
Development and validation of nomograms for predicting survival of locally advanced rectosigmoid junction cancer patients: a SEER database analysis
Highlight box
Key findings
• In this study, we found the risk factors for the overall survival (OS) and cancer-specific survival (CSS) in locally advanced rectosigmoid junction cancer (LARSJC) patients and developed prediction nomograms for OS and CSS.
What is known and what is new?
• Rectosigmoid junction is unique to colon and rectum. However, there is limited research on the prognosis of LARSJC. The optimal treatment approach for LARSJC patients remains uncertain.
• This study represented a comprehensive prognostic analysis of in LARSJC patients and developed prediction nomograms for OS and CSS, respectively.
What is the implication, and what should change now?
• Using our nomograms, it is possible to predict LARSJC patients’ OS and CSS, thereby optimizing individualized treatment options.
Introduction
Colorectal cancer (CRC) ranks as the second leading cause of cancer-related mortality and is the third most prevalent cancer globally, with over 1.8 million new cases and 881,899 deaths reported in 2022 (1). Advances in imaging technology have markedly increased the detection of locally advanced CRC (LACRC) over recent decades (2). Nevertheless, LACRC remains a significant therapeutic challenge in the management of CRC patients (3). Currently, the standard management for LACRC involves a combination of surgical resection, chemotherapy, and/or radiation therapy (4). For locally advanced colon cancer (LACC), the established protocol includes surgical resection followed by adjuvant chemotherapy (5). In contrast, the treatment for locally advanced rectal cancer (LARC) typically begins with chemoradiotherapy, followed by surgical intervention (6). The rectosigmoid junction, linking the sigmoid colon to the upper rectum, has largely been classified with either the rectum or colon in a relevant study, with limited separate evaluation (7). A recent research indicated a progressive increase in the prevalence of the Cytosine-phosphate-Guanine (CpG) island methylator phenotype (CIMP), microsatellite instability-high (MSI-H), and B-Raf proto-oncogene (BRAF) mutations from the rectum to the ascending colon across colorectal subsites (8). Moreover, a prior research has highlighted unique molecular characteristics of the sigmoid-rectal region in comparison to other colonic sites (9). A previous study highlighted that locally advanced rectosigmoid junction cancer (LARSJC) exhibited a higher propensity for lymphatic metastasis compared to sigmoid and rectal cancers, and it is associated with a greater likelihood of distant metastasis (10). One study has identified key prognostic factors for rectosigmoid junction cancer (RSJC), including tumor stage, tumor grade, tumor size, age and histology (11). Additionally, studies suggested that radiotherapy enhanced survival for patients with LARSJC, a group that exhibited different survival outcomes compared to those with LACC and LARC (7,12). The ideal treatment strategy for patients with LARSJC remains debated. Due to the ambiguous anatomical positioning of the rectosigmoid junction, relevant clinical data are scarce, complicating treatment decisions and prognostic predictions. Consequently, developing a robust prediction model for LARSJC is essential to enhance prognostic accuracy for these patients.
As an essential aspect of artificial intelligence, prediction nomograms have shown considerable effectiveness in diagnosing and forecasting various diseases, often outperforming traditional methods in clinical practice (13,14). The rapid development of algorithms has facilitated the use of nomograms to predict survival outcomes in patients with solid tumors by analyzing large and complex clinical datasets (15-17). To date, no nomogram has been established to predict the prognosis of patients with LARSJC. This study sought to create innovative nomograms to forecast overall survival (OS) and cancer-specific survival (CSS) for LARSJC patients using clinicopathological data from the Surveillance, Epidemiology, and End Results (SEER) database. These models aimed to assist clinicians in promptly selecting appropriate treatment strategies to improve patient outcomes. Furthermore, a risk stratification framework was developed to classify newly diagnosed LARSJC patients into distinct groups, facilitating prognosis predictions and aiding in the selection of optimal treatment plans. We present this article in accordance with the TRIPOD reporting checklist (available at https://tcr.amegroups.com/article/view/10.21037/tcr-24-1810/rc).
