
Sublobar resection is non-inferior to lobectomy in octogenarians and older with stage Ia non‑small cell lung cancer
Highlight box
Key findings
• In octogenarians with stage Ia non-small cell lung cancer (NSCLC), lobectomy and sublobar resection show no significant difference in overall survival (OS). Dissecting more than six lymph nodes is an independent prognostic protective factor.
What is known and what is new?
• Surgery with lymph node dissection can provide better long-term survival for such patients compared to radiotherapy
• For patients aged 80+ years with stage Ia NSCLC, sublobar resection offered OS comparable to lobectomy.
What is the implication, and what should change now?
• Performing a sublobar resection with the examination of at least six lymph nodes may be advantageous for these patients.
Introduction
Despite multiple treatment options and the prevalence of early detection and screening, lung cancer remains the most diagnosed cancer (11.6%) and the leading cause of cancer death (18.4%). Additionally, the incidence of lung cancer is increasing (1). Pathologically, lung cancer is categorized into small cell lung cancer (SCLC) and non-small cell lung cancer (NSCLC), with the NSCLC ratio accounting for up to 80% of cases (2). This subtype includes various histological types such as adenocarcinoma, squamous cell carcinoma, and large cell carcinoma (3). The diagnosis of early-stage NSCLC in high-risk patients is on the increase due to advancements in cancer screening and increased longevity (4). It is estimated that 14% of NSCLC patients are older than 80 years (5). The treatment of NSCLC in patients over 80 years old remains controversial due to the higher risk of surgery associated with age-related complications with various diseases, such as poor cardiopulmonary reserve function (6,7). One study highlights that surgical intervention for lung cancer in patients aged 75 years and above is associated with increased mortality rates, emphasizing the need for a comprehensive risk assessment and preoperative management of underlying comorbidities (2). Over the past decade, radiotherapy has increasingly replaced surgery as the predominant treatment approach for early-stage NSCLC in patients 80 years or older (8,9). However, this does not mean that radiotherapy can achieve a better prognosis than surgery. Previous research has suggested that surgery with lymph node dissection can provide better long-term survival for such patients compared to radiotherapy (6). There is still controversy regarding the choice of surgical modalities (10). A study suggested that tumor size and patient age should not serve as the sole criteria for determining the suitability of sublobar resection. Instead, additional factors, including the size of the solid component, the consolidation-to-tumor ratio, and lymph node status, should be taken into account when selecting the most appropriate surgical approach (11). Several studies have indicated that sublobar resection can achieve similar or even better outcomes for patients with early-stage NSCLC, although lobectomy is still considered the gold standard (12,13). A recent study suggested that 80-year-old patients with pathological stage I lung cancer should still undergo lobectomy if they can tolerate surgery, as lobectomy has a better 5-year survival rate (10,14). Unfortunately, the study did not consider the extent of lymph node dissection. In our research, we have observed that lobectomy often involves the dissection of a significant number of lymph nodes, a phenomenon also commonly observed in clinical practice. We aimed to assess how the two surgical approaches impact overall survival (OS) in this population, considering the extent of lymph node dissection. If OS rates are comparable between the two surgical procedures, sublobar resection should be advocated due to its superior preservation of pulmonary function. We present this article in accordance with the STROBE reporting checklist (available at https://tcr.amegroups.com/article/view/10.21037/tcr-2024-2575/rc).
Methods
Study population
The inclusion criteria were as follows: diagnosis between 2004 and 2021, patients aged 80 years or older with early histologically diagnosed primary NSCLC without history of other cancers who underwent lobectomy or sublobar resection (segmentectomy and wedge resection) were enrolled. The exclusion criteria are revealed in Figure 1. Variables extracted from the Surveillance, Epidemiology, and End Results (SEER) database included patient ID, sex, age at diagnosis, race, marital status, the time from diagnosis to treatment, primary tumor site, laterality, International Classification of Disease for Oncology third edition (ICD-O-3), histology code, pathologic grade, American Joint Committee on Cancer T/M stages, lymph nodes examined (LNE), radiation record, chemotherapy record, survival months, and vital status record. The primary outcome was OS. The study was conducted in accordance with the Declaration of Helsinki and its subsequent amendments.
Statistical analysis
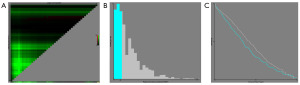
Statistical analyses were performed using R software (version 3.5.3). A P<0.05 was considered statistically significant. Patients were assigned to either lobectomy or sublobar resection (segmentectomy and wedge resection). Survival time was defined from the time of diagnosis to the time of death or last follow-up. The SEER database’s lack of anatomical station data necessitated a quantitative approach. The optimal cutoff values for LNE were determined using X-Tile software (Figure 2), and lymph node status was classified as low dissection group (1–5 lymph nodes) versus high dissection group (6 or more lymph nodes). Differences between groups were determined by the Chi-squared test or Fisher’s exact test for categorical variables and the Kruskal-Wallis test for continuous variables, as appropriate. Cox regression analysis was used to evaluate the effect of patient characteristics and treatment on OS outcomes. A multivariate Cox regression analysis was conducted, focusing on factors deemed significant in univariate analysis (P<0.05). Propensity score matching (PSM) was performed with 1:1 matching of patients in the cohort who underwent lobectomy or sublobar resection based on sex, age at diagnosis, race, marital status, the time from diagnosis to treatment, primary tumor site, laterality, ICD-O-3 histology code, pathologic grade, and LNE. This PSM group was analyzed using similar methods. The Kaplan-Meier curves illustrated patient survival, categorizing them into groups based on lobectomy and sublobar resection. Differences between groups were compared by the log-rank test.
