
The lncRNA MIR22HG suppresses prostate cancer cell proliferation, migration, and epithelial-mesenchymal transition via the miR-4428/PCDH9 axis
Highlight box
Key findings
• Long non-coding RNA (lncRNA) MIR22HG/microRNA-4428 (miR-4428)/PCDH9 axis inhibited the malignant progression of prostate cancer (Pca).
What is known and what is new?
• Previous studies reported that lncRNA MIR22HG was low-expression in many kinds of tumour diseases. The biological functions and mechanism of MIR22HG in Pca remain to be explored.
• In our study, lncRNA MIR22HG acts as a competing endogenous RNA (ceRNA) to regulate PCDH9 through miR-4428. PCDH9 can suppress progression, migration, and epithelial-mesenchymal transition (EMT) of Pca.
What is the implication, and what should change now?
• Our research group found a new ceRNA axis to regulate EMT of Pca. We hope to use animal models to explore the impact of this axis on Pca metastasis in the future.
Introduction
Globally, prostate cancer (Pca) is the second most common cause of death and morbidity among men due to its mortality rate (1). At present, compared to those of other cancers, the incidence of Pca is the fastest growing in males and it is often diagnosed in the late stage (2), during which the disease seriously threatens the physical and mental health of men (3). Therefore, in combination with the current situation, additional novel biomarkers for the treatment of Pca need to be explored.
The Human Genome Project shows that approximately 1% to 2% of human genome sequences encode proteins while the other 98% of DNA transcriptionally output non-coding RNA (ncRNA) (4). There are many types of ncRNAs, including microRNAs (miRNAs), small nuclear RNAs (snRNAs), transfer RNAs (tRNAs), PIWI-interacting RNAs (piRNAs), and long non-coding RNAs (lncRNAs) (5). LncRNAs are precisely regulated and play crucial roles in physiological, pathological and pathophysiological processes (6), as well as in the occurrence, proliferation, drug resistance, angiogenesis and distant metastasis of malignant tumours (7). In vitro and in vivo studies have revealed that functional lncRNAs play important roles in Pca occurrence, development, and progression (8,9).
MIR22HG is located on chromosome 17p13.3. It has been reported that MIR22HG is prone to loss and methylation during tumorigenesis (10). Although the role of MIR22HG in cancer has been preliminarily explored (11-13), it is still necessary to investigate its specific function and molecular mechanism in Pca. Bioinformatic analysis methods were used by Shen et al. and others to determine that low MIR22HG expression in Pca tissue is correlated with the poor Pca patient prognosis (14). The specific mechanism of MIR22HG in Pca has not been previously explored.
In this study, we examined the expression of MIR22HG in Pca tissues and found that it was expressed at low levels in tumour tissues. After verifying its biological function in vitro and in vivo, we discovered that MIR22HG inhibited Pca cell proliferation, migration, and epithelial-mesenchymal transition (EMT) via the microRNA-4428 (miR-4428)/PCDH9 pathway, indicating that MIR22HG may be used as a novel therapeutic target. We present this article in accordance with the MDAR and ARRIVE reporting checklists (available at https://tcr.amegroups.com/article/view/10.21037/tcr-2024-2200/rc).
Methods
Cell culture
Pca cell lines (DU145, PC3, LnCAP, and 22RV1) and the normal prostate epithelial cell line RWPE1 were purchased from the Shanghai Cell Bank and cultured in RPMI 1640 (Gibco, Gaithersburg, MD, USA) supplemented with 10% foetal bovine serum (FBS) at 37 ℃, 5% CO2 and 1×106 cells/well. When the cell confluence reached 90%, the cells were used for subsequent experiments.
Clinical specimens
A protocol was prepared before the study without registration. The Affiliated Drum Tower Hospital of Nanjing University Medical School provided 22 Pca tissues and pair-matched adjacent non-tumorous tissues. The study was conducted in accordance with the Declaration of Helsinki and its subsequent amendments. The study was approved by the Ethics Committee of The Affiliated Drum Tower Hospital of Nanjing University Medical School (No. 2017-044-02) and informed consent was obtained from all individual participants.
Cell transfection
Small interfering RNAs (siRNAs) were synthesized by Shanghai GenePharma Biotechnology Co., (Shanghai, China). The MIR22HG overexpression plasmid, negative control (NC) plasmid, and corresponding strains were synthesized by Shanghai Jikai Company (Shanghai, China). The miR-4428 inhibitor, mimics, and matching NCs (the NC inhibitor and NC mimics) were purchased from GenePharma. Transfection was carried out using Lipofectamine 3000 reagent (Invitrogen, Calrsbad, CA, USA) according to the manufacturer’s instructions. The following siRNA sequences were used: si-MIR22HG, 5'-CCCUUAGCAAACCAUAACATT-3', 5'-UGUUAUGGUUUGCUAAGGGTT-3'; si-NC, 5'-UUCUCCGAACGUGUCACGUTT-3', 5'-ACGUGACACGUUCGGAGAATT-3'. DU145 and PC3 cells were seeded in 6-well plates with a density of 1×105 cells per well by serum-free RPMI1640 medium. NC plasmid and overexpression vector were used to transfect DU145 (named vector group and MIR22HG group, respectively). Furthermore, PC3 cells were also transfected by MIR22HG siRNA and its NC, as well as miR-4428 inhibitor, mimics, and matching NCs. Twenty-four hours later, normal RPMI 1640 medium containing 10% FBS was used to replace the residual medium of each well for 48 hours of incubation at 37 ℃, 5% CO2.
