
Ovarian teratomas causing anti-N-methyl-D-aspartate receptor encephalitis: a case series from west China
Highlight box
Key findings
• Ovarian teratomas (OTs) leading to encephalitis seem to be associated with high expression of N-methyl-D-aspartate receptors (NMDARs) in the squamous epithelium of the tissue, where clusters of lymphocyte infiltration is a unique pathological feature.
What is known and what is new?
• Anti-NMDAR encephalitis is a severe autoimmune disorder of the central nervous system (CNS), characterized by symptoms that arise directly from highly specific pathogenic autoantibodies. One of the clear factors for this encephalitis is OT. When OTs that aberrantly express neural antigens, it may lead to the formation of autoantibodies and finally immune cross-reaction against the CNS.
• The results of this study suggest that T and B cell infiltration is enhanced in teratoma tissue of patients with encephalitis. B cells are continuously activated and proliferate with the help of T cells, forming a germinal center. A large number of plasma cells differentiated from B cells produce NMDAR antibodies, which ultimately cross the blood-brain barrier and act on the CNS, leading to the onset of encephalitis symptoms
What is the implication, and what should change now?
• Some factors NMDAR expression in the squamous epithelium of OTs is significantly increased in patients in the encephalitis group, which triggers a break in immune self-tolerance and leads to NMDARs encephalitis.
• For women without a family history of mental illness but suddenly exhibiting abnormal mental behavior, early screening for OT should be performed. To achieve better treatment outcomes for encephalitis, surgical treatment for OT should be performed as early as possible.
Introduction
Synapses are the basis for the formation of higher functions such as learning and memory. Excitatory synaptic transmission in the brain is mainly mediated by glutamate, and N-methyl-D-aspartate receptor (NMDAR), one of the glutamate receptors, plays an indispensable role in the process of memory and learning (1). NMDAR is an isoform distributed on the surface of nerve cells, usually consisting of two NR1 subunits and two NR2 subunits, which are bound to glycine and to glutamate respectively. The NR2 subunits can be further divided into four isoforms (including 2A, 2B, 2C and 2D) (2).
Anti-N-methyl-D-aspartate receptor encephalitis (anti-NMDARE) was first officially named in 2007 (3). It is a severe autoimmune disorder of the central nervous system (CNS), characterized by symptoms that arise directly from highly specific pathogenic autoantibodies. One of the clear factors for this encephalitis is ovarian teratoma (OT), which accounts for about 35.8% of cases (4). When OTs that aberrantly express neural antigens, it may lead to the formation of autoantibodies and finally immune cross-reaction against the CNS (5). More than 80% of patients with NMDARE recover completely (6), but a small percentage of patients can still have sequelae such as neurological dysfunction, with a mortality rate of about 7% (4).
Because patients tend to seek psychiatric or neurological consultation for their first psychiatric or neurological symptoms, even if they have a combined teratoma, it often takes a long time from their consultation to discover the tumor for surgical intervention, causing some patients to miss the best timing for treatment. Moreover, due to the rarity of teratomas causing anti-NMDARE and the fact that most of the published articles are case reports, histological examination of OTs in encephalitis patients remains limited. Therefore, we analyzed the entire data of the encephalitis patients in West China Second University Hospital, Sichuan University, a tertiary care center in west China and performed immunohistochemistry (IHC) analysis of OTs from patients with or without anti-NMDARE to investigate the differences in the distribution of NMDAR and lymphocyte infiltration in teratoma tissues. We present the following article in accordance with the STROBE reporting checklist (available at https://tcr.amegroups.com/article/view/10.21037/tcr-24-2126/rc).
Methods
Patients and samples
From March 2014 to January 2020, 5,736 patients with OT were admitted to the West China Second University Hospital, Sichuan University for surgical treatment. With the approval of the ethics committee, their admission information was screened. Among all these patients, 13 were diagnosed with encephalitis at the same time. As 5 of 13 patients were excluded because of missing data or unavailable paraffin sections, finally, the remaining 8 patients were included in this research. In the 8 OT with encephalitis patients, 7 were mature teratoma (MT) and 1 was immature teratoma (IMT). The patients who were hospitalized and operated for OT in the same period were selected as the control group, among whom 18 patients were diagnosed as IMT, 20 patients were diagnosed as MT.
This study was conducted in accordance with the Declaration of Helsinki and its subsequent amendments. This study was approved by the Ethics Committee of West China Second University Hospital, Sichuan University [No. 2015(032)] on March 9, 2015. Informed consent was taken from all individual participants.
