expert guidance. Their collaboration is instrumental in ensuring the accuracy and reliability of this study.
Footnote
Reporting Checklist: The authors have completed the TRIPOD reporting checklist. Available at https://tcr.amegroups.com/article/view/10.21037/tcr-2024-2332/rc
Peer Review File: Available at https://tcr.amegroups.com/article/view/10.21037/tcr-2024-2332/prf
Funding: This study was supported by
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tcr.amegroups.com/article/view/10.21037/tcr-2024-2332/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki and its subsequent amendments.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Chen YP, Chan ATC, Le QT, et al. Nasopharyngeal carcinoma. Lancet 2019;394:64-80. [Crossref] [PubMed]
- Siak PY, Khoo AS, Leong CO, et al. Current Status and Future Perspectives about Molecular Biomarkers of Nasopharyngeal Carcinoma. Cancers (Basel) 2021;13:3490. [Crossref] [PubMed]
- Pan JJ, Ng WT, Zong JF, et al. Proposal for the 8th edition of the AJCC/UICC staging system for nasopharyngeal cancer in the era of intensity-modulated radiotherapy. Cancer 2016;122:546-58.
- De Felice F, Musio D, Tombolini V. Osteoradionecrosis and intensity modulated radiation therapy: An overview. Crit Rev Oncol Hematol 2016;107:39-43. [Crossref] [PubMed]
- Koch A, Reinhardt P, Elicin O, et al. Predictive biomarkers of radiotherapy- related dermatitis, xerostomia, mucositis and dysphagia in head and neck cancer: A systematic review. Radiother Oncol 2025;203:110689. [Crossref] [PubMed]
NCCN Guidelines for Treatment of Head and Neck Cancers - Hennequin C, Guillerm S, Quero L. Combination of chemotherapy and radiotherapy: A thirty years evolution. Cancer Radiother 2019;23:662-5. [Crossref] [PubMed]
- Liu HM, Meng CL, Zhao LJ. Survival Outcomes of Local Compared With Systemic First Treatment of Non-Small Cell Lung Cancer Brain Metastases. Front Oncol 2021;11:706409. [Crossref] [PubMed]
- Tubridy EA, Taunk NK, Ko EM. Treatment of node-positive endometrial cancer: chemotherapy, radiation, immunotherapy, and targeted therapy. Curr Treat Options Oncol 2024;25:330-45. [Crossref] [PubMed]
- Wang Y, Li C, Yang F, et al. Random survival forest predicts survival in patients with metastatic laryngeal and hypopharyngeal cancer and the prognostic benefits of surgery and radiotherapy. J Cancer 2025;16:603-21. [Crossref] [PubMed]
- Thompson R, Cheung P, Chu W, et al. Outcomes of extra-cranial stereotactic body radiotherapy for metastatic colorectal cancer: Dose and site of metastases matter. Radiother Oncol 2020;142:236-45. [Crossref] [PubMed]
- Qiu HZ, Zhang X, Liu SL, et al. M1 stage subdivisions based on (18)F-FDG PET-CT parameters to identify locoregional radiotherapy for metastatic nasopharyngeal carcinoma. Ther Adv Med Oncol 2022;14:17588359221118785. [Crossref] [PubMed]
- Jiang Y, Chen K, Yang J, et al. Optimize the number of cycles of induction chemotherapy for locoregionally advanced nasopharyngeal carcinoma: a propensity score matching analysis. J Cancer 2022;13:426-35. [Crossref] [PubMed]
- Hu J, Kong L, Gao J, et al. Use of Radiation Therapy in Metastatic Nasopharyngeal Cancer Improves Survival: A SEER Analysis. Sci Rep 2017;7:721. [Crossref] [PubMed]
- Zhao F, Yang D, Li X. Effect of radiotherapy interruption on nasopharyngeal cancer. Front Oncol 2023;13:1114652. [Crossref] [PubMed]
- Zeng F, Lu T, Xie F, et al. Effects of locoregional radiotherapy in de novo metastatic nasopharyngeal carcinoma: A real-world study. Transl Oncol 2021;14:101187. [Crossref] [PubMed]
- Tulalamba W, Ngernsombat C, Larbcharoensub N, et al. Transcriptomic profiling revealed FZD10 as a novel biomarker for nasopharyngeal carcinoma recurrence. Front Oncol 2022;12:1084713. [Crossref] [PubMed]
- Wang L, Qin X, Zhang Y, et al. The prognostic predictive value of systemic immune index and systemic inflammatory response index in nasopharyngeal carcinoma: A systematic review and meta-analysis. Front Oncol 2023;13:1006233. [Crossref] [PubMed]
- Tang R, Liu S, Mao S, et al. Safety and efficacy of protective stent insertion to prevent carotid blowout syndrome at the distal internal carotid artery in nasopharyngeal carcinoma patients: a comparison with endovascular occlusion. Quant Imaging Med Surg 2024;14:1791-802. [Crossref] [PubMed]
- Li C, Lu J, Luo Y, et al. Case report: Endovascular intervention of internal carotid artery pseudoaneurysm secondary to nasopharyngeal carcinoma radiotherapy. Front Surg 2022;9:1099416. [Crossref] [PubMed]
- Song J, Lan L, Lv Y, et al. Study on carotid artery stenosis after radiotherapy for nasopharyngeal carcinoma. J Cancer Res Clin Oncol 2024;150:273. [Crossref] [PubMed]