RNA isolation and quantitative real-time polymerase chain reaction (qRT-PCR)
Total RNA was extracted from human and animal tissues and from prostate cell lines with TRIzol reagent. The total RNA concentration and purity were determined via Nanodrop. Total RNA was reverse-transcribed into complementary DNA (cDNA) by PCR and stored at −80 ℃ until use. In the qRT-PCR experiment, SYBR Green (Vazyme, Nanjing, China) reagent was used for amplification, and the reaction conditions were 95 ℃ predenaturation for 10 min; 95 ℃ denaturation for 15 s; 60 ℃ annealing for 30 s; and extension at 72 ℃ for 30 s, for a total of 42–45 cycles. All of the reactions involved three pore complexes, and the relative expression of MIR22HG was calculated by the 2−ΔΔCt method. The following primer pairs were used for qRT-PCR: MIR22HG 5'-TGGAGACCAGTGAAGGACCA-3' (forward) and 5'-ACATCTCGGAGGCAGAAAGC-3' (reverse); glyceraldehyde-3-phosphate dehydrogenase (GAPDH): 5'-ACATCTCGGAGGCAGAAAGC-3' (forward) and 5'-TGGTGAAGACGCCAGTGGAC-3' (reverse); miR-4428: 5'-ACACTCCAGCTGAGCGCCAG-3' (forward) and 5'-CACGGTAGCGGTCAGCCATG-3' (reverse); U6: 5'-CTCGCTTCGGCAGCACA-3' (forward) and 5'-AACGCTTCACGAATTTGCGT-3'; and PCDH9: 5'-TCCCAACTCTGATGGGCCTTTGGG-3' (forward) and 5'-GGCTCTGGTCAGGGTGTGCC-3' (reverse).
Transwell assay
The transfected cells in each group were used for functional tests when the cell density reached 70–80%. The cells were washed with 1× phosphate-buffered saline (PBS), fully digested with trypsin, and collected after centrifugation at 800 rpm for 3–5 minutes. A total of 600 µL of serum medium was added to each of the 12 wells, and the Transwell inserts were added to each well. Next, 250 µL of each sample was added to serum-free medium. A total of 2×105 cells were taken from each group and added to the top chamber, and each group had three replicate wells. After 48 hours of culture, cells passed through the membrane were fixed with 10% form-aldehyde for 20 minutes and stained with 0.1% purple crystal for 15 minutes. Before being fixed by formaldehyde, cells that failed to penetrate the membrane were gently scraped off using a cotton swab. Number of invasion cells was counted under an inverted microscope.
Cell Counting Kit-8 (CCK8) assay
Proliferation was monitored via CCK8 assays (Takara, Japan). After the cells were transfected, they were seeded in a 96-well plate at a density of 2×103/well and cultured for 24, 48, 72, or 96 hours. The CCK8 solution was prepared according to the Dulbecco’s modified Eagle medium (DMEM): CCK8 ratio of 9:1. The culture medium was removed after the corresponding time of treatment in each group, 100 µL of CCK8 solution was added to each well, and the cells were incubated at 37 ℃ for 2 hours. An enzyme marker was used to determine the 450 nm optical density (OD) value and a standard curve was established to determine the degree of cell proliferation.
Western blot analysis
Cells were washed three times with pre-cooled PBS at 4 ℃. Total proteins were isolated by using cell lysates and quantified by bicinchoninic acid (BCA) assay methods. After separation by sodium dodecyl sulphate-polyacrylamide gel electrophoresis (SDS-PAGE), proteins were transferred onto polyvinylidene fluoride (PVDF) membranes and incubated with 50 µg/L skim milk powder for 1 hour. Tris-buffered saline with Tween (TBST) solution were used to wash membranes three times. Rabbit anti-mouse E-cadherin, N-cadherin, vimentin, and GAPDH primary antibodies were subsequently added to membranes, respectively. After 12 hours incubation at 4 ℃, all membranes were subjected to three times washing with TBST solution and goat anti-rabbit immunoglobulin G (IgG) antibodies were used to incubate membranes for 2 hours at room temperature. Three times washing with TBST were carried out once again. Finally, the blots were visualized by enhanced chemiluminescence plus and integrated OD was measured through software Lab Works 4.5. In this study, the antibodies used were all from Proteintech Inc. (Wuhan, China): GAPDH (Lot.CL594-60004, 1:10,000), E-cadherin (Lot.20874-1-AP; 1:5,000), N-cadherin (Lot.22018-0-AP; 1:3,000), vimentin (Lot.10366-1-AP; 1:50,000), PCDH9 (Lot.25090-1-AP; 1:600), goat anti-rabbit anti-IgG antibodies (Cat No. SA00001-1; 1:100,000).
