
Intracranial activity of crizotinib: something to rely on?
Central nervous system (CNS) metastases remain a significant problem in the management of patients with ALK-rearranged non-small cell lung cancer (NSCLC). The frequency of CNS involvement in ALK-positive tumors is extremely high; it approaches 25% in treatment-naïve patients (1) and rises to 50% in patients treated with crizotinib (2,3). Crizotinib was previously reported to have only minor intracranial activity (4), with poor CNS penetration suggested as the underlying mechanism (5).
The study published this year by Solomon and colleagues in Journal of Clinical Oncology addressed the question of intracranial efficacy of crizotinib in PROFILE 1014 trial (1). The study confirmed that crizotinib, as compared with platinum-based chemotherapy, is associated with better progression-free survival (PFS) irrespectively of presence or absence of brain metastases at the time of initial diagnosis [HR 0.4 (0.23–0.29); P<0.001] and 0.51 (0.38–0.69; P<0.001) for patients with and without CNS metastases, respectively). Most importantly, the results hinted towards better control of intracranial disease with crizotinib as compared to platinum-based chemotherapy. The effect was even more pronounced in patients with brain metastases treated with radiotherapy (RT) before enrollment. For instance, crizotinib treatment was associated with numerically better intracranial time to tumor progression (IC-TTP) both in the intent-to-treat (ITT) population and the subgroup of patients with treated CNS metastases; however, the results were not statistically significant. Crizotinib treatment was also associated with significantly better intracranial disease-control rate (IC-DCR) in patients with previously treated CNS metastases confirming the results of a combined analysis of PROFILE 1005 and PROFILE 1007 studies (4).
Can the study published by Solomon and colleagues provide us with a “yes or no” answer with regards to intracranial efficacy of crizotinib? The answer to the question is no. It is important to emphasize that although IC-TTP was a protocol-specified end-point, the study was underpowered to demonstrate a statistically significant difference in intracranial effects between crizotinib and chemotherapy. In fact, only 15% of the ITT population had their disease progressed in the CNS. On the other hand, if the existing difference is too small to be picked-up in a large-size cohort study—whether that amount of effect we are looking for in clinics?
Interestingly enough, the intracranial effect of crizotinib was more pronounced in patients with brain metastases at study entry. Is it a pure statistical phenomenon? Imbalances in the baseline patient characteristics, such as male predominance in the CNS metastases subgroup, cannot be responsible for the differences observed. Differences in the schedule assessment between the subgroups may have confounded the results. However, the most possible explanation for larger effect observed with crizotinib in patients with brain metastases treated with RT before the study entry as opposed to patients without CNS metastases is better drug penetration into the CNS resulting from the disruption of the blood-brain barrier by the brain irradiation. Of note, the results observed in that subgroup are in line with the results of the combined analysis of PROFILE 1005 and PROFILE 1007 studies, confirming higher CNS control rate achieved with brain irradiation (4). Solomon and colleagues were first to demonstrate that better CNS control is not a pure radiation effect (since both arms received brain irradiation), but the effect of the combination, supporting the hypothesis of drug penetration improvement following brain RT. Indeed, higher peak (Cmax) crizotinib concentrations in the cerbro-spinal fluid (CSF) may provide a prolonged CNS control in ALK-rearranged tumors (6-8).
Importantly, another scenario of combining RT with crizotinib in order to achieve intracranial control is administration of cranial irradiation after isolated intracranial progression which allows controlling the disease for another 5–7 months (1,4,9).
Overall, crizotinib used as a sole modality has modest intracranial activity and is only marginally superior to chemotherapy in terms of intracranial disease control. Furthermore, its intracranial effects require brain irradiation to be given at some point in the majority of cases in order to control the disease in the CNS. Whole brain radiation therapy (WBRT), on the other hand, delivered early in the disease course frequently results in long-term cognitive decline and substantial neurological morbidity (10).
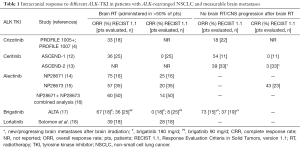
New-generation ALK-inhibitors not only have a broader spectrum of activity in terms of resistant mutations in the ALK gene but also possess better CNS penetration. In particular, alectinib administered at a standard dose produces therapeutic concentrations in the CSF (11). Although the data with regards to intracranial activity of newer compounds is limited, it is very promising (Table 1). Thus, new generation ALK-inhibitors might represent a better alternative to RT in case of intracranial progression during crizotinib treatment (8). Furthermore, it is very possible that new-generation ALK-inhibitors are superior to crizotinib in treatment-naïve patients. According to the results of J-ALEX, a Japanese phase III randomized trial evaluating alectinib versus crizotinib in advanced ALK-positive NSCLC patients naïve to ALK-tyrosine kinase inhibitors (TKI), alectinib is superior to crizotinib in terms of PFS (19). The results of ALEX trial having the same design and conducted in the Caucasian population are highly awaited. Noteworthy, permitting patients with asymptomatic CNS metastases and having time-to-CNS progression as a key secondary end-point, ALEX trial is expected to provide important prospective data on the comparative intracranial efficacy of the two agents.
Full table
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned and reviewed by the Section Editor Shaohua Cui (Department of Pulmonary Medicine, Shanghai Chest Hospital, Shanghai Jiao Tong University, Shanghai, China).
