
Development of a prognostic nomogram and risk factor analysis for survival in H. pylori-positive non-cardia gastric adenocarcinoma patients
Highlight box
Key findings
• The developed nomograms were effective in predicting overall survival (OS) in patients with Helicobacter pylori (H. pylori)-positive non-cardia gastric adenocarcinoma (NCGAC) after radical gastrectomy. Conclusively, determining alcohol consumption, degree of differentiation, and tumor (T)-staging as predictors of 1-, 3-, and 4-year prognosis in H. pylori-positive NCGAC patients were incorporated into our established nomograms.
What is known and what is new?
• Alcohol consumption, advanced T3 + T4 stage, and poorly differentiation status were independent prognostic factors correlated with poor outcomes in patients with H. pylori-positive NCGAC.
• This study is the first to explore the demographic and clinicopathological characteristics distribution and prognostic outcomes of patients with H. pylori-positive NCGAC in the Ningxia Hui Autonomous Region, China.
What is the implication, and what should change now?
• By using our nomogram, it is possible to identify prognostic risk factors for patients with H. pylori-positive NCGAC and estimate their OS, providing new guidance for clinicians.
Introduction
Gastric cancer (GC) ranks as the fifth most commonly diagnosed type of cancer and the third leading cause of cancer-related deaths (1). Gastric adenocarcinoma (GAC), the most prevalent histological subtype of GC, arises from the epithelial cells lining the stomach and accounts for a significant portion of global cancer-related deaths (2). The epidemiology and risk factors for GAC vary depending on the tumor’s location and histological type. Notably, the majority of GAC cases are associated with chronic inflammation induced by Helicobacter pylori (H. pylori) infection (3-5). H. pylori infection and GC both exhibit notably high prevalence in East Asia, underscoring the considerable public health challenge in this region (6).
H. pylori, a Gram-negative bacterium predominantly colonizing the gastric antrum, is widely recognized as a major risk factor for GC (7-9). It is estimated that approximately 89% of newly diagnosed non-cardia GAC (NCGAC) cases are linked to H. pylori infection. In Asian populations, strains carrying the cagA gene are associated with more intense immune response and significant cytoskeletal alterations in gastric epithelial cells (10,11). The impact of H. pylori infection on the prognosis of GC patients remains controversial. While some studies suggest it may act as a protective factor, others associate it with poor clinical outcomes (12-15). Due to factors such as gastroesophageal reflux and the unique anatomical features of the gastric cardia, H. pylori more commonly affects the gastric body and antrum (16,17). However, these studies examining the prognostic impact of tumor location in GC failed to exclude the gastric cardia, leading to a potential oversight of key factors specifically influencing the prognosis of H. pylori-positive GC patients. In particular, the relationship between demographic factors, such as alcohol consumption, and outcomes in H. pylori-positive NCGAC remains unclear, beyond the influence of clinicopathological factors.
Alcohol has been classified as a Group 1 carcinogen by the International Agency for Research on Cancer (IARC) (18). Given its direct exposure to ingested substances, the stomach is especially susceptible to alcohol’s harmful effects, positioning it as a key organ in cancer development. Alcohol consumption is a modifiable risk factor for GC, as ethanol and its metabolites can penetrate and damage the gastric mucosa, potentially triggering cancer (19). Additionally, alcohol may disrupt the gastric mucosa’s pH environment, potentially serving as a protective factor for H. pylori survival in the stomach, thereby amplifying its carcinogenic potential (20,21). However, the impact of alcohol on the prognosis of H. pylori-positive NCGAC remains unclear. While the interaction between alcohol and H. pylori on cancer outcomes is hypothesize, definitive conclusions are lacking, highlighting the need for further research to clarify their role in long-term survival and treatment response in H. pylori-positive NCGAC patients.
Despite recent advances in medical research leading to powerful predictive tools, such as nomograms, there remains a scarcity of specific nomograms for H. pylori-positive NCGAC patients who have undergone surgery (22). Previous studies have developed nomograms that integrate various tumor characteristics and treatment modalities, demonstrating their value in estimating survival and guiding treatment decisions (23-27). In this study, we aimed to fill this gap by conducting a retrospective analysis of clinical data from the General Hospital of Ningxia Medical University. We developed a new nomogram based on demographics, tumor characteristics, and survival data of patients with H. pylori-positive NCGAC. Our nomogram is intended to be a valuable tool for clinicians in guiding treatment decisions and improving the prognosis of H. pylori-positive patients who have undergone radical gastrectomy. We present this article in accordance with the TRIPOD reporting checklist (available at https://tcr.amegroups.com/article/view/10.21037/tcr-24-1776/rc).
