
Differences in esophageal adenocarcinoma survival and treatment modalities by III/IV stage subgroup: a SEER population-based study
Highlight box
Key findings
• Subgroup analysis revealed that higher T stage was associated with poorer prognosis in esophageal adenocarcinoma (EAC) patients in the same III/IVA stage.
• Surgery plus chemotherapy showed significant survival benefits in the early subgroups of III/IVA stage.
What is known and what is new?
• Multimodal treatment has become a critical component in the management of locally advanced EAC.
• For patients in the subgroup of III/IVA stage, higher T stage predicted worse prognosis. Meanwhile, surgery plus chemotherapy in the early subgroups showed better survival benefits.
What is the implication, and what should change now?
• We specify the III/IV stages in the subgroups based on T stage in EAC patients, optimizing the treatment strategies involving surgery and chemotherapy.
Introduction
Esophageal cancer (EC), one of the most lethal malignant tumors, is estimated to have 21,560 new cases and to be responsible for 16,120 deaths in the United States in 2023 (1). Esophageal squamous cell carcinoma (ESCC) and esophageal adenocarcinoma (EAC) are common histological subtypes. The number of EAC cases is increasing in Western countries (2) and the related heath burdens worldwide must urgently be addressed. Currently, the American Joint Committee on Cancer (AJCC) staging system is widely used to determine treatment plans and predict the prognosis of patients. Many studies have focused on this staging system. Inada et al. have concluded that the 8th edition of the AJCC staging system is more advantageous than the 7th edition for patients treated with definitive radiotherapy (3). Hu et al. have revised the N categories of the 8th edition of the AJCC staging system for better clinical decision-making for patients not receiving surgery (4).
Clinically, patients diagnosed with III/IV stage of EC have poor prognosis, owing to severe complications and distant metastasis (DM) (5-8). According to the American Society of Clinical Oncology (ASCO), multimodal management for patients with locally advanced esophageal carcinoma is recommended, and patients with EAC should be offered preoperative chemoradiotherapy or perioperative chemotherapy (9). Qiu et al. have demonstrated metastasis patterns and survival benefits in older patients with DM and diagnosed with EAC, and have concluded that active management such as chemotherapy provides great benefit (10). However, survival differences among various therapy methods among patients with the same tumor-node-metastasis (TNM) stage remain unknown. For example, III stage involves T2N1M0, T3N0-1M0, and T4aN0-1M0, according to the EAC cTNM stage (11). For patients diagnosed with these three subgroups of III stage, we sought to determine how prognosis can be assessed, and which therapy can be offered to achieve optimal therapeutic outcomes.
We postulated that patients with EAC diagnosed with III/IV stage would show survival differences according to T stage. To confirm this hypothesis, we compared the overall survival (OS) and cancer-specific survival (CSS) in the subgroup of patients diagnosed with III/IV stage. Relevant therapeutic methods were compared to determine the survival differences in the III/IV stage subgroups. Clinical statistics were extracted from the Surveillance, Epidemiology, and End Results (SEER) database. We present this article in accordance with the STROBE reporting checklist (available at https://tcr.amegroups.com/article/view/10.21037/tcr-24-2099/rc).
Methods
Study population
The data analyzed in the current study were extracted from the SEER 17 registries research plus database (Nov 2021 Sub, 2000–2019). The study was performed following the Declaration of Helsinki and its subsequent amendments.. Patients were included on the basis of the following criteria: the site recode ICD-O-3 (International Classification of Diseases for Oncology-3)/WHO 2008 was “esophagus”; the behavior code ICD-O-3 was “malignant”; and EAC was histologically diagnosed. The exclusion criteria were as follows: patients with incomplete clinical or demographic information (race, TNM stage, surgery, cause-specific death); endoscopic therapy; patients with stage IVB and received surgery; unknown survival time or survival time equal and shorter than 1 month. Finally, 5,765 patients with III/IV stage were selected for further study. The SEER*Stat software (Version 8.4.0.1) was used to collect clinical data. The flow chart of data screening is shown in Figure 1.
