
Herpes simplex virus-thymidine kinase/ganciclovir suppressed the growth of lung adenocarcinoma cells accompanied by premature senescence
Highlight box
Key findings
• The herpes simplex virus-thymidine kinase/ganciclovir (HSV-TK/GCV) system significantly inhibits the growth, migration, and invasion of lung adenocarcinoma cells both in vitro and in vivo, and this inhibition is accompanied by cell premature senescence.
What is known and what is new?
• Previous research has established that the HSV-TK/GCV system has therapeutic effects on a variety of tumours. The system works by converting a nontoxic prodrug, GCV, into a toxic form that kills dividing cells, making it a promising approach for cancer treatment.
• This study extends current knowledge by focusing on the specific application of the HSV-TK/GCV system in lung adenocarcinoma. It provides novel insights into the system’s ability to induce premature senescence in cancer cells, which is an additional mechanism by which the system may suppress tumour growth. Furthermore, the study introduces the use of adeno-associated virus as a vector to deliver the HSV-TK gene, which could potentially improve the efficiency of gene therapy in cancer treatment.
What is the implication, and what should change now?
• This article focuses on the HSV-TK/GCV system’s effect on lung adenocarcinoma. It implies that this system has great potential in treating lung adenocarcinoma. Based on this, current treatments could change by conducting clinical trials to assess its safety and efficacy and exploring combination therapies.
IntroductionOther Section
Suicide gene therapy, also named virus-directed enzyme prodrug therapy, utilises the vector to transfect the genes that express exogenous enzyme activity into the tumour cells. Then, the enzyme activity transforms non-toxic drugs into toxic substances, thus achieving the goal of killing the tumour cell. The known suicide genes mainly include herpes simplex virus-thymidine kinase (HSV-TK), cytosine deaminase (CD), varicella-zoster virus and nitrate, alanine aminotransferase (GPT) and sulphur dioxide (DEO) in Escherichia coli. Among them, HSV-TK and CD have been the most extensively studied. Ganciclovir (GCV) is a kind of nucleoside analogue. HSV-TK/GCV system has more directive killing effects to tumour than other genes.
HSV-TK/GCV system could code TK via HSV-TK and then metabolise GCV, the nucleoside analogues, into cytotoxic triphosphoric acid substance. Recent data showed that HSV-TK has a killing effect on various tumours, including glioma (1,2), tonsillar carcinoma (3), renal cell carcinoma (4), hepatocellular carcinoma (5), colorectal cancer (6), breast cancer (7,8), prostate cancer (9), and bladder cancer (10). The HSV-TK/GCV killing system could inhibit lung cancer cell proliferation in vitro (11-16) and prolong the survival time of mouse in an in-situ lung tumour model (17). The effect of the HSV-TK/GCV system on lung adenocarcinoma cell lines in vivo was unclear. It is widely acknowledged that transfection efficiency is a critical factor of therapy in vivo, yet it remains a challenge to achieve consistently. The application of traditional method utilizing liposome as the transmitter to import TK gene is limited due to low transfection efficiency in vitro and inoperability in vivo (18). Thus, adeno-associated virus (AAV) was introduced in the present study to overcome the challenges.
HSV-TK/GCV system, mediated by AAV and named AAV-TK, significantly inhibited the growth, metastasis, and migration ability of lung adenocarcinoma cell in vitro and vivo. The ratio of premature senescence cells was also enhanced in vitro. We present this article in accordance with the MDAR and ARRIVE reporting checklists (available at https://tcr.amegroups.com/article/view/10.21037/tcr-24-1815/rc).
MethodsOther Section
Strains, plasmids, and reagents
Human lung adenocarcinoma A549 cell line was purchased from the cell bank of Beijing Union Medical College. Mouse lung adenocarcinoma Lewis cell line was gifted from Feng-Sheng Li of PLA Rocket Rorce Characteristic Medical Center. Endo-free plasmid kit purchased from Tiangen (Beijing, China), Lipofectamine 3000 and OptiMEM were purchased from Invitrogen Corporation (Carlsbad, CA, USA). AAV-TK sequence was from the coding sequence of human herpesvirus 1 strain TK. AAV-TK and the control plasmids of AAV-green fluorescent protein (GFP) were gifted from Dr. Chang Tong of the University of Southern California.