Methods
Study population and inclusion and exclusion criteria
This retrospective cohort study analyzed clinicopathological data for patients with LARSJC from the SEER database, covering the period from January 1, 2010, to December 31, 2017, utilizing SEER*Stat 8.4.0.1 software. LARSJC was characterized as carcinoma of the rectosigmoid junction in patients with stage II (T3-4N0M0) and stage III (T1-4N1-2M0). Collected data included patient age, race, sex, tumor grade, histologic grade, the American Joint Committee on Cancer (AJCC) 7th edition stages (T, N, and M), tumor size, number of lymph nodes harvested, treatment sequence (surgery relative to chemoradiotherapy), preoperative carcinoembryonic antigen (CEA) levels, perineural invasion, tumor deposits, and details regarding radiotherapy and chemotherapy. Inclusion criteria mandated: (I) patients with stage II and III RSJC; (II) those with LARSJC as the sole primary malignancy; and (III) surgical intervention followed by a thorough pathological evaluation. Exclusion criteria involved: (I) patients with multiple primary cancers; (II) those with reports limited to autopsy findings or death certificates; (III) incomplete data on inclusion criteria; (IV) individuals under 18 years; and (V) patients with a survival duration of zero months. Ultimately, 3,571 patients met these criteria, randomly assigned in a 7:3 ratio to a training set of 2,499 and a test set of 1,072 (Table 1). The test set facilitated internal validation of the derived nomogram. The primary endpoints were OS and CSS, with median follow-up reflecting median observed survival time. OS was measured from diagnosis to death from any cause or last follow-up, while CSS tracked from diagnosis to cancer-related death or last follow-up. Prognostic information in the database included survival time and causes of death. The causes of death were divided into (I) dead attributed to this cancer and (II) alive or dead of other causes. So we can get the OS and CSS easily.
Table 1
| Characteristics | All patients (N=3,571) | Training set (N=2,499) | Test set (N=1,072) | P value |
|---|---|---|---|---|
| Sex | 0.14 | |||
| Female | 1,563 (43.8%) | 1,073 (42.9%) | 490 (45.7%) | |
| Male | 2,008 (56.2%) | 1,426 (57.1%) | 582 (54.3%) | |
| Age (years) | 0.36 | |||
| <60 | 1,682 (47.1%) | 1,164 (46.6%) | 518 (48.3%) | |
| ≥60 | 1,889 (52.9%) | 1,335 (53.4%) | 554 (51.7%) | |
| Grade | >0.99 | |||
| Well and moderate | 3,002 (84.6%) | 2,115 (84.6%) | 907 (84.6%) | |
| Poor and undifferentiated | 549 (15.4%) | 384 (15.4%) | 165 (15.4%) | |
| Histology | 0.44 | |||
| Adenocarcinoma | 3,368 (94.3%) | 2,361 (95.2%) | 1,007 (93.9%) | |
| Others | 203 (5.7%) | 138 (4.8%) | 65 (6.1%) | |
| T stage | 0.75 | |||
| T1 | 75 (2.1%) | 50 (2.00%) | 25 (2.33%) | |
| T2 | 268 (7.5%) | 182 (7.28%) | 86 (8.02%) | |
| T3 | 2,664 (74.6%) | 1,875 (75.0%) | 789 (73.6%) | |
| T4 | 564 (15.8%) | 392 (15.7%) | 172 (16.0%) | |
| N stage | 0.20 | |||
| N0 | 1,399 (39.2%) | 1,001 (40.1%) | 398 (37.1%) | |
| N1 | 1,400 (39.2%) | 973 (38.9%) | 427 (39.8%) | |
| N2 | 772 (21.4%) | 525 (21.0%) | 247 (23.0%) | |
| Lymph node dissection | 0.87 | |||
| No | 63 (1.8%) | 43 (1.72%) | 20 (1.87%) | |
| Yes | 3,508 (98.2%) | 2,456 (98.3%) | 1,052 (98.1%) | |
| Radiation | 0.84 | |||
| No | 2,515 (70.4%) | 1,763 (70.5%) | 752 (70.1%) | |
| Yes | 1,056 (29.6%) | 736 (29.5%) | 320 (29.9%) | |
| Chemotherapy | >0.99 | |||
| No | 1,282 (35.9%) | 897 (35.9%) | 385 (35.9%) | |
| Yes | 2,289 (64.1%) | 1,602 (64.1%) | 687 (64.1%) | |
| Treatment sequence | 0.77 | |||
| No | 1,281 (35.8%) | 897 (35.9%) | 384 (35.8%) | |
| Adjuvant | 1,773 (49.6%) | 1,247 (49.9%) | 526 (49.1%) | |
| Neoadjuvant | 517 (14.6%) | 355 (14.2%) | 162 (15.1%) | |
| Carcinoembryonic antigen level | 0.25 | |||
| Negative | 2,072 (58.0%) | 1,466 (58.7%) | 606 (56.5%) | |
| Positive | 1,499 (42.0%) | 1,033 (41.3%) | 466 (43.5%) | |
| Perineural invasion | 0.31 | |||
| No | 2,985 (83.6%) | 2,078 (83.2%) | 907 (84.6%) | |
| Yes | 586 (16.4%) | 421 (16.8%) | 165 (15.4%) | |