Colony formation assay
For colony formation, Pca cells transfected with MIR22HG siRNAs and control siRNA were seeded in a six-well plate at a density of 200 cells/well. After 10 to 14 days of incubation at 37 ℃, 0.1% crystal violet (Sigma-Aldrich, Darmstadt, Germany) and 20% methanol were used as dye solution to fix and stain the colonies. The number of colonies was counted in each well. Clones containing more than 50 cells were counted using a grid. Three independent experiments were performed.
Dual-luciferase reporter gene assay
A dual-luciferase reporter gene plasmid was synthesized by Shanghai Gemma Biotechnology Co., Ltd. (Shanghai, China). The Pca cell line DU145 was cultivated in a 24-well plate and transfected with NC-mimic + MIR22HG-wild type (WT), miR-4428 mimic + MIR22HG-WT, NC-mimic + MIR22HG-mutated (MUT), or miR-4428 mimic + MIR22HG-MUT. The experimental system included serum-free medium, Lipofectamine 3000, 1× Lysate, Luciferase Assay Reagent II (LAR II) warmed to room temperature, Stop & Glo reagent warmed to room temperature, a luciferase vector, and miRNA oligos. After 24 hours of cell transfection, the medium in the wells was removed, the cells were washed with PBS, and the PBS was removed as much as possible.
In vivo tumorigenicity
The corresponding transfected Pca cells were digested with trypsin, mixed to form a single-cell suspension, and centrifuged at 800 rpm for 5 minutes. The Pca cells in each group were washed with serum-free medium three times and counted so that the cells in each group had a density of 1×108 cells/mL. The corresponding treated cell suspension was injected into the left armpit of BALB/c nude mice [male, aged 6–8 weeks, purchased from GemPharmatech (Nanjing, China)]. After 28 days, all the BALB/c nude mice were killed, and the tumours that had formed under the skin were removed and weighed. All animal experiments were performed under a project license (No. UJS-IBCCU-AP2023030817) granted by ethic committee of Jiangsu University Laboratory Animal Research Center, in compliance with the institutional ethic committee’s guidelines for the care and use of animals.
Fluorescence in situ hybridization (FISH)
The FISH probe for the lncRNA MIR22HG was synthesized by GenePharma. Paraffin-embedded sections were dewaxed, rehydrated, digested, and dehydrated using xylene, graded ethanol, and protease K. Then, the FISH probe was added to the hybrid mixture, after which the mixture was incubated overnight. Next, the sections were washed in a dark room with a washing solution containing physiological saline sodium citrate and PBS. The sections were stained with 4',6-diamidino-2-phenylindole (DAPI) for 10 minutes and then observed under a fluorescence microscope.
Statistical analysis
Relevant experimental data were analysed and mapped using GraphPad Prism 8.0 software. All the measurement data were expressed as the mean ± standard deviation. The data between the two groups were compared by an independent sample t-test. We conducted Pearson correlation analysis to determine whether there was a correlation between the two groups of data. P<0.05, P<0.01, and P<0.001 indicated statistical significance.
Results
MIR22HG is downregulated in Pca cells and tissues
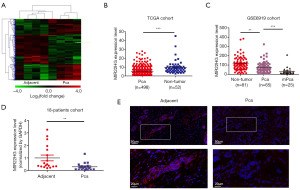
With a lncRNA chip (chip name: Agilent Human lncRNA, 4×180 K, 053726), we determined the expression of lncRNAs in Pca tissue and corresponding paracancerous tissue (Figure 1A). The results showed that the lncRNA MIR22HG was markedly downregulated in Pca tissues compared with normal tissues (Figure 1A, Figure S1A). Additionally, we analysed MIR22HG expression in The Cancer Genome Atlas (TCGA) database (https://portal.gdc.cancer.gov/) and GSE6919 dataset (http://www.ncbi.nlm.nih.gov/geo), and the results showed that MIR22HG expression was significantly lower in Pca tissues than in normal prostate tissues (Figure 1B,1C). In the following study, we collected 18 pairs of Pca tissues and corresponding paracancerous tissues from clinical patients. The qRT-PCR results showed that MIR22HG was downregulated in Pca tissues (Figure 1D). Using FISH to determine MIR22HG expression in carcinomas and adjacent tissues, we found that MIR22HG was localized in the cytoplasm (Figure 1E). A series of results illustrated that there was a marked decrease in MIR22HG expression levels in Pca tissues compared with that in corresponding paracancerous tissues.