Cell transfection
A549 and Lewis cells were collected at logarithmic growth phase (1×105 cells). Solution A (AAV-GFP plasmid) and solution B (Lipofectamine 2000) were prepared in accordance with the manufacturer’s instructions (Invitrogen), mixed for 20 min, incubated with cells at 37 ℃ for 4–6 hours and then replaced with Roswell Park Memorial Institute (RPMI) 1640 medium. Green fluorescence (490 nm) was examined at 24 hours after transfection. AAV-TK was transfected using the same method.
AAV virus packaging
293T cells were inoculated in six-well plates and stay overnight. The packaging plasmids AAV-DJ, helper plasmid, and objective plasmid AAV-GFP or AAV-TK were mixed in 1:1:1 radio. The cells were transfected with 4 µg total plasmid DNA (1.33 µg each of the plasmid DNA). Then, 10 µL Lipofectamine 2000 was diluted into 150 µL OptiMEM and incubated at room temperature for 5 min. The DNA/Lipofectamine mix was incubated for 15 min. The transfection cocktail was then pipetted into the six-well plate drop by drop.
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay
Lewis cells and A549 cells, transfected for AAV-GFP and AAV-TK separately, were used for the MTT assay. AAV-GFP-transfected cells were as the control group. The cells were plated into 96-well plates at a density of 7,000 cells per well and incubated at 37 ℃, in a humidified atmosphere containing 5% CO2. The cells were evaluated at three time points post-transfection: 24, 48, and 72 hours, 20 µL MTT solution was added to each well, respectively, followed by a 4-hour incubation at 37 ℃ in a CO2 incubator. Subsequently, 100 µL of dimethyl sulfoxide (DMSO) was added to dissolve the formazan crystals. The optical density (OD) was then measured at 490 nm using a microplate spectrophotometer.
Colony formation assay
Lewis cells and A549 cells, were trypsinized into suspension 24 hours after separate transfections with AAV-TK and AAV-GFP. A total of 700 cells were resuspended in 3 mL of 10% Dulbecco’s modified Eagle medium (DMEM) culture medium containing double-antibody and seeded into 60 mm petri dishes. Cells were conducted under 37 ℃, 5% CO2 and 95% humidity for 7 days. Subsequently, the cells were washed twice with pre-warmed phosphate-buffered saline (PBS) and fixed with methanol for 20 min. Then, Giemsa dye staining was conducted for 15 min, followed by washing and counting the number of clones under a dissecting microscope. Colony-forming efficiency (CFE) and surviving fraction (SF) were calculated as follow: CFE (%) = (number of clones/inoculated cells) × 100% and SF (%) = (processing cell colony formation rate/control cell colony formation rate) × 100%. Three replicates for each sample and three parallel experiments were designed to ensure the robustness and reproducibility of the results.
Wound-healing assay
A mark pen and a ruler were used to draw straight lines evenly at the back of six-well plates. Lewis cells and A549 cells, transfected with AAV-TK and AAV-GFP, were seeded at a density of 5×105 cells per well in six-well plates. The cells were placed in the incubator for 24 hours to achieve 80–90% confluence. A 200 µL sterilised pipette tip was used to create a straight scratch along the ruler, ensuring that the scratch was as perpendicular as possible to the horizontal plane of the plate’s surface. The exfoliated cells were washed with PBS and serum-free medium was added. Then, they were placed in 37 ℃, 5% CO2, and 95% humidity incubator. Wound closure was monitored at 24 hours post scratch and imaged with a laser scanning LSM 710 Carl Zeiss confocal microscope (×20 magnification). The length (scratch) was quantified with ImageJ software and data were represented as distance.
Invasion assay
The invasion of cells was measured as the number of cells invading through matrigel-coated transwell inserts (19). Briefly, transwell inserts with 8 µm pores were coated with Matrigel (20 µg/well; BD Biosciences, Franklin Lakes, NJ, USA). A total of 2×105 cells were suspended in 200 µL serum-free DMEM and seeded into the upper chamber. DMEM supplemented with 10% foetal bovine serum was placed in the lower chamber as a chemoattractant. The cells were allowed invade for 20 h. Following incubation, the non-invasive cells on the upper side of the membrane were removed using a cotton swab. The invasive cells on the lower surface of the Matrigel-coated membrane were fixed with 100% methanol and stained using 4',6-diamidino-2-phenylindole (DAPI) (Sigma-Aldrich, St. Louis, MO, USA). The invasion was determined by counting cells in five fluorescence microscope fields/well and the extent of invasion was expressed as an average number of cells/microscopic field.