Results
Patient characteristics
A total of 1,735 patients with stage IA NSCLC were included in this study [514 (30%) subjected to sublobar resection and 1,221 (70%) subjected to lobectomy]. The median age of the patient was 82 years, and the median follow-up time was 43 months. Baseline demographics and clinical characteristics for the lobectomy and sublobar resection groups are presented in Table 1. Patients opting for lobectomy, as opposed to sublobar resection, experienced an extended duration between diagnosis and treatment, had significant middle lobe involvement, bigger tumors, more comprehensive lymph node dissection, and were typically younger. A total of 862 patients were PSM, and there was no significant difference in the distribution of confounding factors between the two groups, as shown in Table 2.
Table 1
| Characteristics | Overall (n=1,735) | Sublobar resection [n=514 (30%)] | Lobectomy [n=1,221 (70%)] | P value† |
|---|---|---|---|---|
| Time from diagnosis to treatment in days | 33.00 (0.00, 57.00) | 28.50 (0.00, 55.00) | 35.00 (4.00, 58.00) | 0.006 |
| Sex | 0.28 | |||
| Male | 697 (40.17) | 196 (38.13) | 501 (41.03) | |
| Female | 1,038 (59.83) | 318 (61.87) | 720 (58.97) | |
| Primary site | 0.02 | |||
| Main bronchus | 2 (0.12) | 0 (0.00) | 2 (0.16) | |
| Upper lobe, lung | 1,024 (59.02) | 297 (57.78) | 727 (59.54) | |
| Middle lobe, lung | 102 (5.88) | 17 (3.31) | 85 (6.96) | |
| Lower lobe, lung | 590 (34.01) | 195 (37.94) | 395 (32.35) | |
| Overlapping lesion of lung | 6 (0.35) | 2 (0.39) | 4 (0.33) | |
| Lung, NOS | 11 (0.63) | 3 (0.58) | 8 (0.66) | |
| Laterality | 0.26 | |||
| Right | 1,026 (59.14) | 293 (57.00) | 733 (60.03) | |
| Left | 709 (40.86) | 221 (43.00) | 488 (39.97) | |
| Grade | 0.41 | |||
| I | 427 (24.61) | 125 (24.32) | 302 (24.73) | |
| II | 752 (43.34) | 219 (42.61) | 533 (43.65) | |
| III | 389 (22.42) | 110 (21.40) | 279 (22.85) | |
| IV | 22 (1.27) | 9 (1.75) | 13 (1.06) | |
| Unknown | 145 (8.36) | 51 (9.92) | 94 (7.70) | |
| Histology | <0.001 | |||
| Adenocarcinomas | 1,010 (58.21) | 263 (51.17) | 747 (61.18) | |
| Squamous cell neoplasms | 388 (22.36) | 130 (25.29) | 258 (21.13) | |
| Epithelial neoplasms, NOS | 74 (4.27) | 27 (5.25) | 47 (3.85) | |
| Cystic, mucinous and serous neoplasms | 34 (1.96) | 20 (3.89) | 14 (1.15) | |
| Acinar cell neoplasms | 193 (11.12) | 68 (13.23) | 125 (10.24) | |
| Complex epithelial neoplasms | 36 (2.07) | 6 (1.17) | 30 (2.46) | |
| Maximum tumor diameter (cm) | <0.001 | |||
| ≤1 | 135 (7.78) | 54 (10.51) | 81 (6.63) | |
| >1, ≤2 | 830 (47.84) | 288 (56.03) | 542 (44.39) | |
| >2, ≤3 | 770 (44.38) | 172 (33.46) | 598 (48.98) | |
| Regional nodes examined | <0.001 | |||
| 1–5 | 652 (37.58) | 337 (65.56) | 315 (25.80) | |
| ≥6 | 1,083 (62.42) | 177 (34.44) | 906 (74.20) | |
| Race | 0.47 | |||
| White | 1,521 (87.67) | 456 (88.72) | 1,065 (87.22) | |
| Black | 71 (4.09) | 22 (4.28) | 49 (4.01) | |
| Other | 143 (8.24) | 36 (7.00) | 107 (8.76) | |
| Age (years) | 0.006 | |||
| <85 | 1,383 (79.71) | 388 (75.49) | 995 (81.49) | |
| ≥85 | 352 (20.29) | 126 (24.51) | 226 (18.51) | |
| Marital | 0.62 | |||
| Divorced | 125 (7.20) | 32 (6.23) | 93 (7.62) | |
| Married | 828 (47.72) | 237 (46.11) | 591 (48.40) | |
| Separated | 7 (0.40) | 1 (0.19) | 6 (0.49) | |
| Single (never married) | 159 (9.16) | 52 (10.12) | 107 (8.76) | |
| Unmarried or domestic partner | 5 (0.29) | 1 (0.19) | 4 (0.33) | |
| Widowed | 611 (35.22) | 191 (37.16) | 420 (34.40) | |
| Median household income inflation adjusted to 2022 | 0.17 | |||
| <$50,000 | 63 (3.63) | 19 (3.70) | 44 (3.60) | |
| $50,000–100,000 | 1,228 (70.78) | 348 (67.70) | 880 (72.07) | |
| >$100,000 | 444 (25.59) | 147 (28.60) | 297 (24.32) |
Data are presented as median (IQR) or n (%). †, Wilcoxon rank sum test, Pearson’s Chi-squared test, or Fisher ’s exact test. Grade I, well differentiated; grade II, moderately differentiated; grade III, poorly differentiated; grade IV, undifferentiated. IQR, interquartile range; NOS, not otherwise specified.