MIR22HG inhibits the proliferation, migration, and EMT progression of Pca cells
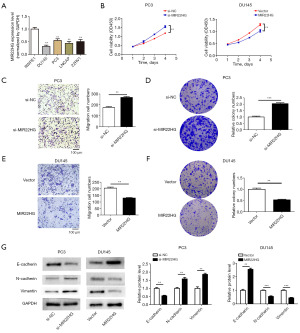
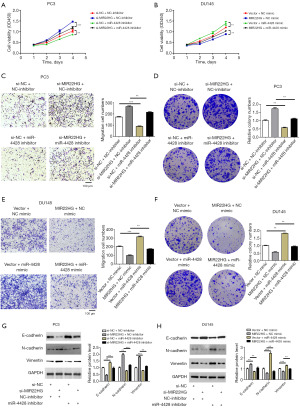
The expression levels of MIR22HG in PC3, DU145, LNCaP, and 22RV1 cells were markedly lower than those in RWPE1 normal prostate epithelial cells (Figure 2A). Among them, the expression of MIR22HG in DU145 cells was the lowest, while the expression in PC3 cells was relatively high. We then selected DU145 and PC3 cells for the next study. Using qRT-PCR to validate transfection efficiency, we found that the expression of MIR22HG was significantly downregulated in PC3 cells transfected with si-MIR22HG but was significantly upregulated in DU145 cells transfected with the MIR22HG overexpression plasmid (Figure S1B,S1C). The CCK8 assays showed that si-MIR22HG enhanced the viability of PC3 cells (Figure 2B). In contrast, overexpression of MIR22HG decreased the proliferation of DU145 cells (Figure 2B). The results from Transwell and colony formation assay experiments showed that after silencing MIR22HG in PC3 cells, cell migration and proliferation significantly increased (Figure 2C,2D), whereas when MIR22HG was overexpressed in DU145 cells, cell migration and proliferation significantly decreased (Figure 2E,2F). The western blot analysis revealed that si-MIR22HG transfection downregulated the expression of the epithelial-related gene E-cadherin and upregulated the interstitial-related genes vimentin and N-cadherin in PC3 cells (Figure 2G). After overexpression of MIR22HG in DU145 cells, the changes in the expression of E-cadherin, vimentin, and N-cadherin were abrogated (Figure 2G). In summary, MIR22HG can inhibit the proliferation, migration, and EMT abilities of Pca cells.
MIR22HG suppresses tumour weight and development in nude mice
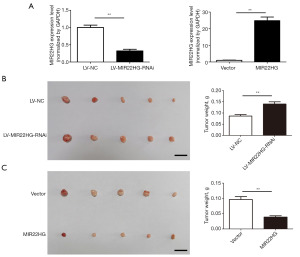
The above results indicated that MIR22HG can inhibit the proliferation of Pca cells in vitro. To determine whether MIR22HG affects tumour growth in vivo, we first constructed a stable MIR22HG-knockdown cell line in PC3 cells and a stable MIR22HG-overexpressing cell line in DU145 cells (Figure 3A). To observe the effect of MIR22HG knockdown or overexpression on the growth of Pca-related transplanted tumours, we injected the cells into mice. Compared to those in the control group, the number of tumours in the MIR22HG-RNA interference (RNAi) lentivirus stable group significantly increased (Figure 3B). Compared with those in the control group, the tumours in the overexpression group were smaller (Figure 3C), proving that MIR22HG inhibits tumour growth in vivo.
miR-4428 is a downstream target of MIR22HG
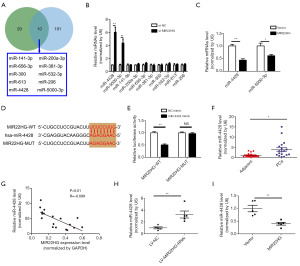
First, we used the ENCORI database (http://starbase.sysu.edu.cn/) and the lncBase database (http://www.microrna.gr/LncBase/) to predict the miRNAs that may target MIR22HG, and the two datasets were compared using a Venn diagram. The results revealed 10 miRNAs that may interact with MIR22HG (Figure 4A). The qRT-PCR results revealed that after MIR22HG was knocked down in PC3 cells, the expression of miR-4428 and miR-5000-3p was upregulated, and the difference between the two miRNAs was statistically significant (Figure 4B). Afterwards, we overexpressed MIR22HG in DU145 cells. According to the qRT-PCR results, miR-4428 and miR-5000-3p expression levels decreased, although the decrease in miR-4428 expression levels was more pronounced (Figure 4C). Bioinformatic analysis was used to predict the possible binding sites of MIR22HG and miR-4428 (Figure 4D). The results of the dual-luciferase reporter assay demonstrated that MIR22HG could bind to miR-4428 (Figure 4E). qRT-PCR analysis revealed that miR-4428 expression was significantly higher in Pca tissues than in normal tissues (Figure 4F). Moreover, Pearson correlation analysis revealed that miR-4428 expression was negatively correlated with the expression level of MIR22HG (Figure 4G). Next, qRT-PCR showed that miR-4428 was upregulated in the transplanted tumours of MIR22HG knockout mice compared with control mice (Figure 4H), while the expression levels of miR-4428 in the transplanted tumours of MIR22HG-overexpressing mice significantly decreased (Figure 4I). MIR22HG expression in Pca tissues was downregulated in vivo, and miR-4428 expression was negatively correlated with MIR22HG expression.