Immunohistochemical study of OT
Paraffin block sections were extracted from the pathology department and then used. The main antibodies (Abs) used were rabbit anti-human CD4 monoclonal antibody (dilution 1:100, Boster, China), rabbit anti-human CD20 polyclonal antibody (dilution 1:100, Boster, China), rabbit anti-human NMDAR2A antibody (dilution 1:100, Boster, China), rabbit anti-human NMDAR2B antibody (dilution 1:100, Tri-Eagle, China), rabbit anti-human NMDAR 1 antibody (dilution 1:100, Boster, China). Slides were dewaxed and hydrated by sequential immersion in xylene and anhydrous ethanol, and peroxidase was blocked using H2O2. The antigen was thermally repaired and incubated with primary and secondary antibodies. The slides were stained with DAB, followed by restaining with hematoxylin and finally dehydrated and sealed and examined microscopically.
Pathological diagnostic criteria were based on the WHO classification of tumors, female genital tumors, 5th edition (7). The staining was evaluated by two pathologists of West China Second University Hospital, Sichuan University respectively, using a semi-quantitative system in which the intensity and percentage of positive cells were taken into account. The intensity of staining was scored by the shade of staining color, i.e., 0 for colorless, 1 for light yellow, 2 for brown, and 3 for dark brown. The scoring criteria for the percentage of positive cells were: ≤5%, 0; 6–25%, 1; 26–50%, 2; ≥50%, 3. The final grade is obtained by multiplying the two ratings: 0, negative (−); 1–2, weakly positive (+); 3–4, positive (++); ≥5, strongly positive (+++).
Statistical analysis
Data analysis was performed using SPSS version 26.0. The mean ± standard deviation was used to describe the measurement data, and the rate (%) was used to describe the enumeration data. Statistical methods such as Kruskal-Wallis H test and Fisher exact test were used for comparison between groups. The significance test level was set at 0.05 (two-sided test).
Results
OT diagnosis by imaging
Of the eight patients, six were directly diagnosed with OT after they got the gynecologic ultrasound (US) screening. While patient 3 and patient 8 were not considered to have OT until the final ultrasound signs matched with the teratoma and were diagnosed by that (Table 1). In patient 3, the first gynecologic ultrasound did not reveal any abnormality, and the abdominal CT only suggested a fatty shadow in the ovary, so a gynecologist was consulted, and the diagnosis of polycystic ovarian syndrome was considered; later, the patient’s treatment was not effective, and her condition gradually worsened, so the ultrasound was performed the second time and a strong echo in the ovary was detected indicating the OT. Also in patient 8, the presence of teratoma was detected by re-examination of the ultrasound because of the poor effect of the treatment.
Table 1
| Supplementary examinations | Patient#1 | Patient#2 | Patient#3 | Patient#4 | Patient#5 | Patient#6 | Patient#7 | Patient#8 |
|---|---|---|---|---|---|---|---|---|
| Accessories ultrasound | Cystic solid mass | Strong echogenicity | Strong echogenicity | Strong echogenicity | Cystic mass and strong echogenicity | Cystic solid mass with calcification | Cystic solid mass with strong echogenicity | Cystic mass |
| Cranial MRI | Meningeal enhancement | Normal | Normal | Normal | Normal | Normal | Bilateral hippocampal abnormal signals | Acute or subacute cerebral infarction |
| Pelvic MRI | — | Normal | — | — | — | — | — | — |
| Accessories CT | Fatty density masses | Calcified foci | Fatty density shadow | Inhomogeneous mass | Low density mass/calcified foci/fatty shadow | Cystic solid mass | Teratoma | Masses, probably malformed tumors |
| Cranial CT | — | Normal | Cerebral sulcus shallowing/slight swelling of brain parenchyma | — | Nodular calcification in the right frontal lobe | Normal | Slight swelling of the brain parenchyma | — |
| EEG | — | Moderate abnormality | — | Normal | Normal | Severe abnormality | Light abnormality | Severe abnormality |
CT, computerized tomography; EEG, electroencephalogram; MRI, magnetic resonance imaging.
Overall, gynecologic ultrasound findings showed strong echogenicity in the ovaries in three patients, mixed cystic and solid masses in three patients and cystic masses in two patients.
General clinical data of patients
The results showed that the differences in teratoma diameter and CA125 were statistically significant between the groups (P<0.05) (Table 2). Further comparison between the groups showed that the IMT group had higher values of teratoma diameter than the rest of the groups and higher values of CA125 than the MT group, and the differences were statistically significant (adjusted P<0.017), while the differences in the rest of the groups were not statistically significant.
Table 2
| Categories | Encephalitis group | IMT group | MT group | F | P |
|---|---|---|---|---|---|
| Age (years) | 25.00±13.45 | 21.89±7.30 | 25.30±9.92 | 0.65 | 0.53 |
| Tumor location | |||||
| Left | 5 | 8 | 6 | ||
| Right | 3 | 9 | 11 | ||
| Bilateral | 0 | 1 | 3 | ||
| Tumor diameter (cm) | 4.33±1.88 | 15.10±4.60 | 6.59±2.93 | 35.20 | <0.001 |
| CA125 (U/mL) | 58.03±79.30 | 85.81±70.13 | 13.56±6.71 | 10.18 | 0.002 |
| CA19-9 (U/mL) | 149.94±344.01 | 47.62±41.69 | 146.93±348.94 | 0.75 | 0.48 |
| AFP (ng/mL) | 2.24±1.48 | 381.83±789.76 | 2.02±1.40 | 2.07 | 0.15 |
| CEA (ng/mL) | 1.39±0.73 | 1.49±1.20 | 1.01±0.85 | 1.13 | 0.33 |
Data are presented as mean ± standard deviation or n. AFP, alpha-fetoprotein; CEA, carcinoembryonic antigen; IMT, immature teratoma; MT, mature teratoma.