β-galactosidase staining experiment
A549 and Lewis cells were seeded at an equivalent concentration in 24-well plates and incubated for a period of 24 hours to allow for attachment and initial growth. Subsequently, the cells were transfected with TK and GFP constructs. Seventy-two hours post-transfection, the cells were subjected to β-galactosidase staining according to the manufacturer’s instructions provided with the staining kit. Following the staining procedure, the cells were incubated at 37 ℃ in the dark for a duration of 2 hours to develop the stain. The stained cells were then observed under a microscope and photographed for documentation. A549 and Lewis cells were seeded in 24-well plates at the same concentration and incubated for 24 hours. The cells were transfected with TK and GFP constructs. Seventy-two hours post-transfection, a β-galactosidase staining kit was used for dyeing in accordance with the instructions. Then, the cells were kept away from light for 2 hours in 37 ℃, observed and photographed.
In-vivo experiments in mice
All animal experiments were performed under a project license (No. [2013]012) granted by the Animal Care and Use Committee of National Institute for Radiological Protection of the Chinese Center for Disease Control and Prevention, in compliance with Chinese national guidelines for the care and use of animals. A protocol was prepared before the study without registration. Six-week-old male C57BL/6 wild-type mice, with an average body weight of approximately 20 g, were purchased from the Beijing Vital River Laboratory Animal Technology Co. (Beijing, China). All mice were littermates and they were maintained under specific pathogen-free conditions at the Animal Centre of Beijing Normal University (Beijing, China), and other irrelevant conditions are the same. Ten male mice were randomly divided into two groups: the AVV-TK group and the AVV-GFP group (n=5 for each group). Gender had no effect on the experiment results, The individual mouse was considered the experimental unit within the study. Pentobarbital was used for mice anaesthesia at a dose of 50 mg/kg, administered by intraperitoneal injection, and continue monitoring the respiratory rate and reflexes of mice to ensure adequate anesthesia. Firstly, the tumour block was generated by subcutaneous injection of Lewis cells into armpit of C57BL/6 (B6) mice, and secondly, the tumour block was divided into small-tumour-block of uniform size. Finally, small-tumour-block was subcutaneously transplanted into the armpit of 8-week-old male C57BL/6 (B6) mice under anaesthetic condition, to establish the murine transplantation tumour model of Lewis cells. After wound healing, the two groups were regularly injected every 2 days with 100 µL of packed GFP and TK virus solution, and with GCV after measuring the tumour volume and weight of mice. Tumours were measured by a calliper every 2 days and the mice were injected six times in total. Each tumour volume in cubic millimetre was calculated using the following formula: V = 0.5 × D × d2 (V, volume; D, longitudinal diameter; d, latitudinal diameter). Finally, the moribund mice were killed humanely in accordance with the Guidelines for Humane End Points for Animals Used in Biomedical Research (20). The tumours were recovered from the moribund or recently deceased mice. The tissue samples were fixed in 10% buffered formalin, embedded in paraffin wax, and stained with haematoxylin and eosin (H&E). Immunohistochemical staining was performed to determine the expression of p16 and proliferating cell nuclear antigen (PCNA) in mouse tumours. To reduce unnecessary errors, injections for all animals are performed by a single experimenter. The research team monitored animals twice daily. Health was monitored by weight (twice weekly), food and water intake, and general assessment of animal activity, panting, and fur condition. The maximum size the tumours allowed to grow in the mice before euthanasia was 2,000 mm.
H&E and immunohistochemical staining
After the mice were euthanised, the tissues from each mouse were removed, fixed for 24 h, embedded in paraffin and then prepared at 3 µm thickness by using a routine procedure (21). The sections were used to perform H&E staining for general histology, and identify the expression of p16 and PCNA by immunohistochemistry. Briefly, after dewaxing, dehydration, rehydration, and antigen repair with microwave were completed, the paraffin sections were blocked with 3% H2O2 deionised water and 5% normal sheep serum in PBS (0.01 M) and subsequently incubated with specific primary antibodies against p16 (1:100) and PCNA (1:100) at 4 ℃ overnight, followed by staining with horseradish peroxidase-conjugated secondary antibodies and counterstaining with haematoxylin to visualise the nuclei. The p16 and PCNA paraffin sections were viewed under a common light microscope equipped with a digital camera and identified by brown colour.