Methods
Patient data
The clinicopathological details of 413 patients who underwent R0 radical gastric surgery from January 2009 to December 2017 in the General Hospital of Ningxia Medical University were retrospectively analyzed.
To be enrolled, patients had to meet the following criteria: (I) pathological diagnosis of NCGAC; (II) pathological diagnosis of H. pylori positive; (III) undergoing R0 radical surgery; (IV) metastasis (M) 0 stage; (V) age over 18 years. Exclusion criteria were: (I) pathological diagnosis of mucinous adenocarcinoma or gastrointestinal stromal tumor; (II) past medical history of additional tumors or secondary primary tumors; (III) M1 stage. A training cohort comprising 70% of the cases and a validation cohort comprising 30% of the cases were used. The median follow-up time for the entire cohort was 90 months. Additionally, the median follow-up time for the training set was 90 months, while for the validation set, it was 87 months.
Data collection
We conducted a retrospective analysis of the demographic and clinicopathological characteristics of 413 patients treated at the General Hospital of Ningxia Medical University. The collected characteristics included age, gender, ethnicity, tumor location, tumor size, history of smoking and alcohol consumption, lymph node metastasis (confirmed by pathological diagnosis), degree of tumor differentiation, Borrmann staging, tumor node metastasis (TNM) stage, and postoperative chemotherapy. In this study, drinkers were defined as individuals who consumed alcohol at least three times per week for a period of 6 months or longer.
The study was conducted in accordance with the Declaration of Helsinki and its subsequent amendments. The study was approved by the Ethics Committee of the General Hospital of Ningxia Medical University (No. KYLL-2022-0306) and informed consent was obtained from all individual participants.
Patients who underwent radical resection of GC were regularly followed up through telephone interviews, text messages, and outpatient records until January 31, 2019. Follow-up assessments included laboratory tests (including tumor marker detection), medical history review, physical examinations, gastroscopy, and chest computed tomography (CT). The survival status of all patients was recorded by accessing the hospital’s inpatient and outpatient medical record systems, as well as follow-up phone and text message logs. Patients who did not respond after three attempts via phone and text were classified as lost to follow up.
The primary outcome of this study was overall survival (OS), calculated from the date of gastrectomy to the date of death or the date of the last follow-up visit. Patients with a survival time of 0 months were excluded from the analysis. Due to privacy considerations, patient survival data were primarily determined by reviewing hospitalization records and conducting follow-up calls. The follow-up period concluded in January 2019.
Immunohistochemistry (IHC) staining of H. pylori
Samples from H. pylori-positive NCGAC patients were utilized to confirm the presence of H. pylori using IHC. Tissue samples were first fixed in formalin, followed by dehydration in anhydrous ethanol and xylene, and then embedded in paraffin. Paraffin-embedded tissues were sectioned and deparaffinized using xylene and anhydrous ethanol.
For antigen retrieval, sections were boiled in EDTA buffer (pH 8.0) for 5 minutes. Endogenous enzyme activity was inactivated using 3% hydrogen peroxide. Subsequently, sections were incubated overnight at 4 ℃ with a primary antibody against H. pylori (ZSGB-BIO, ZA-0127, Beijing, China), followed by incubation with a secondary antibody (ZSGB-BIO, PV9000) for 30 minutes at 37 ℃. Finally, slides were visualized with 3,3-diaminobenzidine and counterstained with hematoxylin for microscopic examination. Positive staining for H. pylori was indicated by the presence of brown or light brown particles under the microscope (Figure S1).
To ensure methodological rigor, negative controls were included in each staining batch to exclude non-specific binding and confirm staining specificity. Reproducibility was maintained through standardized protocols, including consistent antibody concentrations and incubation times. The staining results were independently verified in a blinded manner by three experienced pathologists.
Statistical analysis
Statistical analyses were conducted using R (version 4.1.3). Patients were randomly assigned to two subgroups using the “caret” package, with a 70% allocation for the training set and 30% for the external validation cohort. The training set was utilized for predictive modeling, while the validation cohort served to assess model performance.
Covariates were identified using univariate Cox proportional hazards regression, including variables with P values <0.5 and a variance inflation factor (VIF) <4. Eligible variables were then incorporated into a multivariate Cox proportional hazards model, with results presented using the “Forest” package. Variables with P values <0.05 in the multivariate model were used to construct a time-dependent nomogram to predict 1-, 3-, and 4-year survival.