miR-4428 abrogates the effects of MIR22HG on the proliferation, migration, and EMT of Pca cells
Next, we cotransfected MIR22HG and miR-4428 into PC3 and DU145 cells. The CCK8 assay indicated that miR-4428 inhibitors could attenuate the proliferative effect of si-MIR22HG on PC3 cells (Figure 5A); miR-4428 mimics could attenuate the inhibitory effect of MIR22HG overexpression plasmids on DU145 cells (Figure 5B). The Transwell and colony formation assay results showed that a miR-4428 inhibitor inhibited the migration and proliferation of PC3 cells, and a miR-4428 inhibitor partially abrogated the promotive effect of si-MIR22HG (Figure 5C,5D). In DU145 cells, the miR-4428 mimic promoted cell migration and proliferation, and the miR-4428 mimic partially abrogated the inhibitory effect of the MIR22HG overexpression plasmid on DU145 cell migration and proliferation (Figure 5E,5F). Western blot analysis revealed that a decrease in the miR-4428 level in PC3 cells led to the upregulation of the epithelial marker E-cadherin and the downregulation of the interstitial markers vimentin and N-cadherin. In contrast, miR-4428 downregulation reduced the activation of the EMT signalling pathway caused by MIR22HG siRNA (Figure 5G). Moreover, the upregulation of miR-4428 in DU145 cells activated the EMT signalling pathway, while the miR-4428 mimic partially abrogated the inhibitory effect of MIR22H436G on the EMT signalling pathway (Figure 5H). The results indicated that miR-4428 could abrogate the effects of MIR22HG on Pca cell proliferation, migration, and EMT.
Prediction and validation of the miR-4428 target gene PCDH9
First, our research group used TargetScan, MIDRB, miRPathDB, and EMT-related genes to predict downstream target genes of the MIR22HG/miR-4428 axis. TargetScan predicted 2,065 genes, MIRDB predicted 42 genes, miRPathDB predicted 3,749 genes, and there were 659 EMT-related genes. After the genes predicted from the three databases were compared using jvenn software, 12 shared genes were obtained (Figure 6A). Subsequently, using a Pca cell model with knockdown or overexpression of miR-4428, qRT-PCR was performed using synthetic primers or the 12 predicted target genes (Figure S2). The experimental results confirmed that there were significant changes in the expression of PCDH9 after knockdown or overexpression of miR-4428, among which PCDH9 had the largest significant difference (Figure 6B). Then, we utilized bioinformatic to predict the binding site between miR-4428 and PCDH9 (Figure 6C). The results of the dual-luciferase reporter assay indicated that PCDH9 could bind to miR-4428 (Figure 6D). The expression of miR-4428 was strongly inhibited by the miR-4428 inhibitor but was strongly promoted by MIR22HG knockdown in PC3 cells. We also detected the protein expression of PCDH9 (Figure 6E,6F), and the results indicated that PCDH9 expression was explicitly upregulated by MIR22HG overexpression but was evidently downregulated by the miR-4428 mimic in DU145 cells, while PCDH9 expression was promoted by the miR-4428 inhibitor but was suppressed by MIR22HG knockdown in PC3 cells.
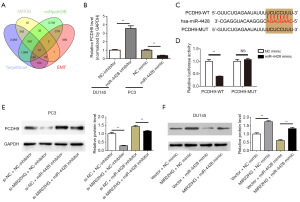
miR-4428 abrogats the effect of PCDH9 in Pca cells
We observed changes in cell proliferation by individually or jointly suppressing (or overexpressing) PCDH9 and miR-4428 in PC3 cells via CCK8 assays. The results showed that si-PCDH9 promoted the proliferation of PC3 cells, but the miR-4428 inhibitor reversed the effect of si-PCDH9 (Figure 7A). Western blot analysis indicated that si-PCDH9 could downregulate the expression of E-cadherin and upregulate the expression of vimentin and N-cadherin. Moreover, the activation of the EMT signalling pathway by si-PCDH9 was accomplished by downregulating miR-4428 (Figure 7B). Next, Transwell and colony formation assays revealed that si-PCDH9 could facilitate the migration and proliferation of PC3 cells, but the miR-4428 inhibitor could partially abrogate the ability of si-PCDH9 to promote migration and proliferation (Figure 7C,7D).

Discussion
The malignant progression of Pca is regulated by multiple signalling pathways and effector factors and is influenced by multiple factors (androgen receptors, miRNA, transcription factors, etc.) (15). It has been found that prostate-specific antigen (PSA) (16), TMPRSS2 (17), miRNA (18), circulating tumour cells (19), circulating tumour DNA (ctDNA) (20), lncRNA (21), and circle RNA (circRNA) (22) can serve as potential diagnostic markers for Pca.