| Tumor deposits | 0.07 | |||
| No | 2,956 (82.8%) | 2,088 (83.6%) | 868 (81.0%) | |
| Yes | 615 (17.2%) | 411 (16.4%) | 204 (19.0%) | |
| Tumor size (mm) | 0.41 | |||
| <56 | 2,475 (69.3%) | 1,743 (69.7%) | 732 (68.3%) | |
| ≥56 | 1,096 (30.7%) | 756 (30.3%) | 340 (31.7%) | |
| Race | 0.74 | |||
| White | 2,799 (78.4%) | 1,957 (78.3%) | 842 (78.5%) | |
| Others | 772 (21.6%) | 542 (21.7%) | 230 (21.5%) |
As this study utilized publicly accessible, de-identified data from the SEER database, ethics committee approval and patient consent were not necessary. The study was conducted in accordance with the Declaration of Helsinki and its subsequent amendments.
Statistical analysis
The Mann-Whitney U test was utilized to compare continuous variables, while categorical data were analyzed using the Chi-squared test. Univariate and multivariate Cox regression analyses were conducted to evaluate odds ratios (OR) and 95% confidence intervals (CI). Prognostic factors with P values below 0.05 in the multivariate model were included in a nomogram to visually predict 1-, 3-, and 5-year survival rates. Hazard ratios (HR) and their corresponding 95% CIs were reported. The optimal tumor size cutoff was determined with X-tile software (version 3.6.1). Model performance was assessed using the concordance index (C-index) and receiver operating characteristic (ROC) curves, alongside area under the curve (AUC) calculations. Calibration plots were employed to compare predicted versus actual survival times at specified intervals. Decision curve analysis (DCA) evaluated the model’s clinical applicability. The development cohort was segmented into risk groups based on total points, and survival differences were analyzed using the Kaplan-Meier method with log-rank tests. Statistical analyses were performed with SPSS 22.0 and R version 4.2.0, and propensity score matching (PSM) was applied to evaluate clinical intervention effects on outcomes. Statistical significance was set at P<0.05. The performance of the prognostic models was evaluated using the AUC from the ROC analysis. An AUC ≥0.7 was considered indicative of a good prognosis prediction model.
Results
Characteristics of LARSJC patients
In summary, this study included 3,571 patients diagnosed with LARSJC, categorized into a training cohort of 2,499 and a test cohort of 1,072. The median age of participants was 62 years, with a median survival time of 52 months. All patients underwent surgery, with 98.2% receiving sufficient number of nodes examined. Positive CEA levels were noted in approximately 42% of cases, and perineural invasion was present in 586 patients (16.4%). Chemotherapy was administered to 2,289 patients (64.1%), while 1,056 patients (29.6%) received radiotherapy, and 517 patients (14.6%) underwent neoadjuvant therapy. Basic characteristics and variance analyses were detailed in Table 1. No significant differences in feature distributions were found between the training and test sets using Mann-Whitney U and Chi-squared (χ2) tests.
Variable feature importance of survival prediction
To identify prognostic factors for OS, we conducted univariate and multivariate Cox regression analyses in the training set (Table 2). Both analyses indicated several risk factors for OS outcomes: age at diagnosis, tumor histology, tumor size, T stage, N stage, CEA level, perineural invasion, tumor deposits, treatment sequence, and chemotherapy. Specifically, T3 stage (HR: 1.56; 95% CI: 1.14–2.14), T4 stage (HR: 3.31; 95% CI: 2.37–4.64), N1 stage (HR: 1.65; 95% CI: 1.39–1.95), and N2 stage (HR: 2.20; 95% CI: 1.82–2.65) were associated with worse OS. Other negative predictors included poor tumor grade (HR: 1.22; 95% CI: 1.00–1.48), larger tumor size (HR: 1.19; 95% CI: 1.01–1.41), older age (HR: 1.89; 95% CI: 1.60–2.23), positive CEA levels (HR: 1.77; 95% CI: 1.51–2.07), and perineural invasion (HR: 1.59; 95% CI: 1.32–1.93). In contrast, adjuvant therapy (HR: 0.09; 95% CI: 0.01–0.60) and neoadjuvant therapy (HR: 0.14; 95% CI: 0.02–0.93) were associated with improved OS.