Model discrimination was evaluated using the area under the receiver operating characteristic (ROC) curve (AUC) and the concordance index (C-index), while calibration was assessed through calibration plots. C-index and AUC values greater than 0.7 indicate good predictive power of the nomogram. Two-sided tests were deemed statistically significant with a P value <0.05. The C-index values for the training set and validation set were calculated using the Dxy function.
Results
Patient characteristics
This study included 413 patients who underwent radical surgery for H. pylori-positive NCGAC. Among these patients, 189 (45.8%) were younger than 60 years, while 224 (54.2%) were 60 years or older. The male-to-female ratio was 2.93:1, with 308 (74.6%) male patients and 105 (25.4%) female patients (Table 1).
Table 1
| Characteristics | Overall | Training cohort (n=289) | Validation cohort (n=124) |
|---|---|---|---|
| Age (years) | |||
| <60 | 224 (54.2) | 152 (52.6) | 72 (58.1) |
| ≥60 | 189 (45.8) | 137 (47.4) | 52 (41.9) |
| Gender | |||
| Male | 308 (74.6) | 209 (72.3) | 99 (79.8) |
| Female | 105 (25.4) | 80 (27.7) | 25 (20.2) |
| Ethnicity | |||
| Han | 340 (82.7) | 237 (82.0) | 103 (84.4) |
| Hui | 71 (17.3) | 52 (18.0) | 19 (15.6) |
| Cigarette smoking | |||
| No | 239 (57.9) | 177 (61.2) | 62 (50.0) |
| Yes | 174 (42.1) | 112 (38.8) | 62 (50.0) |
| Alcohol drinking | |||
| No | 322 (78.0) | 229 (79.2) | 93 (75.0) |
| Yes | 91 (22.0) | 60 (20.8) | 31 (25.0) |
| Blood type | |||
| A + AB | 150 (36.8) | 107 (37.4) | 43 (35.2) |
| B + O | 258 (63.2) | 179 (62.6) | 79 (64.8) |
| Tumor size (cm) | |||
| <5 | 207 (50.7) | 140 (49.3) | 67 (54.0) |
| ≥5 | 201 (49.3) | 144 (50.7) | 57 (46.0) |
| Tumor location | |||
| Upper | 135 (32.7) | 89 (30.8) | 46 (37.1) |
| Middle + low | 278 (67.3) | 200 (69.2) | 78 (62.9) |
| Lymph node metastasis | |||
| No | 222 (55.4) | 148 (53.2) | 74 (60.2) |
| Yes | 179 (44.6) | 130 (46.8) | 49 (39.8) |
| Differentiation degree | |||
| Low | 245 (59.9) | 171 (59.6) | 74 (60.7) |
| Middle + high | 164 (40.1) | 116 (40.4) | 48 (39.3) |
| TNM stage | |||
| I + II | 250 (61.0) | 171 (59.6) | 79 (64.2) |
| III + IV | 160 (39.0) | 116 (40.4) | 44 (35.8) |
| Borrmann classification | |||
| I + II | 314 (77.1) | 219 (77.1) | 95 (77.2) |
| III + IV | 93 (22.9) | 65 (22.9) | 28 (22.8) |
| pT stage | |||
| T1 + T2 | 182 (44.2) | 123 (42.6) | 59 (48.0) |
| T3 + T4 | 230 (55.8) | 166 (57.4) | 64 (52.0) |
| pN stage | |||
| N0 | 222 (54.0) | 150 (52.3) | 72 (58.1) |
| N1 + N2 + N3 | 189 (46.0) | 137 (47.7) | 52 (41.9) |
| Postoperative chemotherapy | |||
| No | 53 (67.1) | 34 (65.4) | 19 (70.4) |
| Yes | 26 (32.9) | 18 (34.6) | 8 (29.6) |
Data are presented as n (%). H. pylori, Helicobacter pylori; NCGAC, non-cardia gastric adenocarcinoma; pN, pathological node; pT, pathological tumor; TNM, tumor, node, metastasis.
The subjects were randomly assigned to two cohorts: a training cohort (n=289) and a validation cohort (n=124). The OS rates at 1, 3, and 4 years were consistent between the training and validation cohorts, with rates of 86.20%, 67.00%, and 65.90%, respectively.