Data collection
Patient demographics and clinical characteristics included age, sex, race, grade, T stage, N stage, M stage, surgery, radiation, and chemotherapy. EAC was identified from the SEER database according to the following codes: 8140, 8144, 8210, 8211, 8255, 8260, 8263, 8310, 8323, 8480, 8481, and 8574. To avoid inadequate sample size, TNM stage was identified on the basis of the 7th and 8th editions of the AJCC staging system together. Only slight differences exist between the above two editions, which have been found not to affect the incorporation and analysis of clinical data (12,13). In terms of surgery, patients were divided into two subgroups: esophagectomy and no surgery. For patients with III/IV stage, endoscopic therapy was not applicable; therefore, patients receiving this therapy were excluded. Meanwhile, surgery is a contraindication for M1 patients. We excluded this group of patients as well. Esophagectomy involved partial or total esophagectomy with or without laryngectomy and gastrectomy. The surgery codes included 30, 40, 50–55, and 80. The endpoint of the study was OS and CSS. OS was measured from the date of diagnosis to the date of death from any cause. CSS was the interval between the initial diagnosis of EAC and the occurrence of EAC-specific death.
Statistical analysis
The R software package “tableone” was used to describe clinical baseline characteristics. The packages “survival” and “plyr” were used to perform the univariable and multivariable Cox regression analysis, and the packages “survminer” and “survival” were used to perform Kaplan-Meier survival analysis. According to the cTNM staging system of EAC (11), stage III was divided into three groups: T2N1M0, T3N0-1M0 and T4aN0-1M0. Stage IVA was divided into five groups: T1N2-3M0, T2N2-3M0, T3N2-3M0, T4aN2-3M0 and T4bN0-3M0. Stage IVB was divided into five groups: T1N0-3M1, T2N0-3M1, T3N0-3M1, T4aN0-3M1 and T4bN0-3M1. Categorical variables described as counts, relative percentages were compared with the chi-square test, and a P-value less than 0.05 was considered statistically significant. All clinical statistics were analyzed in R software (Version 4.2.0).
Results
Patient characteristics
A total of 5,765 patients were extracted from the SEER database according to the strict inclusion and exclusion criteria. All patients were diagnosed with EAC. The demographic and clinicopathological characteristics are shown in Table 1. The median follow-up time was 15 months. Most patients were male (seven times the number of female patients; 88.1%). Regarding race, most patients were White (94.4%). More than half the cases were in T3 stage (63.4%) and had N1 stage (69.3%), whereas a minority of cases had DM (32.6%). T4 stage cases comprised 698 T4a cases and 277 T4b cases. With respect to therapy, most patients were offered chemotherapy and radiation therapy. Patients without surgery were the majority.
Table 1
| Variables | Overall (N=5,765) |
|---|---|
| Age | |
| <65 years | 2,625 (45.5) |
| ≥65 years | 3,140 (54.5) |
| Gender | |
| Female | 686 (11.9) |
| Male | 5,079 (88.1) |
| Race | |
| Black | 146 (2.5) |
| White | 5,444 (94.4) |
| Other | 175 (3.0) |
| Grade | |
| Grade I | 165 (2.9) |
| Grade II | 1,409 (24.4) |
| Grade III/IV | 2,040 (35.4) |
| Unknown | 2,151 (37.3) |
| T stage | |
| T1 | 335 (5.8) |
| T2 | 800 (13.9) |
| T3 | 3,655 (63.4) |
| T4a | 698 (12.1) |
| T4b | 277 (4.8) |
| N stage | |
| N0 | 472 (8.2) |
| N1 | 3,998 (69.3) |
| N2 | 955 (16.6) |
| N3 | 340 (5.9) |
| M stage | |
| M0 | 3,886 (67.4) |
| M1 | 1,879 (32.6) |
| Surgery | |
| No surgery | 3,764 (65.3) |
| Esophagectomy† | 2,001 (34.7) |
| Radiation | |
| No/unknown | 1,488 (25.8) |
| Yes | 4,277 (74.2) |
| Chemotherapy | |
| No/unknown | 722 (12.5) |
| Yes | 5,043 (87.5) |
Data are presented as n (%). †, esophagectomy involved partial or total esophagectomy with or without laryngectomy and gastrectomy.