In our study, a new lncRNA, MIR22HG, was identified through chip screening, and its expression was found to be downregulated in Pca tissue. To further validate these findings, we analysed the expression of MIR22HG in two datasets, a TCGA dataset and the GSE6919 dataset. A significant reduction in MIR22HG expression levels was observed in Pca tissue compared to corresponding adjacent tissues. According to an qRT-PCR analysis of 18 pairs of Pca tissues and adjacent tissues, MIR22HG expression is significantly lower in Pca tissues than in adjacent tissues. Overall, the expression MIR22HG was markedly lower in Pca tissue than adjacent paracancerous tissue, indicating that MIR22HG acts as a tumour suppressor.
The occurrence and development of tumours are complex pathological processes that involve multiple steps and are regulated by multiple factors. Based on cell function experiments, MIR22HG inhibited Pca cell proliferation and migration. During the process of malignant tumour metastasis, EMT can cause primary tumour cells to lose cell polarity, interrupt intercellular adhesion, and achieve migration and invasion (23). Cell models in which MIR22HG was silenced or overexpressed were shown to express EMT-related markers such as vimentin, E-cadherin, and N-cadherin. Significant inhibition of N-cadherin and vimentin expression was observed after MIR22HG treatment, suggesting that MIR22HG may inhibit the progression of Pca EMT. Additionally, MIR22HG was found to significantly inhibit the growth of subcutaneous prostate transplant tumours in animal experimental models, which confirms the lack of conformity of MIR22HG with cell function assays in vitro. In summary, we demonstrated the effects of MIR22HG on Pca cell proliferation, migration, apoptosis, and EMT both in vivo and in vitro.
miRNAs are a class of ncRNAs approximately 22 nucleotides in length that can regulate gene expression at the transcriptional or posttranscriptional level and are important factors involved in regulating gene expression (24). Currently, the human genome is predicted to encode a multitude of novel miRNAs (25). However, in physiological states, miRNA-mediated regulatory effects often fail to affect target proteins, indicating that miRNAs may be inhibited. Salmena et al. discovered for the first time that ncRNA can endogenously compete with other ncRNA molecules, suggesting that lncRNA can act as a molecular sponge that regulates miRNA expression and function by competing for miRNA binding sites (26). A change in lncRNA expression enhances or weakens the regulatory effect of miRNAs on target genes (27,28). In recent years, many studies have validated the regulatory mechanism of lncRNAs-miRNAs-mRNAs (29-31). In turn, lncRNA-mediated competing endogenous RNA (ceRNA) regulatory networks play important roles in Pca occurrence and development (32). Considering the role of the ceRNA mechanism in Pca, we speculate that MIR22HG may suppress the malignant progression of Pca by regulating miRNAs.
To clarify the molecular mechanism by which MIR22HG plays a biological role in Pca, we predicted miRNAs that MIR22HG may target through bioinformatic and further validated miRNAs with altered expression levels in Pca cells with MIR22HG knockdown or overexpression via qRT-PCR and ultimately identified significant changes in miR-4428 expression levels. We then used a dual-luciferase reporter system to evaluate the interaction between MIR22HG and miR-4428, and the results indicated that MIR22HG is able to directly bind to miR-4428. To determine the role of miR-4428 in Pca progression, we used qRT-PCR to measure the differential expression of miR-4428 between Pca tissues and adjacent tissues. The results showed that miR-4428 was significantly overexpressed in Pca tissue. Subsequently, a series of experiments were conducted to evaluate cell proliferation, migration and EMT signalling pathway activity. According to the comprehensive results, miR-4428 can promote EMT activation, migration, and proliferation in Pca. A functional recovery experiment was subsequently conducted to determine whether MIR22HG regulates miR-4428 to affect the proliferation, migration, and EMT signalling pathways in Pca cells. The results showed that the phenotypic changes caused by MIR22HG in Pca cells are regulated by miR-4428.
PCDH9 is a member of non-clustered protocadherins and belongs to the cadherin family (33). A study has shown that PCDH9 is located at 13q21.23 in the whole human tissue genome (34). Numerous studies have shown that PCDH9 prevents the development of neoplastic diseases, including gastric cancer (35), hepatocellular carcinoma (36), ovarian cancer (37), melanoma (38), and glioma (39). However, the biological function of PCDH9 in Pca has not yet been thoroughly researched. On the basis of our research, PCDH9, a downstream gene of MIR22HG/miR-4428, inhibits Pca cell proliferation and migration and promotes EMT in tumour cells.
However, there are some problems and challenges that need to be overcome in the clinical application of lncRNAs. Currently, the intricate regulatory network of lncRNAs remains incompletely elucidated. Additionally, challenges such as off-target effects arising from sequence homology, delivery obstacles related to nanoparticle systems or viral vectors, and the complexity of tumour heterogeneity necessitating personalized approaches, present significant hurdles. Moreover, the incomplete functional characterization of numerous lncRNAs, potential compensatory mechanisms within cancer cells, and the risks of immunogenicity or toxicity associated with delivery systems further complicate the development of therapeutic strategies.
Conclusions
In summary, the lncRNA MIR22HG was downregulated in Pca. Our results demonstrated that the lncRNA MIR22HG inhibited cell proliferation, migration, and EMT by activating the miR-4428/PCDH9 axis.