Prognostic nomogram construction
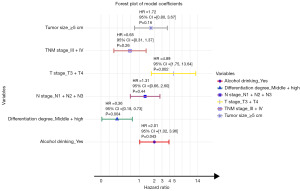
To determine whether baseline characteristics were significantly associated with survival in H. pylori-positive NCGAC patients who underwent gastrectomy, we performed univariate and multivariate analyses. The variables of lymph node metastasis, clinical staging, tumor differentiation grade, tumor size, T stage, and N stage had P values <0.05. Variables with P values <0.5 (including alcohol consumption) were assessed for multicollinearity using the VIF (Figure S2). Variables with VIF <4 (clinical staging, tumor differentiation grade, tumor size, T stage, N stage, and alcohol consumption) were included in the multivariate Cox proportional hazards model. The multivariate Cox model revealed that alcohol consumption [hazard ratio (HR) =2.01, P=0.043], T3 + T4 stage (HR =4.89, P=0.002), and moderate or well-differentiated tumors (HR =0.36, P=0.004) may be independent prognostic factors for H. pylori-positive NCGAC patients (Table 2). A forest plot illustrating the results of the Cox proportional hazards model analysis is presented in Figure 1. This plot provides a clear visualization of the impact of each variable on the HR.
Table 2
| Characteristics | Univariate analysis | Multivariate analysis | |||||
|---|---|---|---|---|---|---|---|
| HR | 95% CI | P | HR | 95% CI | P | ||
| Age | |||||||
| <60 years | Ref | – | – | – | |||
| ≥60 years | 1.133 | 0.646–1.989 | 0.66 | – | – | – | |
| Gender | |||||||
| Male | Ref | – | – | – | |||
| Female | 1.416 | 0.782–2.566 | 0.25 | – | – | – | |
| Ethnicity | |||||||
| Han | Ref | – | – | – | |||
| Hui | 1.002 | 0.500–2.008 | >0.99 | – | – | – | |
| Cigarette smoking | |||||||
| No | Ref | – | – | – | |||
| Yes | 1.104 | 0.627–1.945 | 0.73 | – | – | – | |
| Alcohol drinking | |||||||
| No | Ref | Ref | |||||
| Yes | 1.448 | 0.754–2.780 | 0.27 | 2.013 | 1.023–3.959 | 0.043 | |
| Blood type | |||||||
| A + AB | Ref | – | – | – | |||
| B + O | 1.377 | 0.766–2.473 | 0.29 | – | – | – | |
| Tumor size | |||||||
| <5 cm | Ref | Ref | |||||
| ≥5 cm | 3.733 | 1.907–7.310 | <0.001 | 2.268 | 1.137–4.524 | 0.02 | |
| Tumor location | |||||||
| Upper | Ref | – | – | – | |||
| Middle + low | 1.296 | 0.687–2.433 | 0.43 | – | – | – | |
| Lymph node metastasis | |||||||
| No | Ref | – | – | – | |||
| Yes | 2.844 | 1.539–5.256 | <0.001 | – | – | – | |
| Differentiation degree | |||||||
| Low | Ref | Ref | |||||
| Middle + high | 0.321 | 0.164–0.628 | <0.001 | 0.359 | 0.177–0.726 | 0.004 | |
| TNM stage | |||||||
| I + II | Ref | Ref | |||||
| III + IV | 2.681 | 1.498–4.8 | <0.001 | 0.651 | 0.308–1.375 | 0.26 | |
| Borrmann classification | |||||||
| I + II | Ref | – | – | – | |||
| III + IV | 1.09 | 0.585–2.032 | 0.79 | – | – | – | |
| pT stage | |||||||
| T1 + T2 | Ref | Ref | |||||
| T3 + T4 | 5.69 | 2.553–12.68 | <0.001 | 4.889 | 1.753–13.637 | 0.002 | |
| pN stage | |||||||
| N0 | Ref | Ref | |||||
| Nx | 2.438 | 1.339–4.441 | 0.004 | 1.313 | 0.662–2.604 | 0.44 | |
| Postoperative chemotherapy | |||||||
| No | Ref | – | – | – | |||
| Yes | 1.271 | 0.2124–7.610 | 0.79 | – | – | – | |
CI, confidence interval; HR, hazard ratio; pN, pathological node; pT, pathological tumor; Ref, reference; TNM, tumor, node, metastasis.
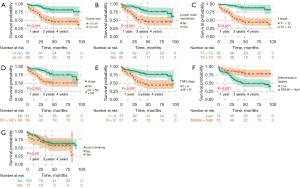
Survival curves for potential individual prognostic factors identified in the multivariate Cox model were generated using the Kaplan-Meier method in R software (Figure 2). A prognostic nomogram was constructed by integrating all independent predictors of OS based on the results of the multivariate analysis (Figure 3).