Predictors of OS and CSS
To evaluate the power of each variable in assessing the prognosis of EAC, we used the Cox proportional hazards model to predict the OS and CSS of EAC cases. Univariate analysis indicated that variables such as age, race, grade, T stage, N stage, M stage, surgery, radiation, and chemotherapy were associated with OS and CSS. Variables with P ≤ 0.05 in univariate analyses were further analyzed in the multivariate analyses. In terms of predicting OS, poor prognosis was associated with age (patients ≥65 compared with <65 years of age), grade (grade III/IV compared with grade I), N stage (N2 and N3 compared with N0) and M stage (M1 compared with M0), whereas good prognosis was associated with T stage (T2 and T3 compared with T1), surgery (esophagectomy compared with no surgery), and chemotherapy (yes compared with no/unknown). In CSS prediction, poor prognosis was associated with age (patients ≥65 years compared with <65 years of age), grade (grade III/IV compared with grade I), N stage (N2 and N3 compared with N0), and M stage (M1 compared with M0), whereas good prognosis was associated with T stage (T2 and T3 compared with T1), surgery (esophagectomy compared with no surgery), and chemotherapy (yes compared with no/unknown). The detailed information is shown in Tables 2,3.
Table 2
| Variables | Univariate analysis | Multivariate analysis | |||
|---|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | ||
| Age (vs. <65 years) | |||||
| ≥65 years | 1.26 (1.18–1.34) | <0.001 | 1.15 (1.08–1.22) | <0.001 | |
| Gender (vs. female) | |||||
| Male | 1.02 (0.93–1.13) | 0.61 | |||
| Race (vs. Black) | |||||
| White | 0.76 (0.63–0.91) | 0.004 | 0.86 (0.71–1.04) | 0.11 | |
| Other | 0.76 (0.59–0.98) | 0.04 | 0.86 (0.66–1.11) | 0.25 | |
| Grade (vs. Grade I) | |||||
| Grade II | 0.99 (0.83–1.18) | 0.91 | 1.00 (0.84–1.19) | 0.98 | |
| Grade III/IV | 1.26 (1.06–1.50) | 0.009 | 1.24 (1.04–1.47) | 0.02 | |
| Unknown | 1.06 (0.89–1.27) | 0.53 | 0.96 (0.80–1.14) | 0.63 | |
| T stage (vs. T1) | |||||
| T2 | 0.45 (0.38–0.52) | <0.001 | 0.63 (0.54–0.75) | <0.001 | |
| T3 | 0.57 (0.49–0.65) | <0.001 | 0.84 (0.72–0.97) | 0.02 | |
| T4a | 0.81 (0.70–0.95) | 0.009 | 0.94 (0.80–1.10) | 0.41 | |
| T4b | 1.14 (0.95–1.36) | 0.17 | 1.17(0.97–1.41) | 0.10 | |
| N stage (vs. N0) | |||||
| N1 | 0.60 (0.54–0.67) | <0.001 | 1.03 (0.91–1.15) | 0.65 | |
| N2 | 0.63 (0.56–0.72) | <0.001 | 1.22 (1.07–1.40) | 0.004 | |
| N3 | 0.86 (0.74–1.01) | 0.06 | 1.49 (1.27–1.75) | <0.001 | |
| M stage (vs. M0) | |||||
| M1 | 2.34 (2.20–2.50) | <0.001 | 1.53 (1.41–1.66) | <0.001 | |
| Surgery (vs. no surgery) | |||||
| Esophagectomy† | 0.35 (0.33–0.38) | <0.001 | 0.46 (0.42–0.50) | <0.001 | |
| Radiation (vs. no/unknown) | |||||
| Yes | 0.62 (0.58–0.66) | <0.001 | 1.07 (0.98–1.17) | 0.12 | |
| Chemotherapy (vs. no/unknown) | |||||
| Yes | 0.37 (0.34–0.41) | <0.001 | 0.41 (0.36–0.47) | <0.001 | |
†, esophagectomy involved partial or total esophagectomy with or without laryngectomy and gastrectomy. CI, confidence interval; HR, hazard ratio; OS, overall survival.