Clinical manifestation of encephalitis patients
The clinical manifestations of the 8 encephalitis patients are shown in Table 3. Three patients (37.5%) had prodromal symptoms like those of infectious encephalitis, typically fever, cough, headache; and 1 of the 3 only had the fever and headache symptoms during the entire course of the disease; 5 patients (62.5%) had simple psychobehavioral disorders such as abnormal excitement and delusion (2 of them were first hospitalized in the psychiatric department); 2 patients (25%) had limbs twitching as the first symptom.
Table 3
| Symptoms | Patient#1 | Patient#2 | Patient#3 | Patient#4 | Patient#5 | Patient#6 | Patient#7 | Patient#8 |
|---|---|---|---|---|---|---|---|---|
| Prodromal symptoms | Fever headache vomiting | Cough, fever | Headache, fever | N | N | N | N | N |
| Primary symptoms | Fever headache vomiting | Abnormal mental behavior | Abnormal mental behavior | Abnormal mental behavior | Abnormal mental behavior | Consciousness limbs twitching | Abnormal mental behavior limbs twitching | Abnormal mental behavior |
| Seizures | N | Y | Intractable | N | N | Intractable | Y | N |
| Psychobehavioral disorders | N | Y | Y | Y | Y | Y | Y | Y |
| Movement disorders | N | Y | Y | N | Y | Y | Y | Y |
| Autonomic dysfunction | N | Y | Y | N | Y | N | Y | N |
| Consciousness disorders | N | Drowsiness | Coma | Drowsiness | N | Drowsiness | Coma | Drowsiness |
| Speech disorders | N | Y | Y | Y | Y | Y | Y | Y |
| First diagnosis | Fever: viral meningitis? | Mental behavior abnormalities: encephalitis? | Impaired consciousness: viral meningitis? | Psychiatric abnormalities to be diagnosed | Uncoordinated psychomotor excitability | Autoimmune encephalitis | Mental behavior abnormalities: encephalitis? | Mental behavior abnormalities: viral encephalitis? |
| Time spent in transfer (days) | 20 | 16 | 39 | 16 | 9 | 8 | 41 | 27 |
| ICU | N | N | Y | N | N | N | N | N |
ICU, intensive care unit; N, none; Y, yes.
In the acute phase, 4 patients (50%) had seizures, and 2 of them progressed to intractable epilepsy; 7 patients (87.5%) were combined with psychiatric and behavioral abnormalities; 6 patients (75%) presented with dyskinesia; 4 patients (50%) had autonomic dysfunction, mainly showing increased heart rate and urinary incontinence; 6 patients (75%) experienced impaired consciousness, including 2 coma cases and 4 drowsiness cases; 7 patients (87.5%) had speech disorders, mainly manifested as speechlessness or their speech cannot be understood.
Among them, patient 3 and patient 7 had to undergo tracheotomy with ventilator-assisted ventilation. Patient 7 took 41 days until she was transferred to our hospital for gynecological surgery, her condition gradually improved after the operation, and the teratoma did not recur during the follow-up period, leaving no neurological symptoms. The disease of patient 3 continued to progress during hospitalization and eventually took 39 days to be transferred to our hospital for surgery. After surgery and being transferred back to the local hospital, she died of multiple organ failure eventually. In this study, the average time from hospitalization to gynecological surgery in the eight patients was 22.00±12.63 days, with the shortest time being 8 days because of positive anti-NMDAR antibodies in the CSF diagnosed in another hospital and the longest being 41 days.
Laboratory test results of encephalitis patients
The results of cerebrospinal fluid (CSF) examination in 8 patients are shown in Table 4, of which patient 6 is only known to have normal CSF results because she was diagnosed and transferred from a local hospital. Five patients had a rise in nucleated cell (NC) count in the range of [10–120]×106/L; 4 patients showed red blood cells (RBCs) with a rise in the range of [3–30]×106/L; 3 patients had a slight rise in trace protein in the range of 0.49–0.53 g/L; 1 patient had a slight rise in glucose level to 4.74 mmol/L; 2 patients had a slight rise in chloride. As for complete blood count (CBC), 6 of them showed an increase in white blood cell (WBC) count in the range of (9.6–13.14)×109/L; 4 patients showed an increase in neutrophil (NEUT) percentage in the range of 76.8–92.2%; 3 showed a decrease in lymphocyte (LYMPH) percentage in the range of 6.1–17.7%. All eight patients’ samples were sent out for autoimmune antibody testing, and one of them also sent blood specimen for autoimmune antibody testing.