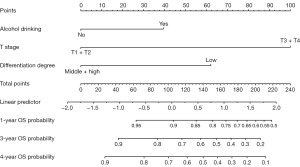
For each patient, the first line of the nomogram displays the points assigned to each variable based on its corresponding value (lines 2–4). By summing these points (line 5), we can determine the probabilities of survival at 1 year, 3 years, and 4 years, as indicated on the survival axes (lines 7–9).
Nomogram calibration and validation
We validated the nomogram and found that the C-index was 0.727 for the training cohort and 0.728 for the test cohort.
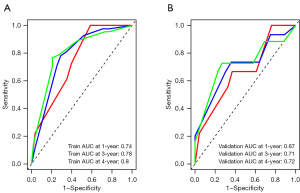
The AUC values for the training set were 0.74, 0.78, and 0.80 for 1-, 3-, and 4-year survival, respectively. In the validation set, the AUC values were 0.67, 0.71, and 0.72 for 1-, 3-, and 4-year survival (Figure 4A,4B).
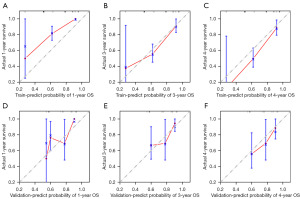
Calibration plots confirmed the predictive effectiveness of the nomogram, demonstrating high agreement between predicted survival and actual survival outcomes (Figure 5A-5F).
Discussion
GC is a serious and potentially fatal disease that affects the survival of millions of people worldwide. H. pylori infection has been established as the primary risk factor for GC, particularly for NCGAC (17,28). In this study, we developed a novel nomogram to predict the survival of H. pylori-positive NCGAC patients who underwent radical gastrectomy and are at higher risk for poor tumor prognosis.
Here, totally 413 patients were randomized into a training group (70%) and a validation group (30%). Our findings showed that alcohol consumption, advanced T3 + T4 stage, and poorly differentiation status were independent prognostic factors correlated with poor outcomes in patients with H. pylori-positive NCGAC, as determined by multivariate Cox proportional hazards models. To identify patients at higher risk of tumor prognosis, we developed a nomogram that considers these three critical factors and predicts survival probabilities for 1 year, 3 years, and 4 years. The C-index for the training cohort was 0.727, and for the validation cohort, it was 0.728. Additionally, the calibration curve and AUC curve for survival probability demonstrated excellent consistency between nomogram predictions and the actual observations. The nomogram we developed could be a valuable tool for clinicians to predict disease prognosis in H. pylori-positive NCGAC patients and help adjust treatment accordingly.
According to previous literature, several nomogram models have been developed for patients with different stages of GC (29-31). Jiang et al. constructed a nomogram for personalized prediction of disease-free survival and OS in GC patients by integrating deep learning models and clinical pathological risk factors (29). Liu et al. predicted the tumor-specific survival in patients with early-onset GC by constructing a nomogram (30). Li et al. predicted the OS feature of GC patients by constructing a risk feature and nomogram model that was based on the number of lymph nodes (31). However, few studies have specifically addressed the prognostic factors and survival outcomes for H. pylori-positive NCGAC. Given that H. pylori primarily colonizes the pylorus and mainly affects the fundus, body, and pyloric region of the stomach, whereas tumors in different locations, such as the cardia versus non-cardia regions, exhibit distinct prognostic implications, the unique anatomical structure of the cardia makes H. pylori-positive NCGAC an independent subgroup for research.
Some studies currently suggested that drinking alcohol increased the incidence of GC (32-34). However, few studies have explored the impact of alcohol consumption on survival, and only one study investigated the correlation between the survival of GC and alcohol consumption (35,36). Likewise, our research found that drinking alcohol also affected the prognosis of H. pylori-positive NCGAC patients. Alcohol can damage the gastric mucosa directly and indirectly, leading to increased acid secretion, hormonal imbalances, depletion of vitamin reserves, and even alterations in the gastric pH environment, thereby promoting carcinogenesis and accelerating cancer progression (37-40). In studies that analyzed GAC outcomes based on anatomical subtypes, high alcohol drinking was also strongly associated with an increased risk of developing NCGAC (41,42). Furthermore, despite modest alcohol consumption, the individuals with ALDH2 polymorphisms are at greater risk of developing alcohol-mediated GC (43). Therefore, the intake of alcohol and the invasion of H. pylori can exacerbate the damage to the gastric mucosa, promoting the progression of NCGAC, which in turn negatively impacted the prognosis of patients.