Table 3
| Variables | Univariate analysis | Multivariate analysis | |||
|---|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | ||
| Age (vs. <65 years) | |||||
| ≥65 years | 1.20 (1.12–1.28) | <0.001 | 1.10 (1.03–1.18) | 0.004 | |
| Gender (vs. female) | |||||
| Male | 1.02 (0.92–1.13) | 0.71 | |||
| Race (vs. Black) | |||||
| White | 0.73 (0.60–0.89) | 0.002 | 0.85 (0.70–1.03) | 0.10 | |
| Other | 0.72 (0.55–0.95) | 0.02 | 0.82 (0.63–1.08) | 0.16 | |
| Grade (vs. Grade I) | |||||
| Grade II | 1.00 (0.83–1.21) | 0.99 | 1.01 (0.84–1.22) | 0.94 | |
| Grade III/IV | 1.27 (1.06–1.53) | 0.01 | 1.24 (1.03–1.49) | 0.02 | |
| Unknown | 1.05 (0.86–1.26) | 0.65 | 0.94 (0.78–1.14) | 0.53 | |
| T stage (vs. T1) | |||||
| T2 | 0.40 (0.34–0.47) | <0.001 | 0.59 (0.50–0.70) | <0.001 | |
| T3 | 0.54 (0.47–0.62) | <0.001 | 0.83 (0.71–0.96) | 0.01 | |
| T4a | 0.82 (0.70–0.96) | 0.02 | 0.96 (0.82–1.13) | 0.64 | |
| T4b | 1.14 (0.95–1.38) | 0.16 | 1.19 (0.98–1.44) | 0.07 | |
| N stage (vs. N0) | |||||
| N1 | 0.56 (0.50–0.62) | <0.001 | 1.00 (0.88–1.12) | 0.96 | |
| N2 | 0.60 (0.53–0.68) | <0.001 | 1.21 (1.05–1.39) | 0.007 | |
| N3 | 0.86 (0.73–1.01) | 0.06 | 1.51 (1.28–1.79) | <0.001 | |
| M stage (vs. M0) | |||||
| M1 | 2.52 (2.35–2.69) | <0.001 | 1.59 (1.46–1.74) | <0.001 | |
| Surgery (vs. no surgery) | |||||
| Esophagectomy† | 0.34 (0.31–0.36) | <0.001 | 0.45 (0.41–0.49) | <0.001 | |
| Radiation (vs. no/unknown) | |||||
| Yes | 0.59 (0.55–0.63) | <0.001 | 1.04 (0.95–1.14) | 0.39 | |
| Chemotherapy (vs. no/unknown) | |||||
| Yes | 0.36 (0.33–0.40) | <0.001 | 0.39 (0.35–0.45) | <0.001 | |
†, esophagectomy involved partial or total esophagectomy with or without laryngectomy and gastrectomy. CI, confidence interval; CSS, cancer-specific survival; HR, hazard ratio.
Subgroup survival analysis
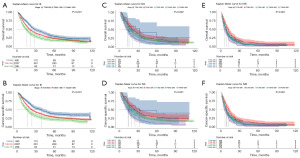
Kaplan-Meier curves were plotted to compare the long-term survival between subgroups of III/IV stage. As shown in Figure 2A,2B, OS and CSS of patients with T2N1M0 in III stage was significantly better when compared to the other two groups (T2N1M0 vs. T3N0-1M0, P<0.001, T2N1M0 vs. T4aN0-1M0, P<0.001). Regarding IVA stage, there was significant survival difference in OS between T2N2-3M0 and T4bN0-3M0 (P=0.01, Figure 2C). Figure 2D revealed that survival difference in CSS was also found between T2N2-3M0, T4aN2-3M0 and T4bN0-3M0 in IVA stage subgroups (T2N2-3M0 vs. T4aN2-3M0, P=0.03, T2N2-3M0 vs. T4bN0-3M0, P=0.005). In terms of IVB stage, we found that patients with T2N0-3M1 had the best OS (T2N0-3M1 vs. T1N0-3M1, P=0.007, T2N0-3M1 vs. T3N0-3M1, P=0.007, T2N0-3M1 vs. T4aN0-3M1, P=0.002, T2N0-3M1 vs. T4bN0-3M1, P<0.001, Figure 2E). Similar results were found in CSS in patients with IVB stage (P=0.008, P=0.001, P<0.001, P<0.001, respectively, Figure 2F). The detailed 3-year and 5-year OS/CSS rates in III/IV stage subgroups are shown in Tables S1,S2.