Table 4
| Laboratory tests | Reference value | Patient#1 | Patient#2 | Patient#3 | Patient#4 | Patient#5 | Patient#6 | Patient#7 | Patient#8 |
|---|---|---|---|---|---|---|---|---|---|
| CSF | |||||||||
| NC, ×106/L | <8 | 120 | 0 | 120 | 19 | 30 | Normal | 10 | 0 |
| RBC, ×106/L | 0 | 30 | 0 | 10 | 3 | 20 | Normal | 0 | 0 |
| Pyocyte | None | None | None | None | None | None | None | None | None |
| Trace protein, g/L | 0.15–0.45 | 0.53 | 0.2 | 0.28 | 0.23 | 0.51 | Normal | 0.28 | 0.49 |
| Glucose, mmol/L | 2.5–4.4 | 2.58 | 4.02 | 2.52 | 3.75 | 3.67 | Normal | 4.74 | 7.3 |
| Chloride, mmol/L | 120–130 | 123.8 | 124.3 | 132.1 | 127.6 | 128.4 | 133.6 | 123.3 | 128 |
| NMDAR Ab titres (CSF) | 1:32 | 1:32 | 1:32 | 1:100 | 1:32 | 1:10 | 1:32 | 1:32 | |
| NMDAR Ab titres (blood) | N/A | 1:100 | N/A | N/A | N/A | N/A | N/A | N/A | |
| CBC | |||||||||
| WBC, ×109/L | 3.5–9.5 | 13.14 | 9.6 | 11.72 | 10.95 | 13.09 | 7.86 | 10.49 | 9.4 |
| NEUT%, % | 40–75 | 68.5 | 70.1 | 73 | 69 | 76.8 | 92.2 | 84.8 | 89.7 |
| LYMPH%, % | 20–50 | 24.2 | 24.9 | 22.1 | 26 | 17.7 | 6.1 | 7.7 | 25.1 |
CBC, complete blood count; CSF, cerebrospinal fluid; LYMPH, lymphocyte; N/A, not applicable; NC, nucleated cell; NEUT, neutrophil; NMDAR, N-methyl-D-aspartate receptor; RBC, red blood cell; WBC, white blood cell.
Follow-up results of patients in the encephalitis group
Eight patients were followed up, and 2 of them were lost. The mean follow-up time was 29.67±9.48 months (17–45 months). Five of the 6 patients had no recurrence of OT and no neurological symptoms remained; patient 3, whose condition deteriorated during hospitalization in West China University Hospital, Sichuan University, was transferred back to the local hospital after OT resection in West China Second University Hospital, Sichuan University and eventually died of multiple organ failure.
Immunohistochemical findings in OTs
Neural tissues were only found in the paraffin section of the IMT patient with encephalitis. We found that NMDARs were expressed differently in the squamous epithelium and the accessory sebaceous glands in all groups, and was generally more strongly expressed in the encephalitis group; The expression of NMDAR was weakly positive to moderately positive in the neural tissue of teratoma in the encephalitis group, while it was mostly negative in the neural tissue of teratoma in the remaining groups (Figure 1). In the encephalitis group, a clear infiltration of lymphocytes could be seen just near the squamous epithelium in the teratoma tissues, whereas in the control group, the lymphocytes were more dispersed and relatively few in number (Figure 2).
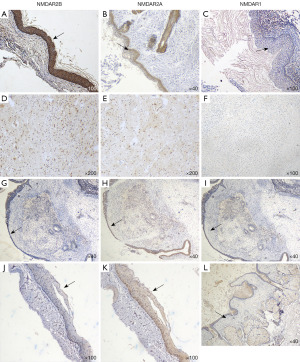
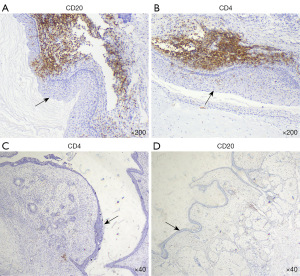
Analysis of the positive expression in different groups
The expression of NMDAR and lymphocytes differed in each group (P<0.05) (Table 5), and then further comparisons were made between the groups separately. The differences in NMDAR, CD4 and CD20 expression were statistically significant between the encephalitis group and the MT group (adjusted P<0.017); the differences in NMDAR2A and CD20 expression were statistically significant between the encephalitis group and the IMT group (adjusted P<0.017).