Table 2
| Characteristics | Overall (n=862) | Sublobar resection [n=431 (50%)] | Lobectomy [n=431 (50%)] | P value† | SMD | 95% CI |
|---|---|---|---|---|---|---|
| Time from diagnosis to treatment in days | 32.00 (0.00, 57.00) | 35.00 (0.00, 58.50) | 29.00 (0.00, 56.00) | 0.09 | 0.06 | −0.08, 0.19 |
| Sex | 0.89 | 0.01 | −0.12, 0.15 | |||
| Male | 337 (39.10) | 170 (39.44) | 167 (38.75) | |||
| Female | 525 (60.90) | 261 (60.56) | 264 (61.25) | |||
| Primary site | 0.97 | 0.050 | −0.09, 0.18 | |||
| Main bronchus | 498 (57.77) | 248 (57.54) | 250 (58.00) | |||
| Upper lobe, lung | 37 (4.29) | 20 (4.64) | 17 (3.94) | |||
| Middle lobe, lung | 320 (37.12) | 160 (37.12) | 160 (37.12) | |||
| Lower lobe, lung | 2 (0.23) | 1 (0.23) | 1 (0.23) | |||
| Overlapping lesion of lung | 5 (0.58) | 2 (0.46) | 3 (0.70) | |||
| Lung, NOS | 498 (57.77) | 248 (57.54) | 250 (58.00) | |||
| Laterality | 0.68 | 0.03 | −0.10, 0.17 | |||
| Right | 485 (56.26) | 246 (57.08) | 239 (55.45) | |||
| Left | 377 (43.74) | 185 (42.92) | 192 (44.55) | |||
| Grade | 0.96 | 0.06 | −0.08, 0.19 | |||
| I | 205 (23.78) | 100 (23.20) | 105 (24.36) | |||
| II | 366 (42.46) | 185 (42.92) | 181 (42.00) | |||
| III | 198 (22.97) | 102 (23.67) | 96 (22.27) | |||
| IV | 12 (1.39) | 6 (1.39) | 6 (1.39) | |||
| Unknown | 81 (9.40) | 38 (8.82) | 43 (9.98) | |||
| Histology | 0.97 | 0.06 | −0.07, 0.20 | |||
| Adenocarcinomas | 452 (52.44) | 224 (51.97) | 228 (52.90) | |||
| Squamous cell neoplasms | 206 (23.90) | 101 (23.43) | 105 (24.36) | |||
| Epithelial neoplasms, NOS | 41 (4.76) | 21 (4.87) | 20 (4.64) | |||
| Cystic, mucinous and serous neoplasms | 24 (2.78) | 11 (2.55) | 13 (3.02) | |||
| Acinar cell neoplasms | 126 (14.62) | 67 (15.55) | 59 (13.69) | |||
| Complex epithelial neoplasms | 13 (1.51) | 7 (1.62) | 6 (1.39) | |||
| Maximum tumor diameter (cm) | >0.99 | 0.01 | −0.13, 0.14 | |||
| ≤1 | 81 (9.40) | 41 (9.51) | 40 (9.28) | |||
| >1, ≤2 | 461 (53.48) | 230 (53.36) | 231 (53.60) | |||
| >2, ≤3 | 320 (37.12) | 160 (37.12) | 160 (37.12) | |||
| Regional nodes examined | 0.58 | 0.04 | −0.09, 0.18 | |||
| 1–5 | 499 (57.89) | 245 (56.84) | 254 (58.93) | |||
| ≥6 | 363 (42.11) | 186 (43.16) | 177 (41.07) | |||
| Race | 0.46 | 0.08 | −0.05, 0.22 | |||
| White | 752 (87.24) | 370 (85.85) | 382 (88.63) | |||
| Black | 37 (4.29) | 21 (4.87) | 16 (3.71) | |||
| Other | 73 (8.47) | 40 (9.28) | 33 (7.66) | |||
| Age (years) | 0.94 | 0.01 | −0.12, 0.14 | |||
| <85 | 664 (77.03) | 333 (77.26) | 331 (76.80) | |||
| ≥85 | 198 (22.97) | 98 (22.74) | 100 (23.20) | |||
| Marital | 0.86 | 0.09 | −0.04, 0.22 | |||
| Divorced | 54 (6.26) | 24 (5.57) | 30 (6.96) | |||
| Married | 397 (46.06) | 199 (46.17) | 198 (45.94) | |||
| Separated | 1 (0.12) | 0 (0.00) | 1 (0.23) | |||
| Single (never married) | 90 (10.44) | 45 (10.44) | 45 (10.44) | |||
| Widowed | 320 (37.12) | 163 (37.82) | 157 (36.43) | |||
| Median household income inflation adjusted to 2022 | 0.69 | 0.06 | −0.07, 0.19 | |||
| <$50,000 | 37 (4.29) | 21 (4.87) | 16 (3.71) | |||
| $50,000–100,000 | 589 (68.33) | 294 (68.21) | 295 (68.45) | |||
| >$100,000 | 236 (27.38) | 116 (26.91) | 120 (27.84) |
Data are presented as median (IQR) or n (%). †, Wilcoxon rank sum test, Pearson’s Chi-squared test, or Fisher ’s exact test. Grade I, well differentiated; grade II, moderately differentiated; grade III, poorly differentiated; grade IV, undifferentiated. CI, confidence interval; IQR, interquartile range; NOS, not otherwise specified; PSM, propensity score matching; SMD, standardized mean difference.