Because the multivariate analysis revealed that surgery and chemotherapy were associated with good prognosis, further stratified analyses were performed to investigate whether the above two therapies might result in survival differences in patients in each III/IV stage subgroup. As described in Figure 3, the statistical difference of the OS was found in patients who received chemotherapy or surgery alone in III stage subgroup (P=0.001 and 0.02, respectively, Figure 3A). Surgery alone or in combination with chemotherapy obviously increased the median period of survival in T2N1M0 in III stage subgroup. However, no survival difference was observed in the subgroup of surgery plus chemotherapy (P=0.07, Figure 3A). Results in CSS subgroup analysis was basically consistent with that in OS subgroup analysis. There was significant survival difference in patients receiving chemotherapy or surgery in III stage subgroup (P<0.001 and P=0.01, respectively, Figure 3B). Our findings pointed out that surgery plus chemotherapy in III stage subgroup had significant survival difference in CSS rather than OS (P=0.02 and 0.07, respectively, Figure 3). As to IVA stage, no differences of OS and CSS was observed between surgery and chemotherapy in subgroup analysis. Nevertheless, surgery plus chemotherapy prolonged the median survival time, especially in T1N2-3M0 in IVA stage subgroup (Figure S1). To patients with M1 stage, subgroup survival difference was found in OS and CSS in chemotherapy group (P<0.001, respectively, Figure S2).

Discussion
The management of EC is a major challenge for physicians. EC ranked 7th in incidence and 6th in mortality in 2020, thus posing a heavy heath burden (14). The morbidity of the two most predominant pathological types of EC has changed considerably in recent years. The incidence of ESCC has generally declined in recent decades in most European countries, due to a decrease in alcohol consumption and tobacco smoking (15). However, EAC cases have sharply increased in recent years because of various factors, such as the cumulative incidence of central obesity, and lower incidence of H. pylori infection resulting in gastroesophageal reflux and consequent damage to esophageal mucous membranes (16). Many studies have revealed links between H. pylori infection and gastroesophageal reflux as well as EAC (17,18).
Patients diagnosed with any cancer can be simply divided into two stages: early stage and late stage. Patients in early stage can gain much better survival benefits than those in late stage. Youssef et al. have indicated that endoscopic submucosal dissection (ESD) is an effective method in the T1b EC, which is associated with safety and low recurrence (19). Qin et al. have identified a survival benefit with the application of endoscopic therapy and surgery among older patients with a TNM stage of cT1N0M0, according to analysis of the SEER database (20). Unfortunately, most patients are diagnosed in late stage and have poor prognosis. Therefore, we focused on the III/IV stage of EC to determine survival differences according to TNM stage subgroup.
In this study, we took advantage of the abundant clinical information in the SEER database. First, we performed univariate and multivariate Cox regression analyses, which indicated that chemotherapy [hazard ratio (HR): 0.41, 95% confidence interval (CI): 0.36–0.47] and surgery (HR: 0.46, 95% CI: 0.42–0.50) were associated with a good prognosis in predicting OS. This result remained consistent in predicting CSS as well. However, no significant difference in predicting OS and CSS was observed when patients received radiotherapy. Liou et al. have indicated that, compared with induction chemotherapy alone, induction chemoradiation for late stage EAC does not prolong survival (21). Some articles have also reported the adverse effects of radiation (22-24).
We further divided stages III and IV into several subgroups according to the 8th edition of the AJCC staging system to compare survival differences. Significant differences between OS and CSS were found for both III stage, IVA stage as well as IVB stage (P<0.001). The Kaplan-Meier curve for III stage and IVA stage indicated that poorer prognosis was associated with higher T stage. Patients with higher T stage may be more likely to have local heart compression, esophagobronchial fistula, and aortoesophageal fistula. These severe complications may be deadly and consequently are associated with poor prognosis.
However, the results for the IVB stage were not consistent with those for III stage and IVA stage. Among the five subgroups of IVB stage, T2N0-3M1 stage was associated with better prognosis than T1N0-3M1. We surmised that these findings might have been due to the unavoidable selection bias. First, compared with patients diagnosed with other stages of IVB, patients with T1N0-3M1 might have had poorer grade differentiation, thus potentially resulting in more chances for vascular and nerve invasion. In addition, all groups were stratified by T stage, whereas the influence of lymph node status was ignored. We postulated that the lymph node metastasis in T1N0-3M1 was much worse than that in T2N0-3M1, thus resulting in a poorer prognosis in T1N0-3M1. Although the role of lymph nodes is meaningful, this study did not focus on that clinical aspect. Many studies have focused on lymph node metastasis. Regarding lymph node clearance, Tian et al. have evaluated the minimum number of examined lymph nodes in performing lymphadenectomy in T1-2 ESCC, and concluded that resection of 14 and 18 lymph nodes was associated with the best survival outcomes for T1 and T2, respectively (25). Radiomics has been rapidly developed over the past several decades and holds great promise for diagnostic and prognostic applications. Wu et al. have built a model to predict lymph node metastasis in ESCC through radiomics (26).
