
Construction and validation of a nomogram prediction model for predicting the risk of chemotherapy-induced myelosuppression after chemotherapy in patients with triple-negative breast cancer: a single-center retrospective case-control study
Highlight box
Key findings
• This study developed a nomogram to predict the risk of chemotherapy-induced myelosuppression (CIM) in triple-negative breast cancer (TNBC) patients. The model showed strong predictive accuracy and clinical utility.
What is known and what is new?
• CIM is a common side effect in TNBC patients after chemotherapy, but reliable tools to predict its occurrence are limited.
• This study provides a new nomogram based on key clinical factors, including bone metastasis, chemotherapy cycles, and pre-chemotherapy neutrophil count, to predict CIM risk with high accuracy.
What is the implication, and what should change now?
• The nomogram can help healthcare providers identify high-risk patients before starting chemotherapy. This allows for personalized treatment planning, reducing the risk of CIM and improving patient outcomes.
Introduction
In 2020, the International Agency for Research on Cancer (IARC) reported that breast cancer has overtaken lung cancer as the most prevalent cancer globally, with an incidence rate of 11.7%, ranking second in mortality rates (1). Breast cancer is typically classified into four molecular subtypes: luminal A, luminal B, human epidermal growth factor receptor 2 (HER-2) enriched, and triple-negative breast cancer (TNBC) (2). TNBC is characterized as invasive breast cancer lacking estrogen receptors, progesterone receptors, and HER-2 (3). This subtype exhibits higher rates of mutations, recurrence, metastasis, and mortality compared to other subtypes, accounting for approximately 15% of all breast cancer cases (4).
TNBC has been a challenging disease to treat compared with other breast cancer subtypes due to its aggressive biological feature and the absence of actionable targeted therapy. Even with the use of poly-chemotherapy in either neoadjuvant or adjuvant settings, the 5-year disease-free survival rate is only around 70%, while the 5-year overall survival (OS) rate is merely 77% (5,6). Adjuvant chemotherapy with anthracyclines and paclitaxel is frequently administered to patients with TNBC. Due to its prolonged regimen and the common use of combination drug protocols, this approach can lead to cumulative drug toxicities in the bloodstream, resulting in chemotherapy-induced myelosuppression (CIM), a common hematologic toxicity with an incidence of 20% to 40% (7,8). CIM has been shown to extend hospitalization periods, increase medical costs, and pose a potential risk to patient survival in severe cases (9,10). Despite the scarcity of clinical studies and validated predictive tools for assessing myelosuppressive risk factors in patients with breast cancer undergoing chemotherapy, it is crucial to promptly identify patients at risk of CIM. Early detection of CIM risk factors in patients with TNBC and implementing preventive measures can support the completion of chemotherapy regimens, improve clinical outcomes, and enhance patients’ overall quality of life. Nomogram prediction models, frequently employed as risk assessment tools in medical research, can facilitate this process (11). Given these considerations, this retrospective case-control study aimed to assess the factors influencing myelosuppression in 316 patients with TNBC from 1 July 2021 to 31 May 2024. Furthermore, the study sought to develop a predictive model to assist healthcare providers in formulating individualized treatment plans at the onset of the disease. We present this article in accordance with the TRIPOD reporting checklist (available at https://tcr.amegroups.com/article/view/10.21037/tcr-24-1513/rc).
Methods
Study population
A retrospective case-control study was conducted on a primary cohort of patients diagnosed with TNBC between 1 July 2021 and 31 May 2024 at the Oncology Center of the Anning First People’s Hospital Affiliated to Kunming University of Science and Technology in Yunnan, China. Out of an initial cohort of 1,335 patients with breast cancer, 1,019 were excluded based on predetermined inclusion and exclusion criteria, resulting in the analysis of medical records and written files of 316 patients with TNBC in a comprehensive and systematic review. These patients constituted the development and validation cohort for this study. The cohort was randomly divided into a development cohort and a validation cohort in an 8:2 ratio, using stratified random sampling to ensure balanced distributions of key variables such as age and chemotherapy regimen between the two cohorts. All patients were staged according to the 8th edition of the American Joint Committee on Cancer (AJCC) staging system (12).
Inclusion and exclusion criteria
Inclusion criteria encompassed female patients aged 18 years or older with confirmed TNBC through pathohistology, cytology, and imaging; who received standardized and complete chemotherapy regimens; had comprehensive medical records; underwent blood cytology analysis before and after each chemotherapy cycle; provided informed consent for treatment; and followed antitumor regimens in accordance with the Chinese Society of Clinical Oncology guidelines. Exclusion criteria included patients with poor overall health unable to withstand chemotherapy, those with primary tumors in other locations, individuals receiving irregular antitumor chemotherapy, patients using leukocyte-boosting drugs preventively before chemotherapy, individuals with autoimmune diseases, hematological disorders, end-stage renal disease, acute inflammation, acute onset of chronic inflammation, patients with incomplete case data (lacking ≥20% clinical data), and participants in clinical trials impacting myelosuppression. The overall flowchart for this study is presented in Figure 1.