Tumor differentiation was identified as a significant factor in evaluating the prognosis of patients with H. pylori-positive NCGAC in this study. Feng et al. observed that the degree of differentiation in GAC was closely linked to prognosis, with tumors that had poorer differentiation typically associated with worse prognosis and shorter survival times (44). Hu et al. noted that different status of tumor differentiation can lead to significant heterogeneity in the prognosis of GC (23). To address this issue, they established corresponding nomograms for GCs of different differentiation states (well differentiated, moderately differentiated and poorly differentiated). Similarly, Zhou et al. also established nomograms for poorly-differentiated GAC (45). Unlike these studies, our study focused on H. pylori-positive NCGAC patients, and we utilized the nomogram model to provide individualized prognostic results and develop personalized treatment plans for patients.
In addition to alcohol consumption and tumor differentiation, T stage played a significant prognostic role in patients with H. pylori-positive NCGAC in this study. The nomogram model also identified T stage as an independent prognostic factor for GC patients with varying status of tumor differentiation (22). T stage also used to guide treatment decisions for GC patients with gastric outlet obstruction after gastrectomy and those with peritoneal metastases (46,47).
Certainly, there are some weaknesses in this study. Firstly, the establishment and validation of the nomogram involved both the training and test cohorts from a single institution. Further multi-center and prospective studies are needed to validate the feasibility of this nomogram model. Secondly, this is a retrospective study, and detailed information on alcohol consumption, such as the specific types of alcohol regularly consumed and the timing of consumption, was not systematically collected. As a result, the study was unable to explore which particular type of alcohol most significantly affects the prognosis of H. pylori-positive NCGAC patients. Finally, only data on H. pylori infection status was collected, and information regarding whether patients underwent eradication therapy was not available, which means the study did not account for the potential impact of H. pylori eradication on patient prognosis. Despite these limitations, the nomogram model predicts patient survival well.
Conclusions
The nomogram developed in this study demonstrated potential as an effective tool for predicting OS in H. pylori-positive NCGAC patients following radical gastrectomy. Alcohol consumption, tumor differentiation, and T stage were identified as independent prognostic factors for NCGAC patients and were found to be significant predictors of 1-, 3-, and 4-year survival in the nomogram model. However, further external validation through multicenter studies and prospective research (focused on treatment strategies for H. pylori-positive NCGAC patients) is necessary to confirm the model’s robustness and its broader applicability in clinical practice.
Acknowledgments
None.
Footnote
Reporting Checklist: The authors have completed the TRIPOD reporting checklist. Available at https://tcr.amegroups.com/article/view/10.21037/tcr-24-1776/rc
Data Sharing Statement: Available at https://tcr.amegroups.com/article/view/10.21037/tcr-24-1776/dss
Peer Review File: Available at https://tcr.amegroups.com/article/view/10.21037/tcr-24-1776/prf
Funding: This research of this project was sponsored by
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tcr.amegroups.com/article/view/10.21037/tcr-24-1776/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki and its subsequent amendments. The study was approved by the Ethics Committee of the General Hospital of Ningxia Medical University (No. KYLL-2022-0306) and informed consent was obtained from all individual participants.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- André T, Tougeron D, Piessen G, et al. Neoadjuvant Nivolumab Plus Ipilimumab and Adjuvant Nivolumab in Localized Deficient Mismatch Repair/Microsatellite Instability-High Gastric or Esophagogastric Junction Adenocarcinoma: The GERCOR NEONIPIGA Phase II Study. J Clin Oncol 2023;41:255-65. [Crossref] [PubMed]