Statistical analysis
All experiments were performed in triplicate or quadruplicate. Animal experiments used biological replicates, and cell experiments used technical replicates. Triplicate measurements were conducted per data point in each experiment. A two-tailed Student’s t-test was used for statistical analysis of comparative data by using SPSS software. P<0.05 values were considered significant.
ResultsOther Section
HSV-TK/GCV system significantly inhibited the viability of A549 and Lewis cells
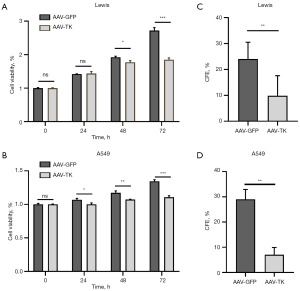
The effect of HSV-TK/GCV system on cell viability was firstly detected in vitro. Lewis cells and A549 cells were used to evaluate the effects of HSV-TK gene expression mediated by AAV-TK transfection. The cells were monitored at three time points post-transfection: 24, 48, and 72 hours, to capture the dynamic changes in cell proliferation. AAV-GFP-transfected cells were as the control group. MTT assays revealed that AAV-TK transfection significantly inhibited Lewis cell proliferation at 72 hours (P<0.05; Figure 1A) and A549 cell proliferation from 24 to 72 hours (P<0.05 in 24, 48, and 72 hours; Figure 1B), compared with the control group. Furthermore, clonogenic assay showed the similar results that AAV-TK transfection inhibited the colony formation capacity of Lewis and A549 cells (P<0.05 in Lewis and P<0.05 in A549; Figure 1C,1D).
HSV-TK/GCV system significantly inhibited the migration capability of Lewis and A549 cells
Wound-healing assays determined the distance moved by a wounded cell monolayer on plastic after AAV-GFP and AAV-TK treatment. The results (Figure 2A-2D) were reported as migration length expressed as the distance moved by the cells. The TK group exhibited decreased migration compared with the controls (P<0.05 in Lewis, Figure 2A,2B and P<0.05 in A549, Figure 2C,2D). Transwell assay, expressed as the number of cell invaded, showed that the inhibited migration capability of AAV-TK group by the HSV-TK/GCV system compared with that of the GFP group (P<0.05 in Lewis and A549, Figure 2E,2F).
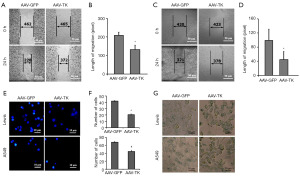
HSV-TK/GCV system could accelerate cell premature senescence
Cell premature senescence is a state of irreversible growth arrest that can be induced by various stresses. It serves as a natural defense mechanism against cancer by preventing the proliferation of damaged cells. Senescent cells typically exhibit characteristic morphological changes and express specific markers, such as increased activity of β-galactosidase and the expression of p16. The β-galactosidase staining experiment results showed that the HSV-TK/GCV system could accelerate cell premature senescence, as shown by the increase in the production of β-galactosidase in the AAV-TK group compared with that in the AAV-GFP group (Figure 2G).