Which multimodal treatment most benefits patients with locally advanced EC has been controversial in the literature. Surgery is the exact choice of treatment methods for locally advanced EC. More and more evidences suggest that neoadjuvant therapy followed by surgery improves outcomes of EAC patients (27,28). Recently, a clinical trial reported the efficacy of perioperative therapy based on fluorouracil, leucovorin, oxaliplatin and docetaxel (FLOT) and the results showed a great OS benefit in locally advanced EAC patients (29). In terms of patients with M1 stage, the treatment strategy is typically palliative, and surgery is not a proper recommendation. Saddoughi et al. performed a relevant retrospective review and suggested that esophagectomy should not be offered for stage IV disease (30). Systemic treatment can alleviate patients’ symptoms and lengthen their living time. It is worth mentioning that adding immune checkpoint blockade (ICB) to chemotherapy has shown great benefits in advanced EAC. Notably, after 3-year follow-up of the phase III CheckMate 649, nivolumab plus chemotherapy continued to show long-term OS and progression-free survival (PFS) benefits (31).
In our study, treatments involving surgery and chemotherapy were further analyzed to determine whether significant differences might exist among treatments in each subgroup of III stage and IV stage. In terms of III stage, our findings revealed that patients who received chemotherapy alone or in combination with surgery had significant survival differences in the subgroup analysis in CSS (P<0.001, P=0.02, respectively). Furthermore, chemotherapy combined with surgery in early stages of III subgroups prominently increased the median survival time. Similar conclusions were observed in the subgroup analysis in OS. However, no survival difference in OS and CSS was found in IVA subgroup analysis. One possible reason was that clinical data was insufficient for subgroup analysis after classifying the IVA stage into five subgroups. Meanwhile, therapy involving surgery and chemotherapy was further stratified. Yet chemotherapy plus surgery increased the median survival time, especially in the early subgroup of IVA stage (T1N2-3M0). Several studies have supported the multimodal treatment strategy in locally advanced EAC (32,33). Concerning M1 stage, our study did not focus on this part excessively because we could not obtain detailed information involving immunotherapy. As mentioned above, ICB in combination with chemotherapy has demonstrated tremendous survival benefits and represented the first-line therapy modality in advanced EAC (31).
This retrospective report has several limitations. First, the information on chemotherapy was not sufficiently detailed in the SEER database, which does not distinguish patients with versus without chemotherapy. Second, the clinical data were tremendously insufficient after stratification by stage and therapy. Third, immunotherapy data in SEER were not available in China, yet immunotherapy is critical for the management of locally advanced or late-stage EC. Finally, selection bias was unavoidable, given that this was a retrospective report.
Conclusions
On the basis of the SEER database, large numbers of clinical cases were extracted to conduct further analysis. We concluded that survival differences did exist among patients diagnosed with the same III/IV stage. Poorer prognosis was generally associated with higher T stage in III/IVA stage. With respect to therapy modalities involving radiation, chemotherapy, and surgery, our findings did not support offering radiation to patients with stage of III/IV. Subgroup analysis revealed that higher T stage was associated with poorer prognosis in EAC patients in the same III/IVA stage. Surgery plus chemotherapy showed significant survival benefits in the early subgroups of III/IVA stage. Whether the decisions regarding treatment modality should take the specific TNM stage into account requires further research based on real-world clinical practice.
Acknowledgments
The authors thank all SEER program staff for their dedication in facilitating this retrospective study.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://tcr.amegroups.com/article/view/10.21037/tcr-24-2099/rc
Peer Review File: Available at https://tcr.amegroups.com/article/view/10.21037/tcr-24-2099/prf
Funding: This work was supported by
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tcr.amegroups.com/article/view/10.21037/tcr-24-2099/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was performed following the Declaration of Helsinki and its subsequent amendments..
