
Construction of a prognostic model for lung adenocarcinoma based on necroptosis genes and its exploration of the potential for tumor immunotherapy
Highlight box
Key findings
• This study identified two different subtypes of lung adenocarcinoma (LUAD), which are characterized by differences in the expression of immune related and necroptosis related genes. Incorporating gene set based subtyping into clinical practice can lead to more accurate prognostic analysis.
• A prognostic model based on two gene sets was developed, which can predict the 1-, 3-, 5-year survival rate and chemotherapy effect of LUAD patients. The model has been verified by many parties that the prognostic model has stable predictive ability in LUAD.
What is known and what is new?
• Necroptosis-related genes (NRGs) are crucial for the onset and progression of LUAD.
• Employing NRGs to develop a prognostic model shows that it is linked to LUAD prognosis.
What is the implication, and what should change now?
• It can effectively predict the prognosis and tumor immune microenvironment of LUAD patients, and provide theoretical support for individualized treatment. Moreover, studies have shown that this prognostic model has stable predictive ability in LUAD.
Introduction
According to the latest statistics from 2022, lung cancer remains the most prevalent malignancy worldwide. It holds the highest global incidence and mortality rates (1). Histologically, lung cancer is classified into small cell lung cancer (SCLC) and non-small cell lung cancer (NSCLC) (2). Among NSCLC subtypes, lung adenocarcinoma (LUAD) is the most prevalent, representing around 40% of all cases (3,4). LUAD’s high incidence and mortality are often attributed to treatment resistance and a generally poor prognosis (5). Despite advancements in chemotherapy, radiotherapy, immunotherapy, and other therapeutic approaches, the overall survival (OS) rate for patients with LUAD remains under five years (6).
Necroptosis is a newly defined mode of programmed cell death that is regulated by receptor-interacting protein kinase 1 (RIPK1) and receptor-interacting protein kinase 3 (RIPK3) as well as downstream effector mixed lineage kinase domain-like protein (MLKL) (7). Unlike traditional apoptosis or necrosis, necroptosis is characterized by membrane disruption and the initiation of an inflammatory response. Although it is a regulated pathway, necroptosis inherently provokes inflammation (8). Recent studies have shown that necroptosis contributes to the development and progression of numerous diseases, including renal ischemia-reperfusion injury (9), sepsis (10) and cancers (11).
Furthermore, a growing body of research (12-14) has uncovered necroptosis-related gene signatures and subtypes that hold promise as prognostic markers and predictors of therapeutic response across multiple tumor types, such as breast cancer (15), clear cell renal carcinoma (11), and pancreatic cancer (16). However, the specific role of necroptosis in LUAD remains poorly understood. We conducted a systematic bioinformatics analysis using publicly available transcriptomic data from The Cancer Genome Atlas (TCGA). A two-gene signature model was constructed, and patients were divided into risk subgroups for survival analysis, immune infiltration analysis and correlation analysis of tumor microenvironment (TME), as well as common drug sensitivity prediction. Then, the mRNA-miRNA-lncRNA regulatory axis associated with LUAD prognosis was constructed. Finally, we validated the modeled genes using the Gene Expression Omnibus (GEO) database and real-time quantitative polymerase chain reaction (RT-qPCR). Overall, the prognostic model based on necrosis-associated genes can effectively predict the prognosis of LUAD patients and provide theoretical support for individualized treatment. We present this article in accordance with the TRIPOD reporting checklist (available at https://tcr.amegroups.com/article/view/10.21037/tcr-24-2165/rc).
Methods
Data acquisition and preprocessing
Transcriptomic and clinical data for LUAD patients were retrieved from both TCGA (https://portal.gdc.cancer.gov/) and the GEO (https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi). The TCGA-LUAD dataset included 541 tumor samples and 59 normal samples. Post-download, raw expression data were converted from Ensembl identifiers to gene symbols and normalized. Samples with incomplete survival data, gene expression profiles, or clinical status were excluded from further analysis. From the GSE68571 dataset, 86 LUAD and 10 non-cancer control samples were selected. Additionally, 417 necroptosis-related genes (NRGs) were identified, including 397 genes screened with a relevance score ≥0.4 from GeneCards (https://www.genecards.org/) and 20 downloaded from Harmonizome (http://xena.ucsc.edu/). Lastly, gene-level copy number data were downloaded from the UCSC Xena platform. The study was conducted in accordance with the Declaration of Helsinki and its subsequent amendments.