Survival analysis
Unadjusted Kaplan-Meier survival curves for unmatched groups are displayed in Figure 3A. Kaplan-Meier survival curves were also drawn separately for the high lymph node dissection group and the low lymph node dissection group (Figure 3B,3C). The OS of the lobectomy group was significantly higher than the sublobar resection group (P=0.02). The median OS was 67.0 months in the sublobar resection group and 77.0 months in the lobectomy group. The 1-, 3-, and 5-year OS rates were 90.79%, 71.38%, and 56.60% in the sublobar resection group, and 89.87%, 76.88%, and 60.94% in the lobectomy group. However, there was no significant difference in OS between the lobectomy and the sublobar resection groups in both the low lymph node dissection group (P=0.058) and the high lymph node dissection group (P=0.41). Univariate and multivariate analyses for OS predictors are shown in Table 3. In univariate analysis, age, median household income, sex, tumor sizes, histology, laterality, grades, number of lymph nodes removed, and surgical modalities were significantly associated with survival outcomes. In multivariate Cox regression analysis, the high lymph node dissection group had a better prognosis [hazard ratio (HR) =0.796; 95% confidence interval (CI): 0.690–0.919; P=0.002]. Younger age, female sex, histology of adenocarcinoma, cystic, mucinous, serous neoplasms, acinar cell neoplasms, right lung cancer, and smaller tumor sizes were independent prognostic factors for better OS.

Table 3
| Characteristics | Overall (n=1,735) | Univariate | Multivariate | |||
|---|---|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | |||
| Age (years) | ||||||
| <85 | 1,383 (79.7) | 1.000 (ref.) | 1.000 (ref.) | |||
| ≥85 | 352 (20.3) | 1.495 (1.278–1.75) | <0.001 | 1.568 (1.337–1.838) | <0.001 | |
| Grade | ||||||
| I | 427 (24.6) | 1.000 (ref.) | 1.000 (ref.) | |||
| II | 752 (43.3) | 1.457 (1.224–1.734) | <0.001 | 1.185 (0.985–1.426) | 0.07 | |
| III | 389 (22.4) | 1.742 (1.440–2.107) | <0.001 | 1.22 (0.984–1.511) | 0.07 | |
| IV | 22 (1.3) | 2.152 (1.342–3.453) | 0.001 | 1.563 (0.931–2.624) | 0.09 | |
| Unknown | 145 (8.4) | 1.138 (0.836–1.549) | 0.41 | 0.87 (0.635–1.193) | 0.39 | |
| Histology | ||||||
| Adenocarcinomas | 1,010 (58.2) | 1.000 (ref.) | 1.000 (ref.) | |||
| Squamous cell neoplasms | 388 (22.4) | 1.645 (1.417–1.911) | <0.001 | 1.433 (1.214–1.692) | <0.001 | |
| Epithelial neoplasms, NOS | 74 (4.3) | 2.025 (1.558–2.631) | <0.001 | 1.910 (1.423–2.564) | <0.001 | |
| Cystic, mucinous and serous neoplasms | 34 (2.0) | 0.53 (0.283–0.991) | 0.047 | 0.477 (0.253–0.896) | 0.02 | |
| Acinar cell neoplasms | 193 (11.1) | 0.609 (0.425–0.872) | 0.007 | 0.571 (0.398–0.820) | 0.002 | |
| Complex epithelial neoplasms | 36 (2.1) | 1.624 (1.102–2.393) | 0.01 | 1.263 (0.847–1.882) | 0.25 | |
| Median household income inflation adjusted to 2022 | ||||||
| <$50,000 | 63 (3.6) | 1.000 (ref.) | 1.000 (ref.) | |||
| $50,000–100,000 | 1,228 (70.8) | 0.689 (0.500–0.948) | 0.02 | 0.84 (0.608–1.160) | 0.29 | |
| >$100,000 | 444 (25.6) | 0.599 (0.426–0.843) | 0.003 | 0.81 (0.572–1.146) | 0.23 | |
| Laterality | ||||||
| Right | 1,026 (59.1) | 1.000 (ref.) | 1.000 (ref.) | |||
| Left | 709 (40.9) | 1.148 (1.006–1.309) | 0.042 | 1.154 (1.009–1.318) | 0.044 | |
| Marital | ||||||
| Divorced | 125 (7.2) | 1.000 (ref.) | ||||
| Married | 828 (47.7) | 1.227 (0.931–1.618) | 0.15 | |||
| Separated | 7 (0.4) | 0.671 (0.164–2.750) | 0.58 | |||