Table 2
| Characteristics | Univariate analysis | Multivariate analysis | |||
|---|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | ||
| Sex (vs. female) | |||||
| Male | 1.17 (0.99–1.36) | 0.053 | |||
| Age (years) (vs. <60) | |||||
| ≥60 | 1.94 (1.65–2.28) | <0.001* | 1.89 (1.60–2.23) | <0.001* | |
| Histology (vs. adenocarcinoma) | |||||
| Others | 1.69 (1.37–2.10) | <0.001* | 1.31 (1.05–1.63) | 0.02* | |
| Reginal lymph node dissection (vs. no) | |||||
| Yes | 0.61 (0.37–0.99) | 0.047* | 0.67 (0.41–1.11) | 0.12 | |
| Radiation (vs. no) | |||||
| Yes | 0.94 (0.79–1.11) | 0.47 | |||
| Chemotherapy (vs. no/unknown) | |||||
| Yes | 0.56 (0.48–0.66) | <0.001* | 0.27 (0.19–0.39) | <0.001* | |
| Treatment sequence (vs. only surgery) | |||||
| Adjuvant therapy | 0.54 (0.47–0.62) | <0.001* | 0.09 (0.01–0.60) | 0.01* | |
| Neoadjuvant therapy | 0.63 (0.52–0.77) | <0.001* | 0.14 (0.02–0.93) | 0.04* | |
| Grade (vs. well and moderate) | |||||
| Poor and undifferentiated | 1.59 (1.32–1.92) | <0.001* | 1.22 (1.00–1.48) | 0.046* | |
| Carcinoembryonic antigen (vs. negative) | |||||
| Positive | 2.14 (1.83–2.50) | <0.001* | 1.77 (1.51–2.07) | <0.001* | |
| Perineural invasion (vs. no) | |||||
| Yes | 2.17 (1.82–2.58) | <0.001* | 1.59 (1.32–1.93) | <0.001* | |
| Tumor deposits (vs. no) | |||||
| Yes | 2.19 (1.84–2.60) | <0.001* | 1.64 (1.35–1.99) | <0.001* | |
| Tumor size (mm) (vs. <56) | |||||
| ≥56 | 1.36 (1.16–1.60) | <0.001* | 1.19 (1.01–1.41) | 0.04* | |
| Race (vs. White) | |||||
| Others | 1.06 (0.95–1.18) | 0.29 | |||
| N stage (vs. N0) | |||||
| N1 | 1.10 (0.95–1.28) | 0.21 | 1.65 (1.39–1.95) | <0.001* | |
| N2 | 1.69 (1.44–1.98) | <0.001* | 2.20 (1.82–2.65) | <0.001* | |
| T stage (vs. T1/T2) | |||||
| T3 | 3.68 (1.53–8.86) | 0.004* | 1.56 (1.14–2.14) | 0.006* | |
| T4 | 8.70 (3.59–21.08) | <0.001* | 3.31 (2.37–4.64) | <0.001* | |
*, statistical significance. CI, confidence interval; HR, hazard ratio.
For CSS, prognostic factors included age at diagnosis, tumor histology, tumor size, T and N stages, CEA level, perineural invasion, tumor deposits, and treatment sequence (Table S1). Notably, T3 stage (HR: 1.94; 95% CI: 1.29–2.91), T4 stage (HR: 4.64; 95% CI: 3.04–7.09), N1 stage (HR: 1.91; 95% CI: 1.56–2.34), N2 stage (HR: 2.64; 95% CI: 2.12–3.29), and race (HR: 1.29; 95% CI: 1.06–1.57) correlated with poorer CSS. Additional negative factors included poor tumor grade (HR: 1.34; 95% CI: 1.11–1.60), larger tumor size (HR: 1.20; 95% CI: 1.03–1.41), older age (HR: 1.59; 95% CI: 1.36–1.86), positive CEA levels (HR: 1.78; 95% CI: 1.53–2.07), and perineural invasion (HR: 1.67; 95% CI: 1.40–2.00). Furthermore, adjuvant therapy (HR: 0.43; 95% CI: 0.35–0.52) and neoadjuvant therapy (HR: 0.57; 95% CI: 0.43–0.76) were associated with better CSS outcomes.