Analysis of variance and copy number variation (CNV) analysis
We employed the R package “limma” to identify NRGs, applying the criteria of |log fold change (FC)| >1 and false discovery rate (FDR) <0.05. The results were depicted using forest and volcano plots. Additionally, a univariate Cox proportional hazards analysis was conducted using the R packages “limma”, “survival”, and “survminer” to assess the associations between gene expression, survival time, and clinical status. Furthermore, CNV data for LUAD-associated genes were obtained, and the frequencies of CNVs in prognostically relevant genes were calculated. These data were visualized using the R package “Circos”, highlighting both CNV frequencies and the corresponding chromosomal locations.
Molecular subtyping of LUAD and gene set variation analysis (GSVA) analysis
Prognostically significant necroptosis- and apoptosis-related genes (P<0.05) were first identified through univariate Cox analysis. To perform unsupervised hierarchical clustering, we utilized the “ConsensusClusterPlus” package in R, employing the “km” algorithm. Genes were randomly classified 1,000 times, and a consistency matrix was generated. The K-value that achieved the best separation was selected for the final subtype classification. Kaplan-Meier (K-M) survival curves were then plotted using the “survival” and “survminer” R packages and principal component analysis (PCA) was performed. Age, gender, and disease stage were compared across the different subtypes to create a subtype heatmap. Finally, GSVA was conducted using the “GSVA” R packages, with a significance threshold set at P<0.05.
Construction and validation of prognostic models
Utilizing the combined dataset from TCGA and GSE68571, which included DENRG expression data and patient survival information, the samples were evenly split into a training set and a test set, each comprising 50% of the total data. Univariate Cox regression analysis was used to identify genes significantly associated with prognosis, and least absolute shrinkage and selection operator (LASSO) regression and multivariate Cox analysis were used to further refine independent necrosis-related prognostic genes in LUAD. Thus, a risk scoring model was established:
where Xi represents the gene expression and Coefi denotes the correlation coefficient of the differentially expressed NRGs (DENRGs). Risk scores were calculated for each sample in the training set. The predictive power of the model was evaluated using K-M survival analysis and ROC curves.
Independent prognostic analysis and nomogram construction
To assess whether the RiskScore could function as an independent prognostic indicator, a multivariate Cox regression analysis was performed. Nomograms were subsequently constructed using the R package “rms” to estimate patient survival probabilities, incorporating patient RiskScore data to facilitate clinical application. The accuracy of these nomograms was evaluated using the R packages “survival”, “survminer”, “timeROC”, and “ggDCA”, which generated calibration plots and decision curve analysis (DCA) to assess the predictive performance for Survival of the patients.
Immunological analysis
Results for immune cell infiltration (ICI) were obtained using CIBERSORT with the “preprocessCore” and “limma” packages, with significance determined by P<0.05. Scoring and assessment of the TME using the ESTIMATE algorithm. To analyze variations in immune cell populations across subtypes, the R packages “GSEABase”, “GSVA”, and “ggpubr” were utilized. The Wilcoxon rank-sum test was applied to compare immune cell differences between risk groups, and the results were visualized using “vioplot”. Correlations between immune cell populations were visualized with the “corrplot” package.
Drug sensitivity analysis
The sensitivity of commonly used cancer therapeutics was assessed using the “OncoPredict” package in R. The Wilcoxon test was used to analyze the differences in drug sensitivity.
Construction of necroptosis-related regulatory axis
In order to predict the miRNA targets of necroptosis-related prognostic gene, we used three miRNA target predicting databases, including miRDB (http://mirdb.org/), StarBase (http://starbase.sysu.edu.cn/), and miRWalk (http://mirwalk.umm.uni-heidelberg.de/). We used two lncRNA databases, StarBase (http://starbase.sysu.edu.cn/) and LncBase module of DIANA tool (https://dianalab.e-ce.uth.gr/html/diana/web/index.php?r=tarbasev8), to predict the lncRNA targets interacting with miRNA.
Statistical analysis
Expression profiling data and clinical information were integrated using Perl (64-bit) version 5.30.0.1, while statistical analyses were performed using R (version 4.0.3). Unless otherwise specified, all parameters were set to default.