| Single (never married) | 159 (9.2) | 0.990 (0.695–1.41) | 0.96 | |||
| Unmarried or domestic partner | 5 (0.3) | 0.748 (0.103–5.402) | 0.77 | |||
| Widowed | 611 (35.2) | 1.231 (0.931–1.629) | 0.15 | |||
| Primary site | ||||||
| Main bronchus | 2 (0.1) | 1.000 (ref.) | ||||
| Upper lobe, lung | 1,024 (59.0) | 0.515 (0.072–3.665) | 0.51 | |||
| Middle lobe, lung | 102 (5.9) | 0.420 (0.058–3.052) | 0.39 | |||
| Lower lobe, lung | 590 (34.0) | 0.497 (0.070–3.548) | 0.49 | |||
| Overlapping lesion of lung | 6 (0.3) | 1.054 (0.123–9.035) | 0.96 | |||
| Lung, NOS | 11 (0.6) | 0.467 (0.054–3.999) | 0.49 | |||
| Race | ||||||
| White | 1,521 (87.7) | 1.000 (ref.) | ||||
| Black | 71 (4.1) | 0.751 (0.518–1.088) | 0.13 | |||
| Other | 143 (8.2) | 0.765 (0.585–1.001) | 0.050 | |||
| Regional nodes examined | ||||||
| 1–5 | 652 (37.6) | 1.000 (ref.) | 1.000 (ref.) | |||
| ≥6 | 1,083 (62.4) | 0.781 (0.685–0.891) | <0.001 | 0.796 (0.690–0.919) | 0.002 | |
| Sex | ||||||
| Male | 697 (40.2) | 1.000 (ref.) | 1.000 (ref.) | |||
| Female | 1,038 (59.8) | 0.615 (0.539–0.701) | <0.001 | 0.633 (0.553–0.724) | <0.001 | |
| Maximum tumor diameter (cm) | ||||||
| ≤1 | 135 (7.8) | 1.000 (ref.) | 1.000 (ref.) | |||
| >1, ≤ 2 | 830 (47.8) | 1.29 (0.964–1.724) | 0.09 | 1.235 (0.921–1.657) | 0.16 | |
| >2, ≤3 | 770 (44.4) | 1.465 (1.096–1.959) | 0.01 | 1.44 (1.070–1.939) | 0.02 | |
| Surgery | ||||||
| Sublobar resection | 514 (29.6) | 1.000 (ref.) | 1.000 (ref.) | |||
| Lobectomy | 1,221 (70.4) | 0.84 (0.727–0.972) | 0.02 | 0.888 (0.754–1.046) | 0.16 | |
| Time from diagnosis to treatment in days | 33.00 (0.00, 57.00) | 1.001 (0.999–1.002) | 0.42 | |||
Data are presented as n (%) or median (IQR), unless otherwise stated. Grade I, well differentiated; grade II, moderately differentiated; grade III, poorly differentiated; grade IV, undifferentiated. CI, confidence interval; HR, hazard ratio; IQR, interquartile range; NOS, not otherwise specified; OS, overall survival; ref., reference.
PSM based on all variables resulted in 431 patients in both the sublobar resection and lobectomy groups (1:1 ratio). The demographics and clinical variables were well-balanced, as shown in Table 2. After PSM, OS showed no obvious difference between the two groups in the Kaplan-Meier survival curves (Figure 4A). Median survival times were 69.0 and 67.0 months in the lobectomy and sublobar resection groups. The 1-, 3-, and 5-year OS rates were 87.69%, 76.43%, and 56.41% in the lobectomy group, and 90.21%, 70.54%, and 55.65% in the sublobar resection group. Likewise, there was no significant difference in OS between the two groups in both the low lymph node dissection (P=0.06) and the high lymph node dissection groups (P=0.37; Figure 4B,4C). OS showed no obvious difference between sublobar resection and lobectomy groups with differing tumor sizes in the Kaplan-Meier survival curves (Figure 5). Univariate and multivariate analyses for OS predictors are shown in Table 4. In univariate analysis, age, grade, histology, race, median household income, sex, tumor sizes, marital, number of lymph node dissections, and surgical modalities were significantly associated with survival outcomes. In multivariate Cox regression analysis, the high lymph node dissection group had a better prognosis (HR =0.765; 95% CI: 0.620–0.944; P=0.01). In addition to this, younger age, female sex, histology of cystic, mucinous, serous neoplasms, and acinar cell neoplasms were independent prognostic factors for better OS.