- Thrift AP, Wenker TN, El-Serag HB. Global burden of gastric cancer: epidemiological trends, risk factors, screening and prevention. Nat Rev Clin Oncol 2023;20:338-49. [Crossref] [PubMed]
- Piscione M, Mazzone M, Di Marcantonio MC, et al. Eradication of Helicobacter pylori and Gastric Cancer: A Controversial Relationship. Front Microbiol 2021;12:630852. [Crossref] [PubMed]
- Kumar S, Metz DC, Ellenberg S, et al. Risk Factors and Incidence of Gastric Cancer After Detection of Helicobacter pylori Infection: A Large Cohort Study. Gastroenterology 2020;158:527-536.e7. [Crossref] [PubMed]
- Zavros Y, Merchant JL. The immune microenvironment in gastric adenocarcinoma. Nat Rev Gastroenterol Hepatol 2022;19:451-67. [Crossref] [PubMed]
- Hooi JKY, Lai WY, Ng WK, et al. Global Prevalence of Helicobacter pylori Infection: Systematic Review and Meta-Analysis. Gastroenterology 2017;153:420-9. [Crossref] [PubMed]
- Pan G, Wang X, Wang Y, et al. Helicobacter pylori promotes gastric cancer progression by upregulating semaphorin 5A expression via ERK/MMP9 signaling. Mol Ther Oncolytics 2021;22:256-64. [Crossref] [PubMed]
- Zhu L, Huang Y, Li H, et al. Helicobacter pylori promotes gastric cancer progression through the tumor microenvironment. Appl Microbiol Biotechnol 2022;106:4375-85. [Crossref] [PubMed]
- Zhao Z, Zhang R, Chen G, et al. Anti-Helicobacter pylori Treatment in Patients With Gastric Cancer After Radical Gastrectomy. JAMA Netw Open 2024;7:e243812. [Crossref] [PubMed]
- Yao P, Kartsonaki C, Butt J, et al. Helicobacter pylori multiplex serology and risk of non-cardia and cardia gastric cancer: a case-cohort study and meta-analysis. Int J Epidemiol 2023;52:1197-208. [Crossref] [PubMed]
- Atherton JC, Blaser MJ. Coadaptation of Helicobacter pylori and humans: ancient history, modern implications. J Clin Invest 2009;119:2475-87. [Crossref] [PubMed]
- Gan C, Wu X, Shen Y, et al. Helicobacter pylori Infection as a Predictor of Treatment Outcomes of Gastric Cancer: A Systematic Review and Meta-Analysis. Dig Dis 2023;41:553-64. [Crossref] [PubMed]
- Li G, Wang Z, Wang Z, et al. Gastric cancer patients with Helicobacter pylori infection have a poor prognosis. J Surg Oncol 2013;108:421-6. [Crossref] [PubMed]
- Morgan R, Cassidy M, DeGeus SWL, et al. Presentation and Survival of Gastric Cancer Patients at an Urban Academic Safety-Net Hospital. J Gastrointest Surg 2019;23:239-46. [Crossref] [PubMed]
- Zhao W, Zhu H, Zhang S, et al. Trop2 is overexpressed in gastric cancer and predicts poor prognosis. Oncotarget 2016;7:6136-45. [Crossref] [PubMed]
- de Martel C, Georges D, Bray F, et al. Global burden of cancer attributable to infections in 2018: a worldwide incidence analysis. Lancet Glob Health 2020;8:e180-90. [Crossref] [PubMed]
- Yang L, Kartsonaki C, Yao P, et al. The relative and attributable risks of cardia and non-cardia gastric cancer associated with Helicobacter pylori infection in China: a case-cohort study. Lancet Public Health 2021;6:e888-96. [Crossref] [PubMed]
- Ferlay J, Soerjomataram I, Ervik M, et al. GLOBOCAN 2012 v1.0, Cancer Incidence and Mortality Worldwide: IARC CancerBase No. 11. Lyon: International Agency for Research on Cancer; 2013. Accessed on 4 May 2015. Available online: http://globocan.iarc.fr
- Siegmund SV, Singer MV. Effects of alcohol on the upper gastrointestinal tract and the pancreas--an up-to-date overview. Z Gastroenterol 2005;43:723-36. [Crossref] [PubMed]
- Brenner H, Rothenbacher D, Bode G, et al. Inverse graded relation between alcohol consumption and active infection with Helicobacter pylori. Am J Epidemiol 1999;149:571-6. [Crossref] [PubMed]
- Hansson LE, Engstrand L, Nyrén O, et al. Helicobacter pylori infection: independent risk indicator of gastric adenocarcinoma. Gastroenterology 1993;105:1098-103. [Crossref] [PubMed]
- Balachandran VP, Gonen M, Smith JJ, et al. Nomograms in oncology: more than meets the eye. Lancet Oncol 2015;16:e173-80. [Crossref] [PubMed]
- Hu L, Yang K, Chen Y, et al. Survival nomogram for different grades of gastric cancer patients based on SEER database and external validation cohort. Front Oncol 2022;12:951444. [Crossref] [PubMed]
- Cui Y, Zhang J, Li Z, et al. A CT-based deep learning radiomics nomogram for predicting the response to neoadjuvant chemotherapy in patients with locally advanced gastric cancer: A multicenter cohort study. EClinicalMedicine 2022;46:101348. [Crossref] [PubMed]