Data collection and explanatory notes
In this extensive investigation, two proficient researchers independently conducted the data collection and review process within the research team. Patient-specific details, including clinical and demographic information such as age, height, weight, body mass index (BMI), Tumor-Node-Metastasis (TNM) stage of tumors, surgical history, hypertension, diabetes mellitus, and coronary artery disease, were extracted from the hospital information system (HIS). The primary laboratory parameters of interest comprised routine blood tests [red blood cells, white blood cells (WBC), platelets (PLT), hemoglobin (HGB), monocytes], albumin levels, alanine aminotransferase (ALT), aspartate aminotransferase (AST), albumin, and total bilirubin levels within one week preceding chemotherapy administration. Additionally, comprehensive data on treatment modalities and responses were obtained, focusing on chemotherapy regimens, cycles, and the occurrence of CIM. Erythrocyte analysis and biochemical analysis were performed using the Sysmex XT-500i automated hematology analyzer and the Sysmex XT-2000i automated biochemical analyzer (Sysmex Corporation, Kobe, Japan).
In this study, hypertension was operationally defined as individuals with a systolic blood pressure (SBP) of 140 mmHg or higher, or a diastolic blood pressure (BP) of 90 mmHg or higher, who are undergoing treatment for hypertension or have received a diagnosis of the condition. Similarly, diabetes mellitus was defined as individuals with a fasting blood glucose level of 7.0 mmol/L or higher who are undergoing treatment for diabetes or have received a previous diagnosis of the disease. Coronary heart disease was defined as definite or probable myocardial infarction or fatal coronary heart disease.
Statistical analysis
Clinical trial data were amassed using Excel 2019. Statistical analyses employed SPSS 26.0 (IBM, Armonk, NY, USA) and the R software package (version 4.2.0). Categorical variables were quantified as values and percentages, while continuous variables were represented as mean ± standard deviation (SD). The χ2 test or Fisher’s exact test evaluated statistical differences between categorical variables, and the two-tailed t-test or the Mann-Whitney U test evaluated continuous variables. The prediction model was constructed using the least absolute shrinkage and selection operator (LASSO) and multivariable logistic regression, followed by nomogram development. The nomogram’s performance was assessed by comparing the predicted probability of CIM formation with the observed probability using the consistency index (C-index). A bootstrapping method with 1,000 resamples was employed. A higher C-index denotes superior predictive accuracy. For model validation, a C-index greater than 0.70 was considered acceptable, while values above 0.80 indicated excellent performance. The model’s discriminative ability was assessed using the receiver operating characteristic (ROC) curve and the area under the curve (AUC), with AUC values greater than 0.70 considered acceptable and values above 0.80 indicating excellent discrimination. The calibration of the nomogram was evaluated by plotting calibration curves, which compared the predicted probabilities with the observed frequencies of CIM. A well-calibrated model will show a close alignment between the predicted and observed probabilities. Decision curve analysis (DCA) evaluated the nomogram’s clinical utility. A P value <0.05 was considered statistically significant, with all tests being two-sided. Any missing data were interpolated using means for continuous variables and plurals for categorical variables.
Diagnostic criteria and grading of CIM
CIM was categorized into grades 0-IV based on the World Health Organization (WHO) toxicity assessment criteria for chemotherapy (13) (Table 1). Grade 0 represented no CIM in the control group, while grades I to IV indicated moderate to severe CIM in the case group
Table 1
| Items | Grade 0 | Grade I | Grade II | Grade III | Grade IV |
|---|---|---|---|---|---|
| Leukocyte (109/L) | ≥4.0 | 3.0–3.9 | 2.0–2.9 | 1.0–1.9 | <1.0 |
| Hemoglobin (g/L) | ≥110 | 95–109 | 80–94 | 65–79 | <65 |
| Platelets (109/L) | ≥100 | 75–99 | 50–74 | 25–49 | <25 |
| Neutrophils (109/L) | >2.0 | 1.5–1.9 | 1.0–1.4 | 0.5–0.9 | <0.5 |
Sample estimates of research objects and development of prediction model
Sample size estimation for the prediction model was based on the “events per variable” (EPV) rule to ensure sufficient statistical power and avoid overfitting. According to this rule, each predictor variable should have at least 5–10 events. In this study, 19 predictor variables were included, and the total number of myelosuppression events was 102, which satisfies the minimum requirement of 5 EPV (102 events >19×5).
Ethical approval
This study was conducted in accordance with the Declaration of Helsinki and its subsequent amendments. All data were anonymized and collected as part of routine practices. The studies involving human participants were reviewed and approved by the Institutional Ethics Committee of the Anning First People’s Hospital Affiliated to Kunming University of Science and Technology (Approval No. 2024-035-01). Informed consent was waived due to the retrospective nature of the study.