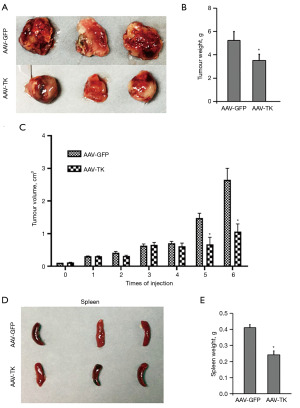
HSV-TK/GCV system could delay growth of tumours in vivo
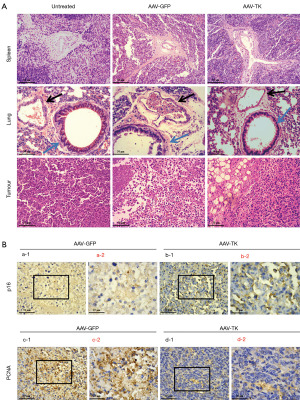
The HSV-TK/GCV system visibly inhibited the tumour growth of Lewis cells in mice in vivo (P<0.05, Figure 3A), and the tumour weights and tumour volume were significantly decreased (P<0.05, Figure 3B,3C) compared with those of the controls. Interestingly, spleen weight was also significantly lighter than that in the AAV-GFP group as control (P<0.05, Figure 3D,3E). H&E (Figure 4A) and immunohistochemical staining assays (Figure 4B) were conducted in tumours and spleens. No significant differences in spleen H&E staining were observed between the untreated, GFP, and TK groups, while the lung H&E staining showed an obvious variation among the three groups. Normal group showed no or rarely inflammatory cell infiltrate around the airway (blue arrow) and large vessels (black arrow). However, GFP group showed increased inflammatory cell infiltration around the airways, and large vessels were distributed into pieces or clusters. TK group exhibited significant reduction in airway and perivascular inflammatory cell infiltration compared to the GFP group. The H&E staining results showed that the HSV-TK/GCV system caused less damage to normal tissue and effectively inhibited tumour growth (Figure 4A). Immunohistochemical staining suggested that the protein level of p16 was elevated and that of PCNA decreased significantly in the AAV-TK group, indicating that the HSV-TK/GCV system could promote premature senescence in lung adenocarcinoma cells (Figure 4B).
DiscussionOther Section
Suicide gene therapy provides a novel concept and approach for tumour therapy. HSV-TK/GCV system exhibits potent therapeutic effects against various types of tumours (22), however, it continues to face theoretical and technical challenges, such as the difficulties to identify target cells, low efficacy of gene transfection and short efficient regulation control of gene expression after transfection (18).
The present study demonstrated that the HSV-TK/GCV system could inhibit cancer cell proliferation and migration in vitro. Furthermore, this system has a therapeutic effect in tumour implanted in mice.
The combination of HSV-TK/GCV and radiofrequency could significantly inhibit HepG2 cell proliferation, as evidenced by the lowest cell proliferation in combination therapy group compared amongst the three control groups (52%±3% vs. 75%±9% vs. 93%±4% vs. 100%±5%, P<0.05) (19). In the present study, cell proliferation was detected by MTT, and the cell transfected with AAV-TK was inhibited in terms of viability (73.5%±3.3% vs. 100%±5.0% in Lewis and 79.5%±4.8% vs. 0.39%±5.0% in A549, P<0.05). The low killing effect was perhaps induced by the different efficiency of transfection in two different cell lines. The colony formation rates of the AAV-TK group were decreased to 42% and 24% than those of the AAV-GFP group in Lewis and A549 cell lines, respectively. The tumour killing effect in vivo in the AAV-TK group also has noticeable effect compared with that in the AAV-GFP group. This is the first study that has shown significantly killing effect in Lewis cell in vivo from the perspective of lung cancer therapy. Those results suggested that the killing effect of HSV-TK is of great theoretical significance to the therapy of lung cancer (13,21).
Laberge et al. (23) revealed that HSV-TK/GCV system could induce senescence. The low concentrations of GCV could induce senescence through the accumulation of nuclear DNA damage, whilst increased concentrations of GCV, similar to those used in vivo, could kill non-dividing senescent cells via mitochondrial DNA (mtDNA) damage and caspase-dependent apoptosis. The present study confirmed that the HSV-TK/GCV system inhibited the proliferation of lung adenocarcinoma, accompanied by senescent cell promotion.
In 1990, Moolten et al. (24) firstly applied TK in cancer therapy. After that, TK gene is widely used in various tumours, such as glioma, tonsillar carcinoma, melanoma, ovarian cancer, liver cancer and gastric cancer (1-16). Through experiments in the cellular level to animal experiments and then to clinical I and II trials, researchers have been equipped with a basic understanding of the clinical effect, limitations and prospects to TK. Fortunately, GCV is widely used in clinical therapy, the dose of GCV could control the cytotoxic effect of HSV-TK. These results exhibit the potential of GCV in enhancing the efficacy of HSV-TK, making the future applications of this system in oncology more promising than ever before.