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Siegel RL, Miller KD, Wagle NS, et al. Cancer statistics, 2023. CA Cancer J Clin 2023;73:17-48. [Crossref] [PubMed]
- Rumgay H, Arnold M, Laversanne M, et al. International Trends in Esophageal Squamous Cell Carcinoma and Adenocarcinoma Incidence. Am J Gastroenterol 2021;116:1072-6. [Crossref] [PubMed]
- Inada M, Nishimura Y, Ishikawa K, et al. Comparing the 7th and 8th editions of the American Joint Committee on Cancer/Union for International Cancer Control TNM staging system for esophageal squamous cell carcinoma treated by definitive radiotherapy. Esophagus 2019;16:371-6.
- Hu K, Kang N, Liu Y, et al. Proposed revision of N categories to the 8th edition of the AJCC-TNM staging system for non-surgical esophageal squamous cell cancer. Cancer Sci 2019;110:717-25.
- Nobel TB, Dave N, Eljalby M, et al. Incidence and Risk Factors for Isolated Esophageal Cancer Recurrence to the Brain. Ann Thorac Surg 2020;109:329-36. [Crossref] [PubMed]
- Matsui K, Kawakubo H, Matsuda S, et al. Clinical Features of Recurrence Pattern with Lung Metastasis After Radical Esophagectomy for Thoracic Esophageal Cancer. World J Surg 2022;46:2270-79. [Crossref] [PubMed]
- Li H, Zhang S, Guo J, et al. Hepatic Metastasis in Newly Diagnosed Esophageal Cancer: A Population-Based Study. Front Oncol 2021;11:644860. [Crossref] [PubMed]
- Wu SG, Zhang WW, Sun JY, et al. Patterns of Distant Metastasis Between Histological Types in Esophageal Cancer. Front Oncol 2018;8:302. [Crossref] [PubMed]
- Shah MA, Kennedy EB, Catenacci DV, et al. Treatment of Locally Advanced Esophageal Carcinoma: ASCO Guideline. J Clin Oncol 2020;38:2677-94. [Crossref] [PubMed]
- Qiu G, Zhang H, Wang F, et al. Metastasis Patterns and Prognosis of Elderly Patients With Esophageal Adenocarcinoma in Stage IVB: A Population-Based Study. Front Oncol 2021;11:625720. [Crossref] [PubMed]
- Rice TW, Ishwaran H, Ferguson MK, et al. Cancer of the Esophagus and Esophagogastric Junction: An Eighth Edition Staging Primer. J Thorac Oncol 2017;12:36-42.
- Rice TW, Patil DT, Blackstone EH. 8th edition AJCC/UICC staging of cancers of the esophagus and esophagogastric junction: application to clinical practice. Ann Cardiothorac Surg 2017;6:119-30.
- Raja S, Ahmad U. Is Newer Actually Better? Where Does the 8th Edition Outperform the 7th Edition of the Esophageal TNM Staging System? Ann Surg Oncol 2021;28:596-7.