Nomogram construction
Building on the multivariate analysis results from the training set, we developed a nomogram model to predict OS and CSS in patients with LARSJC (Figure 1). Each prognostic factor was assigned a score between 0 and 100, reflecting its impact on the model’s predictive accuracy. By aggregating these scores for each patient, we derived a total point value that facilitated the estimation of 1-, 3-, and 5-year OS and CSS probabilities. Importantly, higher total scores were associated with a worse prognosis for patients.
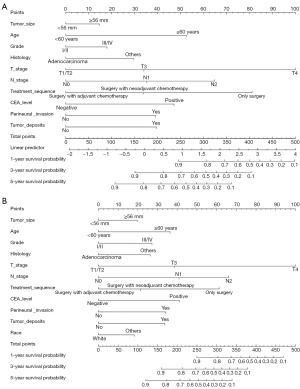
Model validation
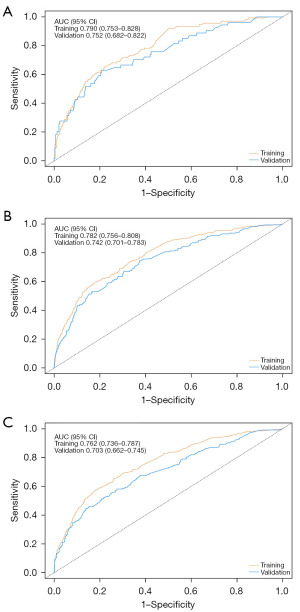
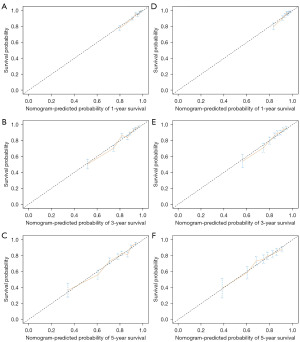
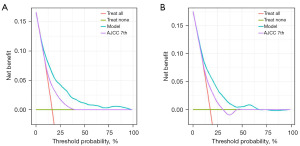
In the training set, the C-index for the nomogram predicting OS was 0.74 (95% CI: 0.72–0.76), notably exceeding the 7th edition of the AJCC staging system, which had a C-index of 0.63 (95% CI: 0.61–0.66). In an independent validation cohort, the C-index was 0.75 (95% CI: 0.73–0.77). The nomogram’s performance was further evaluated using ROC curves, yielding AUC values of 0.790, 0.782, and 0.762 for 1-, 3-, and 5-year OS in the training set, respectively. Likely, in the validation set, the AUC values for 1-, 3-, and 5-year OS were 0.752, 0.742, and 0.703 for 1-, 3-, and 5-year OS, respectively (Figure 2). Calibration plots for these time points confirmed the alignment between actual outcomes and predicted values both in the training set (Figure 3A-3C) and in the validation set (Figure 3D-3F). DCA indicated superior clinical utility of the nomogram over the 7th AJCC staging system in the training set (Figure 4A) and validation set (Figure 4B).
The CSS nomogram also demonstrated strong validation. In the training set, its C-index was 0.75 (95% CI: 0.72–0.77), again surpassing the AJCC’s C-index of 0.63 (95% CI: 0.61–0.66). For the validation cohort, the C-index was 0.74 (95% CI: 0.70–0.77). The ROC curves produced AUC values of 0.718, 0.762, and 0.760 for 1-, 3-, and 5-year CSS in the training set, respectively. And in the validation set, the AUC values for 1-, 3-, and 5-year CSS were 0.739, 0.730, and 0.730 (Figure S1). Calibration plots indicated a strong correlation between predicted and observed outcomes in the training set (Figure S2A-S2C), and consistent findings were also observed in calibration curves for the validation set (Figure S2D-S2F). DCA confirmed the model’s superior clinical utility both in the training set (Figure S3A) and the validation set (Figure S3B).