Results
Characteristics of patients
Following the application of specific inclusion and exclusion criteria, 316 female patients diagnosed with TNBC at the Anning First People’s Hospital Affiliated to Kunming University of Science and Technology, between July 2021 and May 2024, were retrospectively selected for this study. The data were divided into development and validation cohorts at an 8:2 ratio. Demographic information, disease characteristics, treatment details, and laboratory findings of both groups are presented in Table 2. No statistically significant differences were observed between the development and validation cohorts regarding age, platinum-containing regimens, WBC count, PLT count, HGB, neutrophil count, lymphocyte count, monocyte count, ALT, AST, and serum albumin (all P>0.05). Baseline characteristics of the patients in the development cohort are shown in Table 3. The age of the 253 patients with TNBC was 52.05±10.98 years, with a median hospitalization duration of 5 days (range, 4.00–6.00 days). Further analysis revealed significant differences (P<0.05) between the two groups in terms of age, TNM stage, chemotherapy cycles, drug combination quantity, presence of bone metastasis, BMI, WBC, PLT, HGB, neutrophils, monocytes, and albumin levels. By aggregating all case data from the development and validation cohorts, 102 CIM events were identified among 316 patients, resulting in a CIM incidence of 32.28%. The most frequently observed CIM event was myelosuppression of degree I, occurring in 42 cases (41.18% of total events), followed by 37 cases of degree II (36.27%), 16 cases of degree III (15.69%), and 7 cases of degree IV (6.86%). Additionally, the distribution characteristics of the CIM and non-CIM event groups were explored in subgroups of bone metastasis, TNM stage, chemotherapy cycle, hospitalization days, drug combination, and platinum-containing regimens. It was found that 49 (71.01%) patients with bone metastasis experienced CIM events. The highest frequency of CIM events occurred in 54 patients (56.84%) in stage IV of TNM staging, followed by 29 patients (29%) in stage III, 17 patients (17.53%) in stage II, and 2 patients (9.09%) in stage I. Hospitalization days were identified as the most critical factor in the distribution of patients with bone metastases across drug combinations and platinum-containing regimens. Patients with CIM events had prolonged hospital stays, with 14 cases (14/21) hospitalized for 10 days or more, 15 cases (15/33) for 7–8 days, 32 cases (32/113) for 5–6 days, 35 cases (35/136) for 3–4 days, and 6 cases (6/13) for 1–2 days. CIM events occurred in 87 cases (87/102) with combinations of two or more drugs, with the highest rate of CIM events found in platinum-containing regimens (35/48). The characteristic distribution of all CIM-occurring events is shown in Figure 2A-2G.
Table 2
| Characteristic | Entire cohort (N=316) | Development cohort (N=253) | Validation cohort (N=63) | P |
|---|---|---|---|---|
| Age (years) | 52.00 [45.00, 58.00] | 53.00 [44.00, 59.00] | 52.00 [48.00, 54.00] | 0.06 |
| Hospital stay (days) | 5.00 [4.00, 6.00] | 5.00 [4.00, 6.00] | 4.00 [4.00, 5.00] | 0.01 |
| TNM stage | 0.02 | |||
| I | 24 (7.60) | 17 (6.70) | 7 (11.1) | |
| II | 97 (30.7) | 76 (30.0) | 21 (33.3) | |
| III | 100 (31.6) | 74 (29.2) | 26 (41.3) | |
| IV | 95 (30.1) | 86 (34.0) | 9 (14.3) | |
| Surgical history (within the last 12 months) | 0.04 | |||
| No | 203 (64.24) | 170 (67.19) | 33 (52.38) | |
| Yes | 113 (35.76) | 83 (32.81) | 30 (47.62) | |
| Chemotherapy cycles | 4 [2, 6] | 4 [2, 6] | 3 [2, 4] | 0.01 |
| Platinum-containing regimens | 0.68 | |||
| No | 268 (84.81) | 213 (84.19) | 55 (87.30) | |
| Yes | 48 (15.19) | 40 (15.81) | 8 (12.70) | |
| Combination of drug quantities | 0.004 | |||
| 1 | 91 (28.80) | 82 (32.40) | 9 (14.30) | |
| 2 | 198 (62.7) | 147 (58.10) | 51 (81.0) | |
| ≥3 | 27 (8.50) | 24 (9.50) | 3 (4.76) | |
| Bone metastasis | 0.03 | |||
| No | 247 (78.2) | 192 (75.9) | 55 (87.3) | |
| Yes | 69 (21.8) | 61 (24.1) | 8 (12.7) | |
| BMI (kg/m2) | 23.12 [21.21, 25.39] | 23.14 [21.33, 25.64] | 22.44 [20.17, 24.59] | 0.04 |
| Hypertension | 0.004 | |||
| No | 290 (91.77) | 227 (89.72) | 63 (100.00) | |
| Yes | 26 (8.23) | 26 (10.28) | 0 (0.00) | |
| Diabetes mellitus | 0.03 | |||
| No | 298 (94.30) | 235 (92.89) | 63 (100.00) | |
| Yes | 18 (5.70) | 18 (7.11) | 0 (0.00) | |
| Laboratory parameters | ||||
| WBC (109/L) | 5.10 [4.07, 6.80] | 4.99 [4.00, 6.89] | 5.24 [4.40, 6.73] | 0.29 |
| PLT (109/L) | 243.50 [197.00, 310.00] | 240.00 [197.00, 305.00] | 254.00 [201.50, 327.00] | 0.20 |
| HGB (g/L) | 128.50 [120.00, 137.00] | 128.00 [120.00, 136.00] | 130.00 [118.50, 139.00] | 0.87 |
| Neutrophils (109/L) | 3.38 [2.50, 4.89] | 3.36 [2.39, 4.93] | 3.49 [2.83, 4.89] | 0.19 |
| Monocytes (109/L) | 0.37 [0.27, 0.51] | 0.37 [0.27, 0.50] | 0.38 [0.30, 0.52] | 0.37 |
| ALT (g/L) | 27.00 [19.00, 39.00] | 26.00 [19.00, 38.00] | 31.00 [18.00, 41.50] | 0.37 |
| AST (g/L) | 25.00 [20.00, 34.25] | 25.00 [20.00, 34.00] | 26.00 [21.50, 34.50] | 0.27 |
| Albumin (g/L) | 42.90 [40.28, 44.70] | 43.00 [40.30, 45.00] | 42.30 [40.30, 44.45] | 0.44 |
| Total bilirubin (mg/dL) | 7.80 [5.70, 11.33] | 7.80 [5.60, 11.30] | 8.60 [6.30, 11.95] | 0.23 |
Data are presented as median [interquartile range] or n (%). Statistical analysis was performed using Wilcoxon rank sum test, Pearson’s Chi-squared test, and Fisher’s exact test. ALT, alanine aminotransferase; AST, aspartate aminotransferase; BMI, body mass index; HGB, hemoglobin; PLT, platelet; TNM, tumor-node-metastasis; WBC, white blood cell.