Acknowledgments
None.
Footnote
Reporting Checklist: The authors have completed the MDAR and ARRIVE reporting checklists. Available at https://tcr.amegroups.com/article/view/10.21037/tcr-2024-2200/rc
Data Sharing Statement: Available at https://tcr.amegroups.com/article/view/10.21037/tcr-2024-2200/dss
Peer Review File: Available at https://tcr.amegroups.com/article/view/10.21037/tcr-2024-2200/prf
Funding: This research was supported by
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tcr.amegroups.com/article/view/10.21037/tcr-2024-2200/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki and its subsequent amendments. The study was approved by the Ethics Committee of The Affiliated Drum Tower Hospital of Nanjing University Medical School (No. 2017-044-02) and informed consent was obtained from all individual participants. All animal experiments were performed under a project license (No. UJS-IBCCU-AP2023030817) granted by ethic committee of Jiangsu University Laboratory Animal Research Center, in compliance with the institutional ethic committee’s guidelines for the care and use of animals.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Bray F, Laversanne M, Sung H, et al. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2024;74:229-63. [Crossref] [PubMed]
- Zhang L, Liu X, Xia R, et al. Comparison of the clinicopathologic features of prostate cancer in US and Chinese populations. Pathol Res Pract 2022;234:153933. [Crossref] [PubMed]
- Fenton JJ, Weyrich MS, Durbin S, et al. Prostate-Specific Antigen-Based Screening for Prostate Cancer: Evidence Report and Systematic Review for the US Preventive Services Task Force. JAMA 2018;319:1914-31. [Crossref] [PubMed]
- Slack FJ, Chinnaiyan AM. The Role of Non-coding RNAs in Oncology. Cell 2019;179:1033-55. [Crossref] [PubMed]
- Chen LL, Kim VN. Small and long non-coding RNAs: Past, present, and future. Cell 2024;187:6451-85. [Crossref] [PubMed]
- Haghighi R, Castillo-Acobo RY, H, Amin A, et al. A thorough understanding of the role of lncRNA in prostate cancer pathogenesis; Current knowledge and future research directions. Pathol Res Pract 2023;248:154666. [Crossref] [PubMed]
- Adnane S, Marino A, Leucci E. LncRNAs in human cancers: signal from noise. Trends Cell Biol 2022;32:565-73. [Crossref] [PubMed]
- Wang S, Bai Y, Ma J, et al. Long non-coding RNAs: regulators of autophagy and potential biomarkers in therapy resistance and urological cancers. Front Pharmacol 2024;15:1442227. [Crossref] [PubMed]
- Ramnarine VR, Kobelev M, Gibb EA, et al. The evolution of long noncoding RNA acceptance in prostate cancer initiation, progression, and its clinical utility in disease management. Eur Urol 2019;76:546-59. [Crossref] [PubMed]
- Ci Y, Zhang Y, Zhang X. Methylated lncRNAs suppress apoptosis of gastric cancer stem cells via the lncRNA-miRNA/protein axis. Cell Mol Biol Lett 2024;29:51. [Crossref] [PubMed]
- Chen Y, Hu D, Wang F, et al. A systematic framework for identifying prognostic necroptosis-related lncRNAs and verification of lncRNA CRNDE/miR-23b-3p/IDH1 regulatory axis in glioma. Aging (Albany NY) 2023;15:12296-313. [Crossref] [PubMed]
- Deng X, Ye D, Hua K, et al. MIR22HG inhibits breast cancer progression by stabilizing LATS2 tumor suppressor. Cell Death Dis 2021;12:810. [Crossref] [PubMed]
- Huang GD, Liao P, Huang YH, et al. MIR22HG Regulates the Proliferation, Epithelial-Mesenchymal Transition, and Apoptosis in Colorectal Carcinoma. Cancer Biother Radiopharm 2021;36:783-92. [Crossref] [PubMed]
- Shen H, Weng XD, Yang D, et al. Long noncoding RNA MIR22HG is down-regulated in prostate cancer. Math Biosci Eng 2019;17:1776-86. [Crossref] [PubMed]
- Sandhu S, Moore CM, Chiong E, et al. Prostate cancer. Lancet 2021;398:1075-90. [Crossref] [PubMed]
- Özyurt C, Uludağ İ, İnce B, et al. Biosensing strategies for diagnosis of prostate specific antigen. J Pharm Biomed Anal 2022;209:114535. [Crossref] [PubMed]
- Necchi A, Cucchiara V, Grivas P, et al. Contrasting genomic profiles from metastatic sites, primary tumors, and liquid biopsies of advanced prostate cancer. Cancer 2021;127:4557-64. [Crossref] [PubMed]