Table 5
| Categories | Encephalitis group (n=8), n (%) | IMT group (n=18), n (%) | MT group (n=20), n (%) | F | P |
|---|---|---|---|---|---|
| NMDAR1 | 4 (50.0) | 3 (16.7) | 0 | 9.97 | 0.002 |
| NMDAR2A | 8 (100.0) | 6 (33.3) | 9 (45.0) | 10.72 | 0.004 |
| NMDAR2B | 8 (100.0) | 12 (66.7) | 8 (40.0) | 9.25 | 0.01 |
| CD4 | 8 (100.0) | 9 (50.0) | 3 (15.0) | 18.05 | <0.001 |
| CD20 | 8 (100.0) | 6 (33.3) | 6 (30.0) | 12.92 | 0.002 |
There is a single cell expectation <5, choose Fisher’s exact test. IMT, immature teratoma; MT, mature teratoma; NMDAR, N-methyl-D-aspartate receptor.
Further comparison showed that the differences in the degree of positive expression of each marker between the different groups were statistically significant (P<0.05) (Table 6). Two-by-two comparative results showed that there was a statistically significant difference in the degree of positive expression of NMDAR2A, NMDAR2B, CD4 and CD20 between the encephalitis group and the other two control groups (adjusted P<0.017), and a statistically significant difference in the degree of positive expression of NMDAR1 between the encephalitis group and the MT group (adjusted P<0.017). However, further analysis revealed that there was no linear relationship between the CD4/CD20 expression and the NMDAR expression (P>0.05).
Table 6
| Categories | Encephalitis group (n=8), n (%) | IMT group (n=18), n (%) | MT group (n=20), n (%) | H | P |
|---|---|---|---|---|---|
| NMDAR1 | 10.88 | 0.004 | |||
| 0 | 4 (50.0) | 15 (83.3) | 20 (100.0) | ||
| 1+ | 4 (50.0) | 3 (16.7) | 0 | ||
| 2+ | 0 | 0 | 0 | ||
| 3+ | 0 | 0 | 0 | ||
| NMDAR2A | 17.45 | <0.001 | |||
| 0 | 0 | 12 (66.7) | 11 (55.0) | ||
| 1+ | 0 | 3 (16.7) | 7 (35.0) | ||
| 2+ | 8 (100.0) | 3 (16.7) | 2 (10.0) | ||
| 3+ | 0 | 0 | 0 | ||
| NMDAR2B | 18.07 | <0.001 | |||
| 0 | 0 | 6 (33.3) | 12 (60.0) | ||
| 1+ | 1 (12.5) | 9 (50.0) | 6 (30.0) | ||
| 2+ | 3 (37.5) | 3 (16.7) | 2 (10.0) | ||
| 3+ | 4 (50.0) | 0 | 0 | ||
| CD4 | 19.29 | <0.001 | |||
| 0 | 0 | 9 (50.0) | 17 (85.0) | ||
| 1+ | 3 (37.5) | 6 (33.3) | 2 (5.0) | ||
| 2+ | 3 (37.5) | 3 (16.7) | 1 (10.0) | ||
| 3+ | 2 (25.0) | 0 | 0 | ||
| CD20 | 16.11 | <0.001 | |||
| 0 | 0 | 12 (66.7) | 14 (70.0) | ||
| 1+ | 2 (25.0) | 4 (22.2) | 3 (15.0) | ||
| 2+ | 3 (37.5) | 2 (11.1) | 2 (15.0) | ||
| 3+ | 3 (37.5) | 0 | 1 (5.0) |
IMT, immature teratoma; MT, mature teratoma; NMDAR, N-methyl-D-aspartate receptor.
Discussion
Clinical presentation of patients with teratoma causing anti-NMDARE
During the development of anti-NMDARE, it can be roughly divided into three stages according to the clinical manifestations of patients: in the first stage nearly 60–70% of patients will present with a series of atypical symptoms (8,9), such as fever, headache, nausea, and vomiting, which often do not receive sufficient attention from patients or even physicians and are treated as common upper respiratory tract infections. The patient’s condition usually deteriorates sharply within 2 to 4 weeks (10) into the second stage, where prominent psychiatric symptoms such as anxiety, hallucinations, mania, as well as neurologic signs and symptom, including seizures and abnormal movements (8); in the third stage, autonomic dysfunction and impaired consciousness may develop, and there are differences in symptoms between adults and children, as adults are more likely to develop central hypoventilation and undergo tracheotomy and ventilator-assisted ventilation, requiring transfer to the intensive care unit for continuous monitoring (4,11). Although most patients with anti-NMDARE have prodromal symptoms as the early symptom, Kayser et al. (12) retrospectively analyzed the clinical data of 571 patients with anti-NMDARE and found that 23 presented with isolated psychiatric symptoms, of which 10 patients (43%) had an latent tumor during the onset of encephalitis and all were OTs.
Also in our study, 5 patients presented only with mental behavioural abnormalities, which prolonged the time for doctors to make a correct diagnosis, and 2 of them were found to have OT in re-examination sometime later after the initial gynecological ultrasound; these factors significantly prolonged the course of the patients’ disease and delayed the timely teratoma resection.