Table 3
| Characteristics | Overall (N=253) | Control group (N=171) | Case group (N=82) | P |
|---|---|---|---|---|
| Age (years) | 52.05±10.98 | 53.61±10.79 | 48.79±10.71 | <0.001 |
| Hospital stay (days) | 5.00 [4.00, 6.00] | 5.00 [4.00, 6.00] | 5.00 [4.00, 7.75] | 0.04 |
| TNM stage | <0.001 | |||
| I | 17 (6.7) | 15 (8.80) | 2 (2.4) | |
| II | 76 (30.0) | 65 (38.0) | 11 (13.4) | |
| III | 74 (29.20) | 51 (29.80) | 23 (28.0) | |
| IV | 86 (34.0) | 40 (23.40) | 46 (56.1) | |
| Surgical history (within the last 12 months) | 0.33 | |||
| No | 170 (67.19) | 111 (64.91) | 59 (71.95) | |
| Yes | 83 (32.81) | 60 (35.09) | 23 (28.05) | |
| Chemotherapy cycles | 4.00 [2.00, 6.00] | 4.00 [2.00, 5.00] | 5.00 [3.25, 7.00] | <0.001 |
| Platinum-containing regimens | <0.001 | |||
| No | 213 (84.19) | 160 (93.57) | 53 (64.63) | |
| Yes | 40 (15.81) | 11 (6.43) | 29 (35.37) | |
| Combination of drug quantities | <0.001 | |||
| 1 | 82 (32.41) | 69 (40.35) | 13 (15.85) | |
| 2 | 147 (58.10) | 78 (45.61) | 69 (84.15) | |
| ≥3 | 24 (9.49) | 24 (14.04) | 0 (0.00) | |
| Bone metastasis | <0.001 | |||
| No | 192 (75.9) | 152 (88.9) | 40 (48.8) | |
| Yes | 61 (24.1) | 19 (11.1) | 42 (51.2) | |
| BMI (kg/m2) | 23.14 [21.33, 25.64] | 23.63 [22.04, 26.04] | 22.61 [19.85, 25.00] | 0.008 |
| Hypertension | 0.68 | |||
| No | 227 (89.72) | 152 (88.89) | 75 (91.46) | |
| Yes | 26 (10.28) | 19 (11.11) | 7 (8.54) | |
| Diabetes mellitus | 0.44 | |||
| No | 235 (92.89) | 157 (91.81) | 78 (95.12) | |
| Yes | 18 (7.11) | 14 (8.19) | 4 (4.88) | |
| Laboratory parameters | ||||
| WBC (109/L) | 4.99 [4.00, 6.89] | 5.60 [4.42, 7.39] | 4.20 [3.50, 4.91] | <0.001 |
| PLT (109/L) | 240.00 [197.00, 305.00] | 261.00 [220.50, 325.00] | 196.00 [138.50, 238.00] | <0.001 |
| HGB (g/L) | 128.00 [120.00, 136.00] | 131.00 [125.00, 137.00] | 121.50 [114.00, 132.50] | <0.001 |
| Neutrophils (109/L) | 3.36 [2.39, 4.93] | 3.72 [2.75, 5.09] | 2.46 [1.86, 3.75] | <0.001 |
| Monocytes (109/L) | 0.37 [0.27, 0.50] | 0.38 [0.28, 0.50] | 0.33 [0.23, 0.50] | 0.04 |
| ALT (g/L) | 26.00 [19.00, 38.00] | 25.00 [19.00, 36.00] | 27.00 [19.00, 38.75] | 0.52 |
| AST (g/L) | 25.00 [20.00, 34.00] | 23.00 [19.50, 34.50] | 27.50 [21.00, 33.75] | 0.09 |
| Albumin (g/L) | 43.00 [40.30, 45.00] | 43.10 [41.00, 45.00] | 42.00 [39.25, 44.23] | 0.02 |
| Total bilirubin (mg/dL) | 7.80 [5.60, 11.30] | 7.00 [5.40, 10.15] | 9.80 [6.80, 13.85] | <0.001 |
Data are presented as median [interquartile range] or n (%). Statistical analysis was performed using Wilcoxon rank sum test, Pearson’s Chi-squared test, and Fisher’s exact test. ALT, alanine aminotransferase; AST, aspartate aminotransferase; BMI, body mass index; HGB, hemoglobin; PLT, platelet; TNM, tumor-node-metastasis; WBC, white blood cell.