- Sung H, Ferlay J, Siegel RL, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin 2021;71:209-49. [Crossref] [PubMed]
- Morgan E, Soerjomataram I, Rumgay H, et al. The Global Landscape of Esophageal Squamous Cell Carcinoma and Esophageal Adenocarcinoma Incidence and Mortality in 2020 and Projections to 2040: New Estimates From GLOBOCAN 2020. Gastroenterology 2022;163:649-658.e2. [Crossref] [PubMed]
- McColl KEL. What is causing the rising incidence of esophageal adenocarcinoma in the West and will it also happen in the East? J Gastroenterol 2019;54:669-73. [Crossref] [PubMed]
- Emile SH, Elshobaky A, Elbanna HG, et al. Helicobacter pylori, Sleeve Gastrectomy, and Gastroesophageal Reflux Disease; Is there a Relation? Obes Surg 2020;30:3037-45. [Crossref] [PubMed]
- Sugihartono T, Fauzia KA, Miftahussurur M, et al. Analysis of gastric microbiota and Helicobacter pylori infection in gastroesophageal reflux disease. Gut Pathog 2022;14:38. [Crossref] [PubMed]
- Youssef M, Hanna C, Motomura D, et al. Endoscopic submucosal dissection (ESD) outcomes in T1B esophageal cancer: a retrospective study. Surg Endosc 2024;38:2817-25. [Crossref] [PubMed]
- Qin J, Peng Y, Chen W, et al. Comparative study of esophagectomy, endoscopic therapy, and radiotherapy for cT1N0M0 esophageal cancer in elderly patients: A SEER database analysis. Thorac Cancer 2019;10:1511-20. [Crossref] [PubMed]
- Liou DZ, Backhus LM, Lui NS, et al. Induction therapy for locally advanced distal esophageal adenocarcinoma: Is radiation Always necessary? J Thorac Cardiovasc Surg 2018;155:2697-707. [Crossref] [PubMed]
- Gharzai L, Verma V, Denniston KA, et al. Radiation Therapy and Cardiac Death in Long-Term Survivors of Esophageal Cancer: An Analysis of the Surveillance, Epidemiology, and End Result Database. PLoS One 2016;11:e0158916. [Crossref] [PubMed]
- Wang Y, Xu G, Wang J, et al. Relationship of Th17/Treg Cells and Radiation Pneumonia in Locally Advanced Esophageal Carcinoma. Anticancer Res 2017;37:4643-7. [PubMed]
- Geng L, Wu R, Hu H, et al. Clinical application of oral meglumine diatrizoate esophagogram in screening esophageal fistula during radiotherapy for esophageal cancer. Medicine (Baltimore) 2018;97:e0668. [Crossref] [PubMed]
- Tian D, Li HX, Yang YS, et al. The minimum number of examined lymph nodes for accurate nodal staging and optimal survival of stage T1-2 esophageal squamous cell carcinoma: A retrospective multicenter cohort with SEER database validation. Int J Surg 2022;104:106764. [Crossref] [PubMed]
- Wu L, Yang X, Cao W, et al. Multiple Level CT Radiomics Features Preoperatively Predict Lymph Node Metastasis in Esophageal Cancer: A Multicentre Retrospective Study. Front Oncol 2019;9:1548. [Crossref] [PubMed]
- Mamdani H, Birdas T, Jalal SI. Role of surgery following neoadjuvant chemoradiation in patients with lymph node positive locally advanced esophageal adenocarcinoma: a national cancer database analysis. J Gastrointest Oncol 2021;12:1944-50. [Crossref] [PubMed]
- Eyck BM, van Lanschot JJB, Hulshof MCCM, et al. Ten-Year Outcome of Neoadjuvant Chemoradiotherapy Plus Surgery for Esophageal Cancer: The Randomized Controlled CROSS Trial. J Clin Oncol 2021;39:1995-2004. [Crossref] [PubMed]
- Al-Batran SE, Homann N, Pauligk C, et al. Perioperative chemotherapy with fluorouracil plus leucovorin, oxaliplatin, and docetaxel versus fluorouracil or capecitabine plus cisplatin and epirubicin for locally advanced, resectable gastric or gastro-oesophageal junction adenocarcinoma (FLOT4): a randomised, phase 2/3 trial. Lancet 2019;393:1948-57. [Crossref] [PubMed]
- Saddoughi SA, Reinersman JM, Zhukov YO, et al. Survival After Surgical Resection of Stage IV Esophageal Cancer. Ann Thorac Surg 2017;103:261-6. [Crossref] [PubMed]
- Janjigian YY, Ajani JA, Moehler M, et al. First-Line Nivolumab Plus Chemotherapy for Advanced Gastric, Gastroesophageal Junction, and Esophageal Adenocarcinoma: 3-Year Follow-Up of the Phase III CheckMate 649 Trial. J Clin Oncol 2024;42:2012-20. [Crossref] [PubMed]
- Cowzer D, Wu AJ, Sihag S, et al. Durvalumab and PET-Directed Chemoradiation in Locally Advanced Esophageal Adenocarcinoma: A Phase Ib/II Study. Ann Surg 2023;278:e511-8. [Crossref] [PubMed]
- Groth SS, Burt BM, Farjah F, et al. Prognostic value of neoadjuvant treatment response in locally advanced esophageal adenocarcinoma. J Thorac Cardiovasc Surg 2019;157:1682-1693.e1. [Crossref] [PubMed]