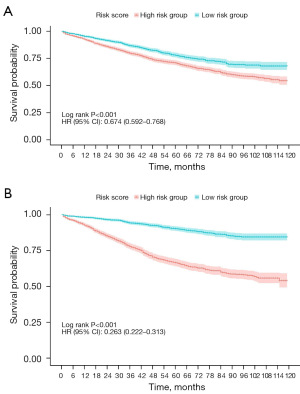
Treatment benefits in different risk groups
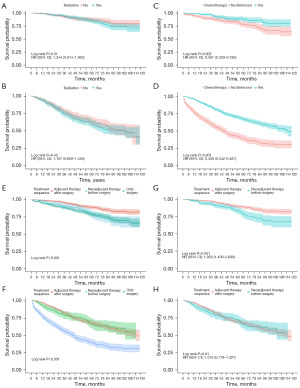
Patients were divided into two subgroups according to the scores derived from the prediction model: the low-risk group (score <214 for OS and <224 for CSS) and the high-risk group (score ≥214 for OS and ≥224 for CSS). Kaplan-Meier survival analysis revealed significant differences in OS and CSS between these groups (Figure 5). Specifically, LARSJC patients with higher risk scores exhibited lower rates of OS and CSS, while those in the low-risk group had improved outcomes (Figure 5). Additionally, we assessed the potential impacts of various treatment modalities on OS and CSS among LARSJC patients in these subgroups after PSM (Figure 6 and Figure S4). The results indicated that chemotherapy markedly enhanced OS for LARSJC patients, whereas radiotherapy did not yield significant survival benefits. Notably, patients receiving adjuvant therapy post-surgery experienced better OS and CSS compared to those who underwent neoadjuvant therapy prior to surgery within the low-risk subgroup.
Discussion
In the latest version of the International Classification of Diseases for Oncology, distinct codes were assigned to cancers of the sigmoid colon, rectosigmoid junction, and rectum, indicating their unique anatomical and pathological characteristics (18). Most clinical frameworks, including the AJCC system, categorize rectosigmoid junction diseases under rectal cancers. Conversely, the Chinese Society of Clinical Oncology (CSCO) suggested that LARSJC should be treated similarly to LACC (19). A precise definition of the rectosigmoid junction remains ambiguous. However, a research indicated that RSJCs exhibited a higher propensity for lymphatic metastasis compared to sigmoid and rectal cancers (20), with the former also showing a greater likelihood of distant metastasis (21). Despite their shared pathological origins, rectosigmoid junction and rectal tumors were associated with different OS outcomes (22), highlighting the need for tailored treatment strategies (23,24). While the efficacy of neoadjuvant chemoradiotherapy for LARC is well-documented, standard management for LACC typically involves adjuvant chemotherapy post-surgery (5,6). Nonetheless, there is limited research focusing on treatment sequences and prognostic factors specifically for LARSJC.
Our study examined prognostic factors and created prediction nomograms for OS and CSS in patients with LARSJC. Independent risk predictors for OS and CSS included T stage, N stage, tumor grade, tumor size, age, histology, elevated CEA levels, tumor deposits, perineural invasion, and treatment sequence; race was identified as a risk factor solely for CSS. We developed and validated the nomogram’s accuracy using C-index, AUC in the ROC analysis, and calibration curves. To assess the clinical utility and potential benefits of the model, DCA was performed (25). Additionally, we established two distinct risk groups based on risk scores to evaluate the advantages of various treatment strategies.
In prior nomograms predicting outcomes for RSJC, the impact of tumor deposits—critical factors associated with recurrence and distant metastasis—was seldom analyzed (7,20,26). Our study demonstrated that tumor deposits correlated with poorer OS and CSS, aligning with findings in colon and rectal cancer (27). Contrary to earlier assertions that RSJC may benefit from neoadjuvant chemoradiation, our research indicated that adjuvant therapy post-surgery was optimal for low-risk LARSJC patients (28), mirroring treatment approaches for LACC. While radiotherapy is routinely utilized in LARC, it is infrequently applied in LACC (29). Our findings revealed no significant survival advantage associated with radiation in the LARSJC patients, potentially due to lower risk of local recurrence in this cohort. Additionally, chemotherapy demonstrated substantial benefits for both risk groups, consistent with previous studies on LACC and LARC (30). Neoadjuvant concurrent therapy is well-established in LARC, yet our analysis suggests that it does not significantly improve survival outcomes in either risk group compared to those undergoing surgery with subsequent adjuvant therapy (31). Further studies are needed to warrant the appropriate cohort for neoadjuvant therapy.