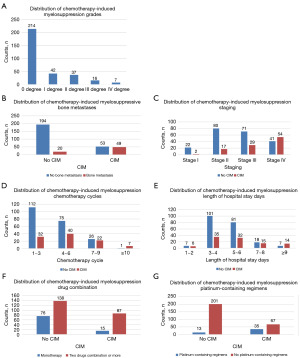
Selection of variables as predictors and derivation of the prediction model
The LASSO regression analysis, conducted on a comprehensive set of clinical indicators encompassing 19 factors, including clinicopathologic features and biomarkers, identified TNM stage, bone metastasis, age, decreased leukocytes before chemotherapy, albumin levels, decreased PLT count before chemotherapy, platinum-containing regimens, number of chemotherapy cycles, presence of decreased neutrophils before chemotherapy, and the number of combined drugs as significant variables, as illustrated in Figure 3A,3B. A multivariate logistic regression analysis was performed to examine the relationship between the occurrence of CIM (coded as “yes” for occurrence and “no” for non-occurrence) and several independent variables. The analysis revealed that five variables remained significant predictors of CIM occurrence in the final model: bone metastasis [odds ratio (OR) 17.602, 95% confidence interval (CI): 7.005–44.232, P<0.001], platinum-containing regimens (OR 9.359, 95% CI: 3.437–25.484, P<0.001), chemotherapy cycles (OR 1.369, 95% CI: 1.155–1.622, P<0.001), decreased neutrophil levels before chemotherapy (OR 29.223, 95% CI: 5.384–158.627, P<0.001), and drug quantity combination (OR 3.425, 95% CI: 1.755–6.684, P<0.001) (Table 4).
Table 4
| Characteristics | β | Odds ratio | 95% CI | P |
|---|---|---|---|---|
| Bone metastasis | 2.868 | 17.602 | 7.005–44.232 | <0.001 |
| Platinum-containing regimens | 2.236 | 9.359 | 3.437–25.484 | <0.001 |
| Chemotherapy cycles | 0.314 | 1.369 | 1.155–1.622 | <0.001 |
| Pre-chemotherapy neutrophil* decrease | 3.375 | 29.223 | 5.384–158.627 | <0.001 |
| Combination of drug quantities | 1.231 | 3.425 | 1.755–6.684 | <0.001 |
*, normal: ≥2.0×109/L; decreased: <2.0×109/L. CI, confidence interval.
Establishment, validation, and evaluation of nomogram for CIM in patients with TNBC
Building on the above analysis, this study utilized five variables—bone metastasis, platinum-containing regimens, chemotherapy cycles, decreased neutrophil levels before chemotherapy, and drug quantity combination—to develop a nomogram model for predicting CIM after chemotherapy in patients with TNBC. The CIM risk nomogram identified decreased neutrophil levels before chemotherapy as the most significant factor, followed by bone metastasis, platinum-containing regimens, drug quantity combination, and chemotherapy cycles. Nomogram weightings for each factor were calculated based on the β coefficients. As illustrated in Figure 4, each independent predictor was mapped to a “points” value at the top of the nomogram, yielding a score within the range of 0 to 100. The total score was then used to accurately predict the CIM risk in patients with TNBC, with higher scores indicating a higher risk of CIM.

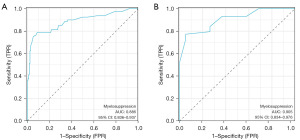
The Bootstrap method was employed for internal validation of the nomogram model. The Hosmer-Lemeshow test result (χ2=11.549, P=0.17) demonstrated a good degree of calibration. ROC analysis was conducted to compare the predictive values of the CIM risk nomogram model. The AUC values for the nomograms were 0.886 (95% CI: 0.836–0.937) in the development cohort (Figure 5A) and 0.905 (95% CI: 0.834–0.976) in the validation cohort (Figure 5B). The nomogram also exhibited excellent calibration in the validation cohort (Figure 6), with χ2=0.122 and P=0.94 in the Hosmer-Lemeshow test, indicating strong calibration ability.
DCAs were conducted on the nomograms for predicting CIM in the development and validation cohorts (Figure 7A,7B). The DCAs indicated that using the prediction nomogram would be more beneficial for predicting CIM than treating all patients as either low-risk or high-risk.

Discussion
The 2024 National Cancer Report, published by the National Cancer Center of China, highlighted that breast cancer was the leading type of cancer among females in China in 2020, with 420,000 new cases. The report also noted a continued increase in the standardized incidence rate of all cancers and a disparity in the 5-year survival rate compared to developed countries like the United States (14). Currently, TNBC lacks molecular targeted therapy and endocrine therapy, making chemotherapy the primary treatment strategy. However, CIM is the most common toxicity, potentially leading to dose-dependent chemotherapy and, in severe cases, interrupting or preventing the completion of a chemotherapy cycle. Therefore, exploring the risk factors of CIM caused by chemotherapy and establishing a prediction model are of great clinical significance. Existing studies on CIM in oncology patients have primarily focused on its incidence, with limited comprehensive analyses on predictive factors and effective prediction models (15,16). To further investigate this aspect, our retrospective exploratory analysis was conducted on a small sample of TNBC patients at a tertiary hospital in southwest China, aiming to identify key risk factors and provide preliminary insights for future predictive model development.
The prevalence of myelosuppression among Chinese patients with breast cancer remains largely unreported (17). In our study of 316 patients with TNBC, the CIM incidence was 32.28% (102/316), lower than that reported in studies on other malignancies (18-20). This discrepancy may be due to various factors, including specific chemotherapeutic agents, dosages, and individual differences in drug metabolism and tolerance across different solid tumors. Our analysis revealed that grade I myelosuppression accounted for the highest occurrence rate among all CIM events, indicating that mild bone marrow suppression, characterized by decreased blood cell production, is the most common manifestation in patients undergoing chemotherapy. Furthermore, increased CIM prevalence was observed in patients with bone metastasis and stage IV disease, those receiving 10 or more chemotherapy cycles, undergoing combination chemotherapy with two or more drugs, and treated with platinum-containing regimens. Additionally, CIM events led to prolonged hospitalization for admitted patients.