Table 4
| Characteristics | Overall (n=862) | Univariate | Multivariate | |||
|---|---|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | |||
| Age (years) | ||||||
| <85 | 664 (77.03) | 1.000 (ref.) | ||||
| ≥85 | 198 (22.97) | 1.568 (1.260–1.951) | <0.001 | 1.670 (1.338–2.084) | <0.001 | |
| Grade | ||||||
| I | 205 (23.78) | 1.000 (ref.) | ||||
| II | 366 (42.46) | 1.357 (1.049–1.754) | 0.02 | 1.049 (0.797–1.382) | 0.73 | |
| III | 198 (22.97) | 1.726 (1.313–2.268) | <0.001 | 1.126 (0.821–1.544) | 0.46 | |
| IV | 12 (1.39) | 2.675 (1.388–5.154) | 0.003 | 1.423 (0.697–2.906) | 0.33 | |
| Unknown | 81 (9.40) | 1.344 (0.894–2.021) | 0.16 | 1.081 (0.711–1.644) | 0.71 | |
| Histology | ||||||
| Adenocarcinomas | 452 (52.44) | 1.000 (ref.) | ||||
| Squamous cell neoplasms | 206 (23.90) | 1.583 (1.279–1.960) | <0.001 | 1.363 (1.065–1.743) | 0.01 | |
| Epithelial neoplasms, NOS | 41 (4.76) | 2.079 (1.461–2.959) | <0.001 | 1.887 (1.267–2.810) | 0.002 | |
| Cystic, mucinous and serous neoplasms | 24 (2.78) | 0.397 (0.176–0.894) | 0.03 | 0.369 (0.163–0.837) | 0.02 | |
| Acinar cell neoplasms | 126 (14.62) | 0.612 (0.397–0.941) | 0.03 | 0.600 (0.387–0.928) | 0.02 | |
| Complex epithelial neoplasms | 13 (1.51) | 1.902 (1.008–3.589) | 0.047 | 1.576 (0.820–3.031) | 0.17 | |
| Median household income inflation adjusted to 2022 | ||||||
| <$50,000 | 37 (4.29) | 1.000 (ref.) | ||||
| $50,000–100,000 | 589 (68.33) | 0.588 (0.388–0.891) | 0.01 | 0.674 (0.437–1.039) | 0.07 | |
| >$100,000 | 236 (27.38) | 0.536 (0.342–0.839) | 0.006 | 0.670 (0.419–1.070) | 0.09 | |
| Laterality | ||||||
| Right | 485 (56.26) | 1.000 (ref.) | ||||
| Left | 377 (43.74) | 1.120 (0.927–1.354) | 0.24 | |||
| Marital | ||||||
| Divorced | 54 (6.26) | 1.000 (ref.) | ||||
| Married | 397 (46.06) | 1.449 (0.915–2.293) | 0.11 | |||
| Separated | 1 (0.12) | 60.802 (7.845–471.248) | <0.001 | |||
| Single (never married) | 90 (10.44) | 1.079 (0.626–1.860) | 0.78 | |||
| Widowed | 320 (37.12) | 1.333 (0.839–2.118) | 0.22 | |||
| Primary site | ||||||
| Upper lobe, lung | 54 (6.26) | 1.000 (ref.) | ||||
| Middle lobe, lung | 397 (46.06) | 0.749 (0.458–1.225) | 0.25 | |||
| Lower lobe, lung | 1 (0.12) | 0.967 (0.792–1.180) | 0.74 | |||
| Overlapping lesion of lung | 90 (10.44) | 2.978 (0.416–21.318) | 0.28 | |||
| Lung, NOS | 320 (37.12) | 1.232 (0.394–3.851) | 0.72 | |||
| Race | ||||||
| White | 752 (87.24) | 1.000 (ref.) | ||||
| Black | 37 (4.29) | 0.775 (0.475–1.263) | 0.31 | |||
| Other | 73 (8.47) | 0.742 (0.491–1.120) | 0.16 | |||
| Regional nodes examined | ||||||
| 1–5 | 499 (57.89) | 1.000 (ref.) | ||||
| ≥6 | 363 (42.11) | 0.726 (0.592–0.891) | 0.002 | 0.765 (0.620–0.944) | 0.01 | |
| Sex | ||||||
| Male | 337 (39.10) | 1.000 (ref.) | ||||
| Female | 525 (60.90) | 0.602 (0.498–0.727) | <0.001 | 0.616 (0.505–0.752) | <0.001 | |
| Maximum tumor diameter (cm) | ||||||
| ≤1 | 81 (9.40) | 1.000 (ref.) | ||||
| >1, ≤2 | 461 (53.48) | 1.353 (0.935–1.959) | 0.11 | 1.176 (0.805–1.716) | 0.40 | |
| >2, ≤3 | 320 (37.12) | 1.645 (1.128–2.400) | 0.01 | 1.405 (0.952–2.074) | 0.09 | |
| Surgery | ||||||
| Lobectomy | 431 (50.00) | 1.000 (ref.) | ||||
| Sublobar resection | 431 (50.00) | 1.111 (0.920–1.341) | 0.27 | 1.146 (0.947–1.387) | 0.16 | |
| Time from diagnosis to treatment in days | 32.00 (0.00, 57.00) | 1.00 (0.998–1.002) | 0.87 | |||
Data are presented as n (%) or median (IQR), unless otherwise stated. Grade I, well differentiated; grade II, moderately differentiated; grade III, poorly differentiated; grade IV, undifferentiated. CI, confidence interval; HR, hazard ratio; IQR, interquartile range; NOS, not otherwise specified; OS, overall survival; PSM, propensity score matching; ref., reference.