There are several limitations in this study. Firstly, the SEER database does not include critical biomarker data, such as MSI and deficient mismatch repair (dMMR) status, which are vital prognostic indicators. Furthermore, it offers only basic therapeutic information, omitting details on specific surgical methods, chemotherapy regimens, radiation doses, and socio-economic factors that may influence survival outcomes. This restricts the analytical depth of our findings. Future researches should aim to integrate these essential factors for a more comprehensive understanding. Additionally, due to the retrospective design, there is a risk of selection bias in patient inclusion. To enhance the validity of our results and reduce bias, prospective cohort studies or randomized controlled trials are needed.
Conclusions
Our research provided an in-depth analysis of OS and CSS in patients with LARSJC, utilizing data from the SEER database. We created and validated distinct prediction nomograms for OS and CSS. While our model shows encouraging results in predicting survival outcomes for LARSJC, further multicenter studies are necessary to confirm its clinical applicability.
Acknowledgments
We would like to thank all the researchers for the SEER program.
Footnote
Reporting Checklist: The authors have completed the TRIPOD reporting checklist. Available at https://tcr.amegroups.com/article/view/10.21037/tcr-24-1810/rc
Peer Review File: Available at https://tcr.amegroups.com/article/view/10.21037/tcr-24-1810/prf
Funding: This research was funded by
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tcr.amegroups.com/article/view/10.21037/tcr-24-1810/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki and its subsequent amendments.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Bray F, Laversanne M, Sung H, et al. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2024;74:229-63. [Crossref] [PubMed]
- Patel SG, Dominitz JA. Screening for Colorectal Cancer. Ann Intern Med 2024;177:ITC49-64. [Crossref] [PubMed]
- Mosele MF, Westphalen CB, Stenzinger A, et al. Recommendations for the use of next-generation sequencing (NGS) for patients with advanced cancer in 2024: a report from the ESMO Precision Medicine Working Group. Ann Oncol 2024;35:588-606. [Crossref] [PubMed]
- Eng C, Yoshino T, Ruíz-García E, et al. Colorectal cancer. Lancet 2024;404:294-310. [Crossref] [PubMed]
- Benson AB, Venook AP, Adam M, et al. Colon Cancer, Version 3.2024, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw 2024;22:e240029. [Crossref] [PubMed]
- Benson AB, Venook AP, Adam M, et al. NCCN Guidelines® Insights: Rectal Cancer, Version 3.2024. J Natl Compr Canc Netw 2024;22:366-75. [Crossref] [PubMed]
- Yanlong W, Yunxiao W, Yibing W. A postsurgical prognostic nomogram for patients with lymph node positive rectosigmoid junction adenocarcinoma. BMC Gastroenterol 2023;23:159. [Crossref] [PubMed]
- Zhu Q, Zhu C, Zhang X, et al. Comprehension of rectosigmoid junction cancer molecular features by comparison to the rectum or sigmoid colon cancer. J Gastrointest Oncol 2023;14:1307-19. [Crossref] [PubMed]
- Vieira LM, Jorge NAN, de Sousa JB, et al. Competing Endogenous RNA in Colorectal Cancer: An Analysis for Colon, Rectum, and Rectosigmoid Junction. Front Oncol 2021;11:681579. [Crossref] [PubMed]
- Shen Q, Liu D, Liu S. Response to Neoadjuvant Chemoradiotherapy as a Predictor of Long-Term Survival in Patients with Locally Advanced Rectosigmoid Junction Cancer: An Analysis Based On SEER Database. Iran J Public Health 2023;52:1935-41. [Crossref] [PubMed]
- Khan M, Chandasir A, Yasinzai AQK, et al. Rectosigmoid Junction Cancer; The Role of Preoperative and Postoperative Radiation With Novel Nomogram in Predicting Survival in the United States. Clin Colorectal Cancer 2024;S1533-0028(24)00100-2.