- Ma L, Chen G, Wang D, et al. A nomogram to predict survival probability of gastric cancer patients undergoing radical surgery and adjuvant chemotherapy. Front Oncol 2022;12:893998. [Crossref] [PubMed]
- Talebi A, Borumandnia N, Doosti H, et al. Development of web-based dynamic nomogram to predict survival in patients with gastric cancer: a population-based study. Sci Rep 2022;12:4580. [Crossref] [PubMed]
- Chen Y, Jia Y, Peng Z, et al. The prognostic role of tumor size in stage T1 gastric cancer. World J Surg Oncol 2022;20:135. [Crossref] [PubMed]
- Chen Z, Zheng Y, Fan P, et al. Risk factors in the development of gastric adenocarcinoma in the general population: A cross-sectional study of the Wuwei Cohort. Front Microbiol 2022;13:1024155. [Crossref] [PubMed]
- Jiang Y, Zhang Z, Yuan Q, et al. Predicting peritoneal recurrence and disease-free survival from CT images in gastric cancer with multitask deep learning: a retrospective study. Lancet Digit Health 2022;4:e340-50. [Crossref] [PubMed]
- Liu H, Li Z, Zhang Q, et al. Multi institutional development and validation of a nomogram to predict prognosis of early-onset gastric cancer patients. Front Immunol 2022;13:1007176. [Crossref] [PubMed]
- Li H, Lin D, Yu Z, et al. A nomogram model based on the number of examined lymph nodes-related signature to predict prognosis and guide clinical therapy in gastric cancer. Front Immunol 2022;13:947802. [Crossref] [PubMed]
- Deng W, Jin L, Zhuo H, et al. Alcohol consumption and risk of stomach cancer: A meta-analysis. Chem Biol Interact 2021;336:109365. [Crossref] [PubMed]
- Li Y, Eshak ES, Shirai K, et al. Alcohol Consumption and Risk of Gastric Cancer: The Japan Collaborative Cohort Study. J Epidemiol 2021;31:30-6. [Crossref] [PubMed]
- Yoo JE, Shin DW, Han K, et al. Association of the Frequency and Quantity of Alcohol Consumption With Gastrointestinal Cancer. JAMA Netw Open 2021;4:e2120382. [Crossref] [PubMed]
- Kim SA, Choi BY, Song KS, et al. Prediagnostic Smoking and Alcohol Drinking and Gastric Cancer Survival: A Korean Prospective Cohort Study. Korean J Gastroenterol 2019;73:141-51. [Crossref] [PubMed]
- Minami Y, Kanemura S, Oikawa T, et al. Associations of cigarette smoking and alcohol drinking with stomach cancer survival: A prospective patient cohort study in Japan. Int J Cancer 2018;143:1072-85. [Crossref] [PubMed]
- Chari S, Teyssen S, Singer MV. Alcohol and gastric acid secretion in humans. Gut 1993;34:843-7. [Crossref] [PubMed]
- Knoll MR, Kölbel CB, Teyssen S, et al. Action of pure ethanol and some alcoholic beverages on the gastric mucosa in healthy humans: a descriptive endoscopic study. Endoscopy 1998;30:293-301. [Crossref] [PubMed]
- Blot WJ. Alcohol and cancer. Cancer Res 1992;52:2119s-23s. [PubMed]
- Aberle NS 2nd, Burd L, Zhao BH, et al. Acetaldehyde-induced cardiac contractile dysfunction may be alleviated by vitamin B1 but not by vitamins B6 or B12. Alcohol Alcohol 2004;39:450-4. [Crossref] [PubMed]
- Wang PL, Xiao FT, Gong BC, et al. Alcohol drinking and gastric cancer risk: a meta-analysis of observational studies. Oncotarget 2017;8:99013-23. [Crossref] [PubMed]
- Sung H, Ferlay J, Siegel RL, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin 2021;71:209-49. [Crossref] [PubMed]
- Matsuo K, Oze I, Hosono S, et al. The aldehyde dehydrogenase 2 (ALDH2) Glu504Lys polymorphism interacts with alcohol drinking in the risk of stomach cancer. Carcinogenesis 2013;34:1510-5. [Crossref] [PubMed]
- Feng F, Liu J, Wang F, et al. Prognostic value of differentiation status in gastric cancer. BMC Cancer 2018;18:865. [Crossref] [PubMed]
- Zhou X, Dong Z, Zhang C, et al. A novel nomogram for predicting survival of patients with poorly differentiated gastric adenocarcinoma. Transl Cancer Res 2021;10:886-98. [Crossref] [PubMed]
- Wei C, Li C, Chen X, et al. Development and verification of a nomogram for predicting the prognosis of resectable gastric cancer with outlet obstruction. BMC Cancer 2022;22:1154. [Crossref] [PubMed]
- Yang J, Su H, Chen T, et al. Development and validation of nomogram of peritoneal metastasis in gastric cancer based on simplified clinicopathological features and serum tumor markers. BMC Cancer 2023;23:64. [Crossref] [PubMed]