Independent risk factors for CIM occurrence after chemotherapy were comprehensively screened by retrospectively analyzing demographics, medical history, and laboratory indices of 316 patients with TNBC for 19 readily available clinical factors, which do not impose additional financial burdens on patients. A nomogram prediction model for CIM in patients with TNBC was developed according to standard procedures. The primary focus of this study was to facilitate the prediction of CIM likelihood after chemotherapy using the developed nomogram model.
The findings of this study indicate a significantly elevated risk of CIM in patients with bone metastasis compared to those without (OR 17.602; 95% CI: 7.005–44.232; P<0.001). This increased risk is attributed to the infiltration of tumor cells into the bone marrow via the bloodstream, where they interact with osteoclasts, osteoblasts, and other cellular components. This interaction results in bone tissue destruction, impairment of the hematopoietic system, fibrosis formation in the bone marrow, and accelerated apoptosis of hematopoietic cells, ultimately increasing the likelihood of developing CIM (21,22). Currently, clinicians empirically select bone-targeting drugs to manage breast cancer bone metastases and mitigate the risk of osteonecrosis and fractures. However, this approach may also reduce the likelihood of CIM to some extent, necessitating further studies for validation. Research has indicated that platinum-based combinations exhibit greater efficacy compared to monotherapy in patients with breast cancer (23,24). However, platinum-based drugs, which primarily disrupt DNA synthesis, induce DNA cross-linking, modulate cell cycle progression, and trigger apoptosis of tumor cells (25), also compromise normal bone marrow hematopoiesis and heighten the risk of myelosuppression due to the absence of targeted drug benefits. This study showed that platinum-containing chemotherapeutic regimens carried nearly a tenfold higher risk of CIM than platinum-free regimens (OR 9.359; 95% CI: 3.437–25.484; P<0.001), consistent with findings from previous studies (26,27). Additionally, in-depth analyses of single-agent and multi-agent chemotherapy regimens, as well as chemotherapy cycles, revealed that multi-agent combination chemotherapy (OR 3.425; 95% CI: 1.755–6.684; P<0.001) and multi-cycle chemotherapy regimens (OR 1.369; 95% CI: 1.155–1.622; P<0.001) result in the accumulation of chemotherapeutic drugs, high blood drug concentrations, and increased CIM risk. Therefore, close attention to chemotherapy regimens, especially platinum-containing ones, and early nursing intervention is essential for patients with malignant tumors.
Neutropenia is a frequent and serious complication in patients treated with myelosuppressive chemotherapy (28). Studies (29,30) have confirmed that the most common manifestation of CIM is neutrophil reduction, and patients with pre-chemotherapy abnormalities in leukocytes, hemoglobin, neutrophils, and platelets are more likely to develop CIM. These low levels reflect impaired bone marrow hematopoietic function, indicating a lower immune state and increased CIM risk. LASSO regression and multivariate logistic regression analyses identified pre-chemotherapy neutropenia as a significant risk factor (OR 29.223; 95% CI: 5.384–158.627; P<0.001). This finding does not diminish the predictive value of other laboratory indicators, which remain important for future large-scale, multicenter studies. Therefore, healthcare professionals should closely monitor pre-chemotherapy laboratory indicators, strengthen prophylactic use of leukocyte-boosting drugs, and promptly correct anemia to ensure the completion of the chemotherapy cycle.
Nomograms, visual tools that predict the incidence of clinical events (31), were employed in this study. The five predictors incorporated were bone metastasis, platinum-containing regimens, chemotherapy cycles, decreased neutrophils before chemotherapy, and drug quantity combination, to create a straightforward and user-friendly chart. The nomogram demonstrated good discriminatory power in both the development and validation cohorts, validating its clinical utility. Healthcare professionals, as well as patients and their families, can use the total scores of these risk factors to predict the probability of CIM in patients with TNBC. Apart from serum neutrophils, which are uncontrollable, healthcare professionals should enhance the management of controllable factors, such as active bone protection with phosphates, intensive rehydration therapy (e.g., saline, dextrose), selecting alternative platinum-containing chemotherapeutic regimens, appropriate dose control of chemotherapeutic agents, and careful consideration of medication combinations. Additionally, proactive blood cytology testing can help prevent CIM after chemotherapy.
Although not yet reported in the literature, variables such as age, low BMI, hypoproteinemia, and TNM stage are potential independent risk factors for CIM. With advancing age, bone marrow hematopoietic function gradually deteriorates, reducing its capacity to withstand chemotherapy and regenerate. Additionally, the detoxification ability of the liver and the creatinine clearance ability of the kidneys decline in elderly patients, leading to the accumulation of chemotherapeutic drugs and higher blood drug concentrations, which in turn increase the risk of CIM (20,32,33). Our multivariate analysis produced similar results; most patients were under 60, highlighting a limitation in our current study. Low BMI (<18.5 kg/m2) and low protein levels generally indicate poorer nutrition (34,35), which is theorized to correlate with poorer health outcomes, increased comorbidities, reduced efficacy of adjuvant therapies, and a higher likelihood of myelosuppression at the same dose, making these patients more susceptible to severe myelosuppression. While our univariate analysis found statistically significant differences, multivariate analyses did not yield the expected findings, indicating that pre-chemotherapy nutritional enhancement should be prioritized. Similarly, higher TNM stages indicate more advanced tumor progression and a higher risk of multi-organ metastasis, which can damage normal bone marrow hematopoietic cells, thus increasing the risk of myelosuppression. Considering that this study is retrospective, discrepancies in patient history collection might exist, but the final prediction model factors are relatively simple and easy to determine.