Therefore, in young women without a family history of psychiatric disorders who suddenly present with abnormal psychiatric behavior, it is important to consider the possibility that the patient may have an OT and routine tumor screening should be performed as the prognosis of these patients depends heavily on early diagnosis and treatment.
Assistant examination in patients with teratoma causing anti-NMDARE
OT is a common type of ovarian germ cell tumor that originates from germ cells and is divided into MT and IMT. Routine detection of blood tumor markers such as CA125, CA19-9, AFP, CEA, etc. can help to identify benign and malignant teratomas. According to the current published literature, there is no significant difference in the course of encephalitis caused by either type of teratoma, but surprisingly, one investigator reported a case in which the onset of encephalitis began 3 days after resection of an IMT (13). It is also worth noting that if preoperative tumor markers or imaging suggest a high likelihood of malignancy of the combined teratoma in patients with anti-NMDARE, timely initiation of adjuvant chemotherapy may help to shorten the course of the disease (10,13).
The results of routine CSF examination in these patients are often non-specific and may show inflammatory reactions in the early stage. A study in China compared the CSF of patients with NMDARE with that of patients with viral encephalitis and found that the number of leukocytes in the CSF of patients with viral encephalitis was significantly higher, and the rate of plasma cells in the CSF of patients with NMDARE was higher, while the ratio of polymorphonuclear cells was lower (14). Espinola-Nadurille et al. (15) analyzed the clinical data of 29 anti-NMDARE patients and found that only 13 patients (44.8%) had CSF characterized like an inflammatory reaction (with an increase in cells and/or proteins) and that this group of patients had a delay in receiving immunotherapy because their CSF results were normal, also they spent twice as long as patients with abnormal CSF before undergoing EEG and MRI, all of which could delay the treatment.
As for imaging, just as found in this study, although most patients with anti-NMDARE have no abnormal findings on cranial CT, MRI or EEG, or their abnormal presentation is usually non-specific and mild, it is still crucial for the screening of OTs. Overall, the sensitivity and specificity of ultrasound for diagnosing MTs range between 58–92.7% and 87.5–99%, respectively (16). The sonograms of most OTs can be divided into these patterns (17): simple cyst with a thick wall, with a nodular projection (Rokitansky nodule) inside; either a parenchymal-like mass with bone, calcification, and fat-like substances, or a mixed one with a series of features such as lipid stratification or dense fine dots.
However, for women suspected of having anti-NMDARE, even if the first ultrasound screening for OT is negative, the diagnosis of it cannot be easily excluded, because even extremely small or even microscopically visible OTs can cause severe encephalitis or even death of the patient, and in emergency situations, it is worth discussing direct surgical removal of the ovaries even if the imaging is negative in order to save the patient’s life (18-20).
NMDAR and lymphocytes expression in various groups
The pathogenesis of teratoma combined with anti-NMDARE has not been fully revealed. Some researchers have found that exogenous antibodies to NMDAR primarily stain areas of neural tissues and also found some specific neuronal cells that are distinctly different from the normal morphology (21-28), but it has also been found that exogenous antibodies to NMDAR can significantly stain only squamous epithelium or cause both components to be stained (29-31). In addition, findings with regard to the cell types of lymphocyte infiltration and the pattern of lymphocyte distribution vary, with some finding significant infiltration of immune cells in neural tissue components and others finding lymphocyte infiltration in the periphery of squamous epithelium where NMDAR receptors are overexpressed (24,25,27,31).
In our study, IHC results showed that NMDAR was expressed in all groups of squamous epithelium and sebaceous glands with different levels of intensity, but the highest expression intensity was found in the encephalitis group; also in the only teratoma containing neural tissues in the encephalitis group, unlike other studies, no morphologically abnormal neurons were found, while neural tissues showed mild to moderate staining. Although the results of the correlation analysis suggested that there was no linear relationship between the expression of NMDAR and lymphocytes infiltration, there were still apparent clusters of lymphocytes near the squamous epithelium of the encephalitis group, while lymphocytes in the control group were mostly scattered.
Due to the low incidence of teratomas causing anti-NMDARE, the current studies are retrospective and there are inconsistent descriptions of teratoma histopathology in patients with encephalitis, so the true mechanisms of why OTs are so common while so few people actually get encephalitis are currently unanswered. Tan et al. (32) have tested iGluRs in inflamed human skin tissues and observed a significant upregulation of their mRNA and protein levels. Meanwhile, a study by Makuch et al. (33) found that B cells capable of producing NMDAR antibodies were continuously being generated in the blood circulation of these patients, and that B cells infiltrated in OTs of patients were also capable of producing NMDAR antibodies after being cultured.
Therefore, we speculate that due to some factors NMDAR expression in the squamous epithelium of OT is significantly increased in patients in the encephalitis group, which triggers a break in immune self-tolerance and leads to a different inflammatory response process in teratoma tissues than in regular OT patients; T and B cell infiltration is enhanced in teratoma tissues of encephalitis patients, and B cells, aided by T cells, continuously activate and proliferate and form germinal centers, and a large number of plasma cells differentiated from B cells produce NMDAR antibodies, which eventually cross the blood-brain barrier and act on the CNS eventually leading to the onset of encephalitis symptoms.