- Guan X, Jiang Z, Ma T, et al. Radiotherapy dose led to a substantial prolongation of survival in patients with locally advanced rectosigmoid junction cancer: a large population based study. Oncotarget 2016;7:28408-19. [Crossref] [PubMed]
- Zheng R, Wang X, Zhu L, et al. A deep learning method for predicting the origins of cervical lymph node metastatic cancer on digital pathological images. iScience 2024;27:110645. [Crossref] [PubMed]
- Swanson K, Wu E, Zhang A, et al. From patterns to patients: Advances in clinical machine learning for cancer diagnosis, prognosis, and treatment. Cell 2023;186:1772-91. [Crossref] [PubMed]
- Tang S, Zhang H, Liang J, et al. Prostate cancer treatment recommendation study based on machine learning and SHAP interpreter. Cancer Sci 2024;115:3755-66. [Crossref] [PubMed]
- Yang XG, Yang SS, Bao Y, et al. Novel machine-learning prediction tools for overall survival of patients with chondrosarcoma: Based on recursive partitioning analysis. Cancer Med 2024;13:e70058. [Crossref] [PubMed]
- Wu Y, Zhang Y, Duan S, et al. Survival prediction in second primary breast cancer patients with machine learning: An analysis of SEER database. Comput Methods Programs Biomed 2024;254:108310. [Crossref] [PubMed]
- Lo Greco MC, La Rocca M, Marano G, et al. Integrated Intensified Chemoradiation in the Setting of Total Neoadjuvant Therapy (TNT) in Patients with Locally Advanced Rectal Cancer: A Retrospective Single-Arm Study on Feasibility and Efficacy. Cancers (Basel) 2023;15:921. [Crossref] [PubMed]
- Chen L, Hu H, Yuan Y, et al. CSCO guidelines for colorectal cancer version 2024: Updates and discussions. Chin J Cancer Res 2024;36:233-9. [Crossref] [PubMed]
- Zhang C, Zhao S, Wang X. A Postsurgical Prognostic Nomogram for Locally Advanced Rectosigmoid Cancer to Assist in Patient Selection for Adjuvant Chemotherapy. Front Oncol 2021;11:772482. [Crossref] [PubMed]
- Tsai MH, Cabral DN, Grunert C, et al. Colorectal cancer survival disparities in the five regions of Georgia. PLoS One 2024;19:e0301027. [Crossref] [PubMed]
- Levy L, Smiley A, Latifi R. Adult and Elderly Risk Factors of Mortality in 23,614 Emergently Admitted Patients with Rectal or Rectosigmoid Junction Malignancy. Int J Environ Res Public Health 2022;19:9203. [Crossref] [PubMed]
- Langenfeld SJ, Davis BR, Vogel JD, et al. The American Society of Colon and Rectal Surgeons Clinical Practice Guidelines for the Management of Rectal Cancer 2023 Supplement. Dis Colon Rectum 2024;67:18-31. [Crossref] [PubMed]
- Hashiguchi Y, Muro K, Saito Y, et al. Japanese Society for Cancer of the Colon and Rectum (JSCCR) guidelines 2019 for the treatment of colorectal cancer. Int J Clin Oncol 2020;25:1-42. [Crossref] [PubMed]
- Sande SZ, Li J, D'Agostino R, et al. Statistical inference for decision curve analysis, with applications to cataract diagnosis. Stat Med 2020;39:2980-3002. [Crossref] [PubMed]
- Zheng X, Cen W, Zhu J, et al. Prognostic Value of Tumor Deposits in Stage III Colorectal Cancer Patients with Different N Stages: A Population-Based, Retrospective, Cohort Study. Ann Surg Oncol 2023;30:8067-73. [Crossref] [PubMed]
- Alonso MD, Moreno FM, Mansilla CV, et al. Prognostic Value of Tumor Deposits in Patients with Colorectal Cancer. J Cancer 2024;15:4789-800. [Crossref] [PubMed]
- Li F, Qu R, Meng Y, et al. Sigmoid take-off in rectosigmoid cancer as a landmark identifying benefit from neoadjuvant chemoradiation: A retrospective comparative cohort study. Asian J Surg 2023;46:4330-6. [Crossref] [PubMed]
- Hitchcock KE, Miller ED, Shi Q, et al. Alliance for clinical trials in Oncology (Alliance) trial A022101/NRG-GI009: a pragmatic randomized phase III trial evaluating total ablative therapy for patients with limited metastatic colorectal cancer: evaluating radiation, ablation, and surgery (ERASur). BMC Cancer 2024;24:201. [Crossref] [PubMed]
- Yao S, Han Y, Yang M, et al. It's high-time to re-evaluate the value of induced-chemotherapy for reinforcing immunotherapy in colorectal cancer. Front Immunol 2023;14:1241208. [Crossref] [PubMed]
- Guan B, Xu M, Zheng R, et al. Novel biomarkers to predict treatment response and prognosis in locally advanced rectal cancer undergoing neoadjuvant chemoradiotherapy. BMC Cancer 2023;23:1099. [Crossref] [PubMed]