Conflicts of Interest: Elizabeth Dudnik—honoraria for lectures: Pfizer and Roche; Nir Peled—paid membership of advisory boards: Pfizer.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Solomon BJ, Cappuzzo F, Felip E, et al. Intracranial Efficacy of Crizotinib Versus Chemotherapy in Patients With Advanced ALK-Positive Non-Small-Cell Lung Cancer: Results From PROFILE 1014. J Clin Oncol 2016;34:2858-65. [Crossref] [PubMed]
- Camidge DR, Bang YJ, Kwak EL, et al. Activity and safety of crizotinib in patients with ALK-positive non-small-cell lung cancer: updated results from a phase 1 study. Lancet Oncol 2012;13:1011-9. [Crossref] [PubMed]
- Otterson GA, Riely GJ, Shaw AT, et al. Clinical characteristics of ALK+ NSCLC patients (pts) treated with crizotinib beyond disease progression (PD): Potential implications for management. J Clin Oncol 2012;30:abstr 7600.
- Costa DB, Shaw AT, Ou SH, et al. Clinical Experience With Crizotinib in Patients With Advanced ALK-Rearranged Non-Small-Cell Lung Cancer and Brain Metastases. J Clin Oncol 2015;33:1881-8. [Crossref] [PubMed]
- Costa DB, Kobayashi S, Pandya SS, et al. CSF concentration of the anaplastic lymphoma kinase inhibitor crizotinib. J Clin Oncol 2011;29:e443-5. [Crossref] [PubMed]
- Kim YH, Ozasa H, Nagai H, et al. High-dose crizotinib for brain metastases refractory to standard-dose crizotinib. J Thorac Oncol 2013;8:e85-6. [Crossref] [PubMed]
- Peled N, Zach L, Liran O, et al. Effective crizotinib schedule for brain metastases in ALK rearrangement metastatic non-small-cell lung cancer. J Thorac Oncol 2013;8:e112-3. [Crossref] [PubMed]
- Dudnik E, Siegal T, Zach L, et al. Durable brain response with pulse-dose crizotinib and ceritinib in ALK-positive non-small cell lung cancer compared with brain radiotherapy. J Clin Neurosci 2016;26:46-9. [Crossref] [PubMed]
- Weickhardt AJ, Scheier B, Burke JM, et al. Local ablative therapy of oligoprogressive disease prolongs disease control by tyrosine kinase inhibitors in oncogene-addicted non-small-cell lung cancer. J Thorac Oncol 2012;7:1807-14. [Crossref] [PubMed]
- Attia A, Page BR, Lesser GJ, et al. Treatment of radiation-induced cognitive decline. Curr Treat Options Oncol 2014;15:539-50. [Crossref] [PubMed]
- Gadgeel SM, Gandhi L, Riely GJ, et al. Safety and activity of alectinib against systemic disease and brain metastases in patients with crizotinib- resistant ALK-rearranged non-small-cell lung cancer (AF-002JG): Results from the dose-finding portion of a phase 1/2 study. Lancet Oncol 2014;15:1119-28. [Crossref] [PubMed]
- Kim DW, Mehra R, Tan DS, et al. Activity and safety of ceritinib in patients with ALK-rearranged non-small-cell lung cancer (ASCEND-1): updated results from the multicentre, open-label, phase 1 trial. Lancet Oncol 2016;17:452-63. [Crossref] [PubMed]
- Crinò L, Ahn MJ, De Marinis F, et al. Multicenter Phase II Study of Whole-Body and Intracranial Activity With Ceritinib in Patients With ALK-Rearranged Non-Small-Cell Lung Cancer Previously Treated With Chemotherapy and Crizotinib: Results From ASCEND-2. J Clin Oncol 2016;34:2866-73. [Crossref] [PubMed]
- Shaw AT, Gandhi L, Gadgeel S, et al. Alectinib in ALK-positive, crizotinib-resistant, non-small-cell lung cancer: a single-group, multicentre, phase 2 trial. Lancet Oncol 2016;17:234-42. [Crossref] [PubMed]
- Ou SH, Ahn JS, De Petris L, et al. Alectinib in Crizotinib-Refractory ALK-Rearranged Non-Small-Cell Lung Cancer: A Phase II Global Study. J Clin Oncol 2016;34:661-8. [Crossref] [PubMed]
- 16th World Conference on Lung Cancer. J Thorac Oncol 2015. Available online: http://wclc2015.iaslc.org/wp-content/uploads/2015/11/WCLC-2015-Abstract-Book_vF_FOR-JTO-Website_low-res_REV-NOV-2015.pdf
- Kim DW, Tiseo M, Ahn MJ, et al. Brigatinib (BRG) in patients (pts) with crizotinib (CRZ)-refractory ALK+ non-small cell lung cancer (NSCLC): First report of efficacy and safety from a pivotal randomized phase (ph) 2 trial (ALTA). J Clin Oncol 2016;34:abstr 9007.
- Solomon BJ, Bauer TM, Felip E, et al. Safety and efficacy of lorlatinib (PF-06463922) from the dose-escalation component of a study in patients with advanced ALK+ or ROS1+ non-small cell lung cancer (NSCLC). J Clin Oncol 2016;34:abstr 9009.
- Nokihara H, Hida T, Kondo M, et al. Alectinib (ALC) versus crizotinib (CRZ) in ALK-inhibitor naive ALK-positive non-small cell lung cancer (ALK+ NSCLC): Primary results from the J-ALEX study. J Clin Oncol 2016;34:abstr 9008.