Conclusions
In conclusion, the number of patients examined in this study was limited, and the practical significance of the histopathological findings we performed remains to be studied further and more comprehensively by including more patients. This is the first multi-case group study about OTs causing anti-NMDARE in southwestern China. However, regardless of the pathogenesis, the ability of physicians to identify the presence of OTs early and accurately in young women who present with this type of paraneoplastic encephalitis and to take action is essential to improve patients’ prognosis.
Acknowledgments
The authors thank the patients for their agreement to the publication of the report.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://tcr.amegroups.com/article/view/10.21037/tcr-24-2126/rc
Data Sharing Statement: Available at https://tcr.amegroups.com/article/view/10.21037/tcr-24-2126/dss
Peer Review File: Available at https://tcr.amegroups.com/article/view/10.21037/tcr-24-2126/prf
Funding: This work was supported by
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tcr.amegroups.com/article/view/10.21037/tcr-24-2126/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This study was conducted in accordance with the Declaration of Helsinki and its subsequent amendments. This study was approved by the Ethics Committee of West China Second University Hospital, Sichuan University [No. 2015(032)] on March 9, 2015. Informed consent was taken from all individual participants.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Hansen KB, Yi F, Perszyk RE, et al. Structure, function, and allosteric modulation of NMDA receptors. J Gen Physiol 2018;150:1081-105. [Crossref] [PubMed]
- Huang Q, Xie Y, Hu Z, et al. Anti-N-methyl-D-aspartate receptor encephalitis: A review of pathogenic mechanisms, treatment, prognosis. Brain Res 2020;1727:146549. [Crossref] [PubMed]
- Dalmau J, Tüzün E, Wu HY, et al. Paraneoplastic anti-N-methyl-D-aspartate receptor encephalitis associated with ovarian teratoma. Ann Neurol 2007;61:25-36. [Crossref] [PubMed]
- Titulaer MJ, McCracken L, Gabilondo I, et al. Treatment and prognostic factors for long-term outcome in patients with anti-NMDA receptor encephalitis: an observational cohort study. Lancet Neurol 2013;12:157-65. [Crossref] [PubMed]
- Ciano-Petersen NL, Cabezudo-García P, Muñiz-Castrillo S, et al. Current Status of Biomarkers in Anti-N-Methyl-D-Aspartate Receptor Encephalitis. Int J Mol Sci 2021;22:13127. [Crossref] [PubMed]
- Seery N, Butzkueven H, O’Brien TJ, et al. Contemporary advances in anti-NMDAR antibody (Ab)-mediated encephalitis. Autoimmun Rev 2022;21:103057. [Crossref] [PubMed]
- Herrington CSE, & Editorial Board, WHO. C. O. T. (2020). WHO Classification of Tumours Female Genital Tumours. (5th ed.) International Agency for Research on Cancer. Available online: https://publications.iarc.who.int/Book-And-Report-Series/Who-Classification-Of-Tumours/Female-Genital-Tumours-2020
- Dalmau J, Lancaster E, Martinez-Hernandez E, et al. Clinical experience and laboratory investigations in patients with anti-NMDAR encephalitis. Lancet Neurol 2011;10:63-74. [Crossref] [PubMed]
- Wang W, Li JM, Hu FY, et al. Anti-NMDA receptor encephalitis: clinical characteristics, predictors of outcome and the knowledge gap in southwest China. Eur J Neurol 2016;23:621-9. [Crossref] [PubMed]
- Nizam A, Menzin AW, Whyte JS. Anti-NMDA receptor encephalitis with neurologic sequelae refractory to conservative therapy with complete response to adjuvant therapy. Gynecol Oncol Rep 2020;33:100597. [Crossref] [PubMed]
- Graus F, Titulaer MJ, Balu R, et al. A clinical approach to diagnosis of autoimmune encephalitis. Lancet Neurol 2016;15:391-404. [Crossref] [PubMed]
- Kayser MS, Titulaer MJ, Gresa-Arribas N, et al. Frequency and characteristics of isolated psychiatric episodes in anti–N-methyl-d-aspartate receptor encephalitis. JAMA Neurol 2013;70:1133-9. [Crossref] [PubMed]
- Chetram DK, Pan K, Elfasi A, et al. Anti-NMDAR encephalitis presenting after immature teratoma resection. BMJ Case Rep 2021;14:e244637. [Crossref] [PubMed]
- Huang L, Liang B, Li XL, et al. Comparison of clinical and CSF cytology features of anti-N-methyl-D-aspartate receptor encephalitis and virus encephalitis. Acta Universitatis Medicinalis Anhui 2016;51:140-2.