Despite the strong performance of our nomogram, the first study of its kind among national and international related studies, several limitations must be addressed. Firstly, the limited number of patients with TNBC, all from the same hospital, introduces selection bias. Secondly, as this predictive modeling study involved only internal data validation, our results require further confirmation through large-sample, multicenter, prospective cohort studies. Thirdly, our model was specific to patients with TNBC, and its applicability to other solid tumors remains uncertain. Therefore, future studies with multiple centers, larger sample sizes, and more detailed information are needed to validate these results.
Conclusions
The new methodology was employed to develop and validate the first straightforward and accurate nomogram for predicting CIM occurrence in patients with TNBC. By utilizing five laboratory and clinical parameters, clinicians can efficiently and accurately assess the individual risk of CIM development in patients with TNBC before chemotherapy, enabling timely and appropriate clinical decision-making for optimal disease management.
Acknowledgments
None.
Footnote
Reporting Checklist: The authors have completed the TRIPOD reporting checklist. Available at https://tcr.amegroups.com/article/view/10.21037/tcr-24-1513/rc
Data Sharing Statement: Available at https://tcr.amegroups.com/article/view/10.21037/tcr-24-1513/dss
Peer Review File: Available at https://tcr.amegroups.com/article/view/10.21037/tcr-24-1513/prf
Funding: None.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tcr.amegroups.com/article/view/10.21037/tcr-24-1513/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The research adhered to the principles outlined in the Declaration of Helsinki and its subsequent amendments and received approval from the Ethics Committee of the Anning First People’s Hospital Affiliated to Kunming University of Science and Technology (Approval No. 2024-035-01). Informed consent was waived for this retrospective study.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Cao W, Chen HD, Yu YW, et al. Changing profiles of cancer burden worldwide and in China: a secondary analysis of the global cancer statistics 2020. Chin Med J (Engl) 2021;134:783-91. [Crossref] [PubMed]
- He Y, Jiang Z, Chen C, et al. Classification of triple-negative breast cancers based on Immunogenomic profiling. J Exp Clin Cancer Res 2018;37:327. [Crossref] [PubMed]
- Choi H, Kim K. Theranostics for Triple-Negative Breast Cancer. Diagnostics (Basel) 2023;13:272. [Crossref] [PubMed]
- Lin Y, Zhang J, Li Y, et al. CTPS1 promotes malignant progression of triple-negative breast cancer with transcriptional activation by YBX1. J Transl Med 2022;20:17. [Crossref] [PubMed]
- Hu H, Kaklamani V. Updates on the preoperative immunotherapy for triple-negative breast cancer. Transl Breast Cancer Res 2023;4:17. [Crossref] [PubMed]
- Song XQ, Shao ZM. Identification of immune-related prognostic biomarkers in triple-negative breast cancer. Transl Cancer Res 2024;13:1707-20. [Crossref] [PubMed]
- Partridge AH, Burstein HJ, Winer EP. Side effects of chemotherapy and combined chemohormonal therapy in women with early-stage breast cancer. J Natl Cancer Inst Monogr 2001;135-42. [Crossref] [PubMed]
- Ashrafi F, Salmasi M. Comparison of the effects of pegylated granulocyte-colony stimulating factor and granulocyte-colony stimulating factor on cytopenia induced by dose-dense chemotherapy in breast cancer patients. J Res Med Sci 2018;23:73. [Crossref] [PubMed]
- Li N, Wang Y, Wang A, et al. STS1 and STS2 Phosphatase Inhibitor Baicalein Enhances the Expansion of Hematopoietic and Progenitor Stem Cells and Alleviates 5-Fluorouracil-Induced Myelosuppression. Int J Mol Sci 2023;24:2987. [Crossref] [PubMed]
- Tian H, Qin W, Wu W, et al. Effects of Traditional Chinese Medicine on Chemotherapy-Induced Myelosuppression and Febrile Neutropenia in Breast Cancer Patients. Evid Based Complement Alternat Med 2015;2015:736197. [Crossref] [PubMed]
- Wang WH, You LL, Huang KZ, et al. A nomogram model for predicting ocular GVHD following allo-HSCT based on risk factors. BMC Ophthalmol 2023;23:28. [Crossref] [PubMed]
- Chun YS, Pawlik TM, Vauthey JN. 8th Edition of the AJCC Cancer Staging Manual: Pancreas and Hepatobiliary Cancers. Ann Surg Oncol 2018;25:845-7.
- World Health Organization. Haemoglobin concentrations for the diagnosis of anaemia and assessment of severity. 2011.