Discussion
Currently, there are many treatment methods for early NSCLC patients over 80 years old, among which surgical treatment is superior to other treatment methods (6). Surgical treatment primarily includes lobectomy, wedge resection, and segmentectomy (15). There are no specific criteria for the extent of lymph node dissection (16). Based on the data in this study, we observed that sublobar resection was often accompanied by less or even no lymph node dissection, while lobectomy was often accompanied by more lymph node dissection. Therefore, we discuss the effect of surgical procedures and the extent of lymph node dissection on the prognosis of these patients and try to guide clinical work.
A recent randomized controlled trial (JCOG0802) indicated that for tumors smaller than 2 cm, patients undergoing segmentectomy had improved survival rates compared to those undergoing lobectomy (13). Unfortunately, this study did not include patients over the age of 80 years. A recent multicenter study suggested that the OS of sublobar resection with a secure surgical margin was comparable to that of lobectomy in patients aged 80 years or older with early peripheral NSCLC (2–4 cm) who could tolerate lobectomy (17). Based on these studies, we speculated that there is no significant difference in OS between sublobar resection and lobectomy for stage IA NSCLC in patients over the age of 80 years. However, an analysis of the National Cancer Database (NCDB) by Chan et al. concluded that lobectomy was beneficial for patients with stage I NSCLC who underwent surgery at age 80 or older (10). However, this study did not consider the number or extent of lymph node dissection. Currently, numerous studies have substantiated the significant role of the number of lymph node dissections in predicting the prognosis of lung cancer (18,19). Our perspective is that the number of lymph nodes dissected could significantly influence the outcomes of the study; hence, its inclusion in our research. Owing to the limitations of the SEER database, we were unable to retrieve detailed information regarding utilizing established LNE classification criteria, such as the International Association for the Study of Lung Cancer (IASLC) lymph node mapping system. Consequently, X-Tile software was utilized to determine the optimal cut-off value for the number of lymph node dissections, thereby enhancing the differentiation of survival curves. In our study, PSM and multivariate Cox regression analyses showed no significant difference in survival between lobectomy and sublobar resection in patients over 80 years of age with stage IA NSCLC, and the number of dissected lymph nodes was an independent prognostic factor. Additionally, there was no significant difference in survival between the two surgical procedures in the low lymph node dissection group and the high lymph node dissection group. Therefore, sublobar resection may be a better choice, as it can preserve lung function and reduce complications, especially for patients over 80 years old (20).
There are limitations of our study that must be acknowledged. Firstly, the target population based on the pathological stage, making it difficult to interpret the clinical relevance of the findings. However, we consider that if comparisons are made based on the same clinical stage, the postoperative pathological stage may vary significantly, which could also affect survival prognosis. Comparing the survival prognosis of the two surgical methods under such circumstances may lead to biased results. The clinical stage may vary significantly due to different examination methods. Secondly, extensive lymph node dissection seemed to lead to a better prognosis for these patients. However, we prefer to raise two points of concern. First, patients undergoing extensive lymph node dissection often undergo lobectomy, are usually younger, and may have fewer underlying diseases (21). Second, among these patients, those with higher staging may be classified as stage IA, leading to poorer survival outcomes. Consequently, patients with less lymph node dissection may experience poorer survival (22). Thirdly, the limitations of this study also include many inherent constraints observed in large-scale database research, such as the retrospective nature of the data and patient selection bias. For example, there is no determination of the reasons behind the choice of surgical procedure, and there is no data on pulmonary function testing, cardio-vascular diseases, and diabetes mellitus, which could play a significant role in determining the surgical procedure. It is hoped that with further improvement in the accuracy of clinical staging, more multi-center randomized controlled trials, this issue can be gradually addressed.
Conclusions
In octogenarians and older with stage Ia NSCLC, no significant difference in OS exists between lobectomy and sublobar resection. The dissection of more than six lymph nodes serves as an independent protective factor for prognosis. For patients aged 80 years and older diagnosed with stage Ia NSCLC, it is recommended to perform a sublobar resection accompanied by the dissection of a minimum of six lymph nodes.