The experiments in vivo and in vitro observably showed that the HSV-TK/GCV system inhibited the growth of tumour cells and had the same or even smaller damage to normal tissues compared with the control group. The proliferation and migration capability of cells were inhibited by the HSV-TK/GCV system. The results also verified that the level of premature senescence in AAV-TK was higher than AAV-GFP group. Immunohistochemical staining revealed an increase in p16 and a decrease in PCNA within mouse tumours treated with AAV-TK, which was associated with senescence and proliferation. In conclusion, the HSV TK/GCV system could significantly inhibit the growth and metastasis of lung adenocarcinoma, accompanied by cell premature senescence. This finding may lead to the clinical application of the HSV-TK/GCV system in lung cancer.
Nevertheless, the research of cancer suicide gene therapy is still in its nascent stages. Although this approach has demonstrated promising outcomes in vitro and in animal experiments, the killing effect and mechanism to human subjects have not been fully understand. In addition, the application in clinical practice still faces many challenges. As research progresses, HSV-TK/GCV system therapy is expected to act as a promising therapeutic strategy for clinical treatment of advanced, recurrent, and metastatic tumours to improve the life quality of patients and extend survival time.
ConclusionsOther Section
This study demonstrates that the HSV-TK/GCV system effectively inhibits the growth, migration, and invasion of lung adenocarcinoma cells both in vitro and in vivo. Furthermore, the system also induced premature senescence in lung adenocarcinoma cells. This system shows the potential as a potential therapeutic approach for the treatment of lung adenocarcinoma.
AcknowledgmentsOther Section
None.
FootnoteOther Section
Reporting Checklist: The authors have completed the MDAR and ARRIVE reporting checklists. Available at https://tcr.amegroups.com/article/view/10.21037/tcr-24-1815/rc
Data Sharing Statement: Available at https://tcr.amegroups.com/article/view/10.21037/tcr-24-1815/dss
Peer Review File: Available at https://tcr.amegroups.com/article/view/10.21037/tcr-24-1815/prf
Funding: This work was supported by
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tcr.amegroups.com/article/view/10.21037/tcr-24-1815/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All animal experiments were performed under a project license (No. [2013]012) granted by the Animal Care and Use Committee of National Institute for Radiological Protection of the Chinese Center for Disease Control and Prevention, in compliance with Chinese national guidelines for the care and use of animals.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
ReferencesOther Section
- Seth R, Khan AA, Pencavel TD, et al. Adenovirally delivered enzyme prodrug therapy with herpes simplex virus-thymidine kinase in composite tissue free flaps shows therapeutic efficacy in rat models of glioma. Plast Reconstr Surg 2015;135:475-87. [Crossref] [PubMed]
- Chien YC, Chen JC, Lin WC, et al. Using [18F]FBAU for imaging brain tumor progression in an F98/tk-luc glioma-bearing rat model. Oncol Rep 2014;32:691-9. [Crossref] [PubMed]
- Goh AR, Shin SP, Jung NR, et al. Low-dose cisplatin converts the tumor microenvironment into a permissive state for HSVtk-induced antitumor immunity in HPV16-related tonsillar carcinoma. Cancer Lett 2015;356:743-50. [Crossref] [PubMed]
- Xiao X, Jin R, Li J, et al. The antitumor effect of suicide gene therapy using Bifidobacterium infantis-mediated herpes simplex virus thymidine kinase/ganciclovir in a nude mice model of renal cell carcinoma. Urology 2014;84:982.e15-20. [Crossref] [PubMed]
- Zhu R, Chen D, Lin D, et al. Adenovirus vector-mediated herpes simplex virus-thymidine kinase gene/ganciclovir system exhibits anti-tumor effects in an orthotopic hepatocellular carcinoma model. Pharmazie 2014;69:547-52. [PubMed]
- Higashi K, Hazama S, Araki A, et al. A novel cancer vaccine strategy with combined IL-18 and HSV-TK gene therapy driven by the hTERT promoter in a murine colorectal cancer model. Int J Oncol 2014;45:1412-20. [Crossref] [PubMed]
- Zhan Y, Yu B, Wang Z, et al. A fiber-modified adenovirus co-expressing HSV-TK and Coli.NTR enhances antitumor activities in breast cancer cells. Int J Clin Exp Pathol 2014;7:2850-60. [PubMed]
- Castillo-Rodríguez RA, Arango-Rodríguez ML, Escobedo L, et al. Suicide HSVtk gene delivery by neurotensin-polyplex nanoparticles via the bloodstream and GCV Treatment specifically inhibit the growth of human MDA-MB-231 triple negative breast cancer tumors xenografted in athymic mice. PLoS One 2014;9:e97151. [Crossref] [PubMed]
- Rojas-Martínez A, Manzanera AG, Sukin SW, et al. Intraprostatic distribution and long-term follow-up after AdV-tk immunotherapy as neoadjuvant to surgery in patients with prostate cancer. Cancer Gene Ther 2013;20:642-9. [Crossref] [PubMed]
- Jiang L, Ren J, Xiao X, et al. Proteomic analysis of bladder cancer by iTRAQ after Bifidobacterium infantis-mediated HSV-TK/GCV suicide gene treatment. Biol Chem 2013;394:1333-42. [Crossref] [PubMed]
- Yu Y, Sun FL, Chen XS. The proliferative activity influence of HSV-TK/ACV transfected by cationic liposme to lung caner cell. Practical Oncology Journal 2005;19:11-3.