- Zheng RS, Chen R, Han BF, et al. Cancer incidence and mortality in China, 2022. Zhonghua Zhong Liu Za Zhi 2024;46:221-31. [Crossref] [PubMed]
- Zhang QL, Wu TT, Han Y, et al. Chemotherapy-Induced Myelosuppression in Esophageal Cancer Patients: Risks and Suggestions for Its Management. Curr Med Sci 2022;42:530-7. [Crossref] [PubMed]
- Du Y, Liu Y, Fang R, et al. Risk Prediction of Myelosuppression Following First-line Chemotherapy in Colorectal Cancer. J Cancer 2025;16:1379-96. [Crossref] [PubMed]
- Cui L, Huang J, Zhan Y, et al. Association between the genetic polymorphisms of the pharmacokinetics of anthracycline drug and myelosuppression in a patient with breast cancer with anthracycline-based chemotherapy. Life Sci 2021;276:119392. [Crossref] [PubMed]
- Zhou J, Niu G, Pei Y, et al. The effect and clinical efficacy of lienal polypeptide injection combined with FOLFOX chemotherapy regimen in colon cancer patients. Oncol Lett 2016;12:3191-4. [Crossref] [PubMed]
- Wang XB, Wu DJ, Chen WP, et al. Impact of radiotherapy on immunological parameters, levels of inflammatory factors, and clinical prognosis in patients with esophageal cancer. J Radiat Res 2019;60:353-63. [Crossref] [PubMed]
- Dong Y, Hu C, Liu J, et al. Construction of an auxiliary scoring model for myelosuppression in patients with lung cancer chemotherapy based on random forest algorithm. Am J Transl Res 2023;15:4155-63.
- Bendre MS, Margulies AG, Walser B, et al. Tumor-derived interleukin-8 stimulates osteolysis independent of the receptor activator of nuclear factor-kappaB ligand pathway. Cancer Res 2005;65:11001-9. [Crossref] [PubMed]
- Chen WZ, Shen JF, Zhou Y, et al. Clinical characteristics and risk factors for developing bone metastases in patients with breast cancer. Sci Rep 2017;7:11325. [Crossref] [PubMed]
- Larsen MS, Yde CW, Christensen IJ, et al. Carboplatin treatment of antiestrogen-resistant breast cancer cells. Int J Oncol 2012;41:1863-70. [Crossref] [PubMed]
- Egger SJ, Chan MMK, Luo Q, et al. Platinum-containing regimens for triple-negative metastatic breast cancer. Cochrane Database Syst Rev 2020;10:CD013750. [Crossref] [PubMed]
- He SS, Wu QJ, Gong CY, et al. Enhanced efficacy of combination therapy with adeno-associated virus-delivered pigment epithelium-derived factor and cisplatin in a mouse model of Lewis lung carcinoma. Mol Med Rep 2014;9:2069-76. [Crossref] [PubMed]
- Langer CJ, Manola J, Bernardo P, et al. Cisplatin-based therapy for elderly patients with advanced non-small-cell lung cancer: implications of Eastern Cooperative Oncology Group 5592, a randomized trial. J Natl Cancer Inst 2002;94:173-81. [Crossref] [PubMed]
- Chirgwin J, Chua SL. Management of breast cancer with nanoparticle albumin-bound (nab)-paclitaxel combination regimens: a clinical review. Breast 2011;20:394-406. [Crossref] [PubMed]
- Bond TC, Szabo E, Gabriel S, et al. Meta-analysis and indirect treatment comparison of lipegfilgrastim with pegfilgrastim and filgrastim for the reduction of chemotherapy-induced neutropenia-related events. J Oncol Pharm Pract 2018;24:412-23. [Crossref] [PubMed]
- Epstein RS, Basu Roy UK, Aapro M, et al. Cancer Patients' Perspectives and Experiences of Chemotherapy-Induced Myelosuppression and Its Impact on Daily Life. Patient Prefer Adherence 2021;15:453-65. [Crossref] [PubMed]
- Ramalingam SS, Khuri FR. The role of the taxanes in the treatment of older patients with advanced stage non-small cell lung cancer. Oncologist 2009;14:412-24. [Crossref] [PubMed]
- Ma K, Li J, Shen G, et al. Development and Validation of a Risk Nomogram Model for Predicting Contrast-Induced Acute Kidney Injury in Patients with Non-ST-Elevation Acute Coronary Syndrome Undergoing Primary Percutaneous Coronary Intervention. Clin Interv Aging 2022;17:65-77. [Crossref] [PubMed]
- Long J, Wang X, Yuan J, et al. Reference intervals of complete blood count parameters for individuals aged 80 to 89 years in Guizhou, China: A STROBE-compliant retrospective study. Medicine (Baltimore) 2022;101:e30859. [Crossref] [PubMed]
- Wang K, Jiang L, Hu A, et al. Vertebral-specific activation of the CX3CL1/ICAM-1 signaling network mediates non-small-cell lung cancer spinal metastasis by engaging tumor cell-vertebral bone marrow endothelial cell interactions. Theranostics 2021;11:4770-89. [Crossref] [PubMed]
- Zhang X, Fang H, Zeng Z, et al. Preoperative Prognostic Nutrition Index as a Prognostic Indicator of Survival in Elderly Patients Undergoing Gastric Cancer Surgery. Cancer Manag Res 2021;13:5263-73. [Crossref] [PubMed]
- Bade AB, Mega TA, Negera GZ. Malnutrition is Associated with Delayed Sputum Culture Conversion Among Patients Treated for MDR-TB. Infect Drug Resist 2021;14:1659-67. [Crossref] [PubMed]