Acknowledgments
We thank the Surveillance, Epidemiology, and End Results (SEER) Program registries in creating and regularly refreshing the SEER database, which plays a critical role.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://tcr.amegroups.com/article/view/10.21037/tcr-2024-2575/rc
Peer Review File: Available at https://tcr.amegroups.com/article/view/10.21037/tcr-2024-2575/prf
Funding: This work was supported by
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tcr.amegroups.com/article/view/10.21037/tcr-2024-2575/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This study was granted exemption by the Ethics Committee of the Affiliated Huai’an Hospital of Xuzhou Medical University because of the publicity and anonymity of SEER data. The study was conducted in accordance with the Declaration of Helsinki and its subsequent amendments.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Global incidence, prevalence, years lived with disability (YLDs), disability-adjusted life-years (DALYs), and healthy life expectancy (HALE) for 371 diseases and injuries in 204 countries and territories and 811 subnational locations, 1990-2021: a systematic analysis for the Global Burden of Disease Study 2021. Lancet 2024;403:2133-61. [Crossref] [PubMed]
- Kaprin A, Pikin O, Ryabov A, et al. Surgical intervention for lung cancer in patients aged 75 and above: potential associations with increased mortality rates-a single-center observational study. J Cardiothorac Surg 2024;19:471. [Crossref] [PubMed]
- Zhou T, Zhang LY, He JZ, et al. Review: Mechanisms and perspective treatment of radioresistance in non-small cell lung cancer. Front Immunol 2023;14:1133899. [Crossref] [PubMed]
- Singareddy A, Flanagan ME, Samson PP, et al. Trends in Stage I Lung Cancer. Clin Lung Cancer 2023;24:114-9. [Crossref] [PubMed]
- Peng Y, Wo Y, Liu P, et al. Identified optimal candidates for pulmonary resection in octogenarians with non-small cell lung cancer: a web-based predictive model. J Thorac Dis 2023;15:1142-54. [Crossref] [PubMed]
- Ni L, Lin G, Zhang Z, et al. Surgery versus radiotherapy in octogenarians with stage Ia non-small cell lung cancer: propensity score matching analysis of the SEER database. BMC Pulm Med 2022;22:411. [Crossref] [PubMed]
- Ettinger DS, Wood DE, Aisner DL, et al. NCCN Guidelines® Insights: Non-Small Cell Lung Cancer, Version 2.2023. J Natl Compr Canc Netw 2023;21:340-50. [Crossref] [PubMed]
- Aoki S, Onishi H, Karube M, et al. Comparative Analysis of Photon Stereotactic Radiotherapy and Carbon-Ion Radiotherapy for Elderly Patients with Stage I Non-Small-Cell Lung Cancer: A Multicenter Retrospective Study. Cancers (Basel) 2023;15:3633. [Crossref] [PubMed]
- Yun J, Cho JH, Hong TH, et al. Sublobar Resection versus Stereotactic Body Radiation Therapy for Clinical Stage I Non-Small Cell Lung Cancer: A Study Using Data from the Korean Nationwide Lung Cancer Registry. Cancer Res Treat 2023;55:1171-80. [Crossref] [PubMed]
- Chan EY, Amirkhosravi F, Nguyen DT, et al. Lobectomy Provides the Best Survival for Stage I Lung Cancer Patients Despite Advanced Age. Ann Thorac Surg 2022;114:1824-32. [Crossref] [PubMed]
- Liu K, Lin X, Chen X, et al. Development and validation of a deep learning signature for predicting lymphovascular invasion and survival outcomes in clinical stage IA lung adenocarcinoma: A multicenter retrospective cohort study. Transl Oncol 2024;42:101894. [Crossref] [PubMed]
- Ding H, Song N, Zhang P, et al. Wedge resection plus adequate lymph nodes resection is comparable to lobectomy for small-sized non-small cell lung cancer. Front Oncol 2022;12:1022904. [Crossref] [PubMed]
- Saji H, Okada M, Tsuboi M, et al. Segmentectomy versus lobectomy in small-sized peripheral non-small-cell lung cancer (JCOG0802/WJOG4607L): a multicentre, open-label, phase 3, randomised, controlled, non-inferiority trial. Lancet 2022;399:1607-17. [Crossref] [PubMed]
- Pagès PB. Lobectomy or Segmentectomy for Elderly? What's the Best? Ann Thorac Surg 2022;114:1832-3. [Crossref] [PubMed]
- Mamudu L, Salmeron B, Odame EA, et al. Disparities in localized malignant lung cancer surgical treatment: A population-based cancer registry analysis. Cancer Med 2023;12:7427-37. [Crossref] [PubMed]
- Zhiqiang W, Shaohua M. Perioperative outcomes of robotic-assisted versus video-assisted thoracoscopic lobectomy: A propensity score matched analysis. Thorac Cancer 2023;14:1921-31. [Crossref] [PubMed]
- Mimae T, Saji H, Nakamura H, et al. Sublobar Resection for Non-Small Cell Lung Cancer in Octogenarians: A Prospective, Multicenter Study. Ann Thorac Surg 2023;116:543-51. [Crossref] [PubMed]
- Huang L, Petersen RH. Impact of number of dissected lymph nodes on recurrence and survival following thoracoscopic segmentectomy for clinical stage I non-small cell lung cancer. Lung Cancer 2024;193:107846. [Crossref] [PubMed]
- Moonen L, Derks JL, Hillen LM, et al. Disease relapse in relation to lymph node sampling in lung carcinoid patients. Endocr Relat Cancer 2024;31:e230202. [Crossref] [PubMed]
- Bao M, Lang Z, Wang Z, et al. Changes in pulmonary function in lung cancer patients after segmentectomy or lobectomy: a retrospective, non-intervention, observation study. Eur J Cardiothorac Surg 2023;64:ezad256. [Crossref] [PubMed]
- Brown S, Kokosis G, Graziano FD, et al. Immediate Lymphatic Reconstruction with Vascularized Omentum Lymph Node Transplant: Reducing the Risk of Both Painful Contracture and Lymphedema. Plast Reconstr Surg Glob Open 2024;12:e5747. [Crossref] [PubMed]
- Jiang C, Zhang Y, Fu F, et al. A Shift in Paradigm: Selective Lymph Node Dissection for Minimizing Oversurgery in Early Stage Lung Cancer. J Thorac Oncol 2024;19:25-35. [Crossref] [PubMed]