- Espinola-Nadurille M, Bautista-Gomez P, Flores J, et al. Non-inflammatory cerebrospinal fluid delays the diagnosis and start of immunotherapy in anti-NMDAR encephalitis. Arq Neuropsiquiatr 2018;76:2-5. [Crossref] [PubMed]
- Saleh M, Bhosale P, Menias CO, et al. Ovarian teratomas: clinical features, imaging findings and management. Abdom Radiol (NY) 2021;46:2293-307. [Crossref] [PubMed]
- Saida T, Mori K, Masumoto T, et al. Ovarian and non-ovarian teratomas: a wide spectrum of features. Jpn J Radiol 2021;39:143-58. [Crossref] [PubMed]
- Anderson D, Nathoo N, Henry M, et al. Oophorectomy in NMDA receptor encephalitis and negative pelvic imaging. Pract Neurol 2020; Epub ahead of print. [Crossref] [PubMed]
- Hayashi M, Motegi E, Honma K, et al. Successful Laparoscopic Resection of 7 mm Ovarian Mature Cystic Teratoma Associated with Anti-NMDAR Encephalitis. Case Rep Obstet Gynecol 2014;2014:618742. [Crossref] [PubMed]
- Masghati S, Nosratian M, Dorigo O. Anti-N-methyl-aspartate receptor encephalitis in identical twin sisters: role for oophorectomy. Obstet Gynecol 2014;123:433-5. [Crossref] [PubMed]
- Dalmau J, Gleichman AJ, Hughes EG, et al. Anti-NMDA-receptor encephalitis: case series and analysis of the effects of antibodies. Lancet Neurol 2008;7:1091-8. [Crossref] [PubMed]
- Seki M, Suzuki S, Iizuka T, et al. Neurological response to early removal of ovarian teratoma in anti-NMDAR encephalitis. J Neurol Neurosurg Psychiatry 2008;79:324-6. [Crossref] [PubMed]
- Nolan A, Buza N, Margeta M, et al. Ovarian Teratomas in Women With Anti-N-methyl-D-Aspartate Receptor Encephalitis: Topography and Composition of Immune Cell and Neuroglial Populations Is Compatible With an Autoimmune Mechanism of Disease. Am J Surg Pathol 2019;43:949-64. [Crossref] [PubMed]
- Chefdeville A, Treilleux I, Mayeur ME, et al. Immunopathological characterization of ovarian teratomas associated with anti-N-methyl-D-aspartate receptor encephalitis. Acta Neuropathol Commun 2019;7:38. [Crossref] [PubMed]
- Iemura Y, Yamada Y, Hirata M, et al. Histopathological characterization of the neuroglial tissue in ovarian teratoma associated with anti-N-methyl-D-aspartate (NMDA) receptor encephalitis. Pathol Int 2018;68:677-84. [Crossref] [PubMed]
- Day GS, Laiq S, Tang-Wai DF, et al. Abnormal neurons in teratomas in NMDAR encephalitis. JAMA Neurol 2014;71:717-24. [Crossref] [PubMed]
- Tabata E, Masuda M, Eriguchi M, et al. Immunopathological significance of ovarian teratoma in patients with anti-N-methyl-d-aspartate receptor encephalitis. Eur Neurol 2014;71:42-8. [Crossref] [PubMed]
- Tüzün E, Zhou L, Baehring JM, et al. Evidence for antibody-mediated pathogenesis in anti-NMDAR encephalitis associated with ovarian teratoma. Acta Neuropathol 2009;118:737-43. [Crossref] [PubMed]
- Clark RM, Lynch MP, Kolp R, et al. The N-methyl-D-aspartate receptor, a precursor to N-methyl-D-aspartate receptor encephalitis, is found in the squamous tissue of ovarian teratomas. Int J Gynecol Pathol 2014;33:598-606. [Crossref] [PubMed]
- Jiang XY, Lei S, Zhang L, et al. Co-expression of NMDA-receptor subunits NR1, NR2A, and NR2B in dysplastic neurons of teratomas in patients with paraneoplastic NMDA-receptor-encephalitis: a retrospective clinico-pathology study of 159 patients. Acta Neuropathol Commun 2020;8:130. [Crossref] [PubMed]
- Xiao Y, Li J, Liu L, et al. Clinicopathological characteristics of dysplastic teratomous neuroglia with anti-N-methyl-d-aspartate receptor encephalitis. Clin Immunol 2020;210:108271. [Crossref] [PubMed]
- Tan PH, Yang LC, Chiang PT, et al. Inflammation-induced up-regulation of ionotropic glutamate receptor expression in human skin. Br J Anaesth 2008;100:380-4. [Crossref] [PubMed]
- Makuch M, Wilson R, Al-Diwani A, et al. N-methyl-D-aspartate receptor antibody production from germinal center reactions: Therapeutic implications. Ann Neurol 2018;83:553-61. [Crossref] [PubMed]