- Imaizumi K, Hasegawa Y, Kawabe T, et al. Bystander tumoricidal effect and gap junctional communication in lung cancer cell lines. Am J Respir Cell Mol Biol 1998;18:205-12. [Crossref] [PubMed]
- Tang XJ, Wang YP, Zhou QH, et al. Construction of Tumor Cells Specific Expression Vector of Suicide Gene of HSV-TK Driven by hTERT Promotor. Life Science Research 2007;11:273-8.
- Wang WD, Chen ZT, Li DZ, et al. Experimental study on lung carcinoma-targeted suicide gene therapy induced by irradiation. Zhonghua Jie He He Hu Xi Za Zhi 2003;26:84-7. [PubMed]
- Kang BG, Chen H, Yang Q, et al. Effect of suicide gene TK/GCV and radiation on apoptosis of lung adenocarcinoma cells. Medical Recapitulate 2014;20:529-31.
- Fukunaga M, Takamori S, Hayashi A, et al. Adenoviral herpes simplex virus thymidine kinase gene therapy in an orthotopic lung cancer model. Ann Thorac Surg 2002;73:1740-6. [Crossref] [PubMed]
- Delgado O, Batten KG, Richardson JA, et al. Radiation-enhanced lung cancer progression in a transgenic mouse model of lung cancer is predictive of outcomes in human lung and breast cancer. Clin Cancer Res 2014;20:1610-22. [Crossref] [PubMed]
- Hossain JA, Latif MA, Ystaas LAR, et al. Long-term treatment with valganciclovir improves lentiviral suicide gene therapy of glioblastoma. Neuro Oncol 2019;21:890-900. [Crossref] [PubMed]
- Wang J, Shi Y, Bai Z, et al. Radiofrequency hyperthermia-enhanced herpes simplex virus-thymidine kinase/ganciclovir direct intratumoral gene therapy of hepatocellular carcinoma. Int J Hyperthermia 2017;33:170-7. [Crossref] [PubMed]
- National Research Council. Guidelines for Humane End Points for Animals Used in Biomedical Research. Washington: National Academies Press; 2011.
- Freeman SM, Abboud CN, Whartenby KA, et al. The "bystander effect": tumor regression when a fraction of the tumor mass is genetically modified. Cancer Res 1993;53:5274-83. [PubMed]
- Hosseindoost S, Dehpour AR, Dehghan S, et al. Fluoxetine enhances the antitumor effect of olfactory ensheathing cell-thymidine kinase/ganciclovir gene therapy in human glioblastoma multiforme cells through upregulation of Connexin43 levels. Drug Dev Res 2023;84:1739-50. [Crossref] [PubMed]
- Laberge RM, Adler D, DeMaria M, et al. Mitochondrial DNA damage induces apoptosis in senescent cells. Cell Death Dis 2013;4:e727. [Crossref] [PubMed]
- Moolten FL, Wells JM. Curability of tumors bearing herpes thymidine kinase genes transferred by retroviral vectors. J Natl Cancer Inst 1990;82:297-300. [Crossref] [PubMed]