- Doghish AS, Ismail A, El-Mahdy HA, et al. A review of the biological role of miRNAs in prostate cancer suppression and progression. Int J Biol Macromol 2022;197:141-56. [Crossref] [PubMed]
- Merseburger AS, Waldron N, Ribal MJ, et al. Genomic Testing in Patients with Metastatic Castration-resistant Prostate Cancer: A Pragmatic Guide for Clinicians. Eur Urol 2021;79:519-29. [Crossref] [PubMed]
- Shaya J, Nonato T, Cabal A, et al. Analysis of the Prognostic Significance of Circulating Tumor DNA in Metastatic Castrate Resistant Prostate Cancer. Clin Genitourin Cancer 2021;19:564.e1-564.e10. [Crossref] [PubMed]
- Kan Y, Li B, Yang D, et al. Emerging Roles of Long Non-coding RNAs as Novel Biomarkers in the Diagnosis and Prognosis of Prostate Cancer. Discov Med 2021;32:29-37. [PubMed]
- Liu X, Tong Y, Xia D, et al. Circular RNAs in prostate cancer: Biogenesis,biological functions, and clinical significance. Mol Ther Nucleic Acids 2021;26:1130-47. [Crossref] [PubMed]
- Dongre A, Weinberg RA. New insights into the mechanisms of epithelial-mesenchymal transition and implications for cancer. Nat Rev Mol Cell Biol 2019;20:69-84. [Crossref] [PubMed]
- Gebert LFR, MacRae IJ. Regulation of microRNA function in animals. Nat Rev Mol Cell Biol 2019;20:21-37. [Crossref] [PubMed]
- Gao F, Wang F, Chen Y, et al. The human genome encodes a multitude of novel miRNAs. Nucleic Acids Res 2025;53:gkaf070. [Crossref] [PubMed]
- Salmena L, Poliseno L, Tay Y, et al. A ceRNA hypothesis: the Rosetta Stone of a hidden RNA language? Cell 2011;146:353-8. [Crossref] [PubMed]
- Palcau AC, Canu V, Donzelli S, et al. CircPVT1: a pivotal circular node intersecting Long Non-Coding-PVT1 and c-MYC oncogenic signals. Mol Cancer 2022;21:33. [Crossref] [PubMed]
- Wei L, Sun J, Zhang N, et al. Noncoding RNAs in gastric cancer: implications for drug resistance. Mol Cancer 2020;19:62. [Crossref] [PubMed]
- Li Y, Zhong H, Luo L, et al. Integrated analysis of the lncRNA-miRNA-mRNA ceRNA network in nasopharyngeal carcinoma. Transl Cancer Res 2024;13:4372-88. [Crossref] [PubMed]
- Cui C, Xu B, Liu H, et al. Exploring the Role of SMPD3 in the lncRNA-miRNA-mRNA Regulatory Network in TBI Progression by Influencing Energy Metabolism. J Inflamm Res 2024;17:10835-48. [Crossref] [PubMed]
- Li S, Wang M, Liu B, et al. Analysis of lncRNA-miRNA-mRNA interactions identified a novel biomarker LINC00657 to improve prognosis prediction of papillary thyroid carcinoma. Int Immunopharmacol 2024;137:112432. [Crossref] [PubMed]
- Xu YH, Deng JL, Wang G, et al. Long non-coding RNAs in prostate cancer: Functional roles and clinical implications. Cancer Lett 2019;464:37-55. [Crossref] [PubMed]
- Zhang Y, Zhu Y, Chen Y, et al. Nuclear translocation of cleaved PCDH9 impairs gastric cancer metastasis by downregulating CDH2 expression. iScience 2024;27:109011. [Crossref] [PubMed]
- Zhu P, Lv J, Yang Z, et al. Protocadherin 9 inhibits epithelial-mesenchymal transition and cell migration through activating GSK-3β in hepatocellular carcinoma. Biochem Biophys Res Commun 2014;452:567-74. [Crossref] [PubMed]
- Chen Y, Xiang H, Zhang Y, et al. Loss of PCDH9 is associated with the differentiation of tumor cells and metastasis and predicts poor survival in gastric cancer. Clin Exp Metastasis 2015;32:417-28. [Crossref] [PubMed]
- Zhang T, Guan G, Chen T, et al. Methylation of PCDH19 predicts poor prognosis of hepatocellular carcinoma. Asia Pac J Clin Oncol 2018;14:e352-8. [Crossref] [PubMed]
- Izycka N, Sterzynska K, Januchowski R, et al. Semaphorin 3A (SEMA3A), protocadherin 9 (PCdh9), and S100 calcium binding protein A3 (S100A3) as potential biomarkers of carcinogenesis and chemoresistance of different neoplasms, including ovarian cancer - review of literature. Ginekol Pol 2019;90:223-7. [Crossref] [PubMed]
- Cai G, Zou R, Yang H, et al. Circ_0084043-miR-134-5p axis regulates PCDH9 to suppress melanoma. Front Oncol 2022;12:891476. [Crossref] [PubMed]
- Wang C, Tao B, Li S, et al. Characterizing the role of PCDH9 in the regulation of glioma cell apoptosis and invasion. J Mol Neurosci 2014;52:250-60. [Crossref] [PubMed]