Cell culture
Human normal lung epithelial cell line BEAS-2B (Cat. No. SNL-257, Haixing Biosciences, Suzhou, China) and LUAD cell lines A549 (Cat. No. TCH-C116, Haixing Biosciences) and NCL-H1299 (Cat. No. TCH-C269, Haixing Biosciences) were sourced from Suzhou Starfish Biotechnology Co., Ltd. (Suzhou, China). Authentication of these cell lines was rigorously verified through short tandem repeat (STR) analysis, ensuring the integrity and reliability of the cells. All cell lines were cultured in Roswell Park Memorial Institute (RPMI) 1640 medium (GIBCO, Rockville, MD, USA) supplemented with 10% fetal bovine serum (FBS; GIBCO, Rockville, MD, USA) and 1% penicillin-streptomycin (Hyclone, Logan, UT, USA), and maintained in a 5% CO2 incubator at 37 ℃.
RNA Extraction and real-time quantitative polymerase chain reaction (RT-qPCR) analysis
RNA was extracted using Trizol reagent (Cat. No. 15596026CN, Invitrogen, Massachusetts, USA) according to the manufacturer’s protocol. Complementary DNA (cDNA) was synthesized using a reverse transcription kit (Cat. No. RR047A, Takara, Kyoto, Japan). Subsequently, RT-qPCR was performed in triplicate with a PCR kit (Cat. No. RR820A, Takara). Primers were designed using the NCBI database, and the sequences used for determining the relative expression of target RNA are as follows:
PLK1: forward 5'-AAGAGATCCCGGAGGTCTTA-3'; reverse 5'-TCATTCAGGAAAAGGTTGCC-3'.
KL: forward 5'-TGGATCACCATCGACAACCC-3'; reverse 5'-GACTTTGGCATGAGCCAGGA-3'.
GAPDH: forward 5'-TAAAGGCATCCTGGGCTACACT-3'; reverse 5'-TTACTCCTTGGAGGCCATGTAG-3'.
Results
Identification of DENRGs and CNV analysis
The expression profiles of 417 necrosis-associated genes were analyzed, and 88 genes that were significantly differentially expressed between normal (n=59) and tumor samples (n=541) were identified. Of these, 61 genes were upregulated and 27 were downregulated, with the 50 most significantly altered genes displayed in heatmaps and volcano plots (Figure 1A,1B). Univariate Cox regression analysis further identified eight differentially expressed DENRGs as being significantly associated with patient prognosis. Among these, five genes with hazard ratios (HRs) greater than 1 were classified as high-risk, while three genes with HR less than 1 were considered low-risk, as shown in the forest plot (Figure 1C). CNV analysis revealed that the majority of differential DENRGs exhibited copy number gains, while a smaller subset, including CDC7 and KL, showed copy number losses (Figure 1D). These genes were mapped across various chromosomes, as depicted in Figure 1E. Additionally, a prognostic network map (Figure 1F) illustrated the interactions and prognostic relevance of the identified NRGs in patients with LUAD.
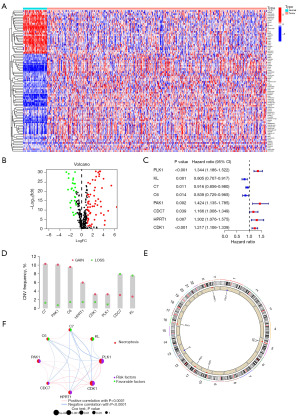
Pathway analysis of LUAD based on NRGs for sample typing and staging
It was determined that the optimal number of clusters provided relatively stable clustering results when the optimal number of clusters was 2 according to the cumulative distribution function (CDF) curve (Figure 2A,2B). PCA showed that the expression of these 8 DENRGs could clearly classify the LUAD samples into two different subtypes (Figure 2C). The K-M curves showed that patients in group B had a longer median survival time than those in group A (P<0.001) (Figure 2D). Further differential expression analysis demonstrated that all eight DENRGs showed significant variation between the two subtypes (Figure 2E). In subtype B, three genes—KL, C7, and C6—were upregulated, while five genes—PAK1, HPRT1, CDC7, PLK1, and CDK1—were downregulated (Figure 2F). These findings suggest that patients in subtype B may have a more favorable prognosis. GSVA of pathways (Figure 2G) revealed that immune-related pathways, such as arachidonic acid metabolism and cell adhesion molecules (CAMs), were enriched in subtype B, while pathways related to the cell cycle, proteasome, and pyrimidine metabolism were suppressed.
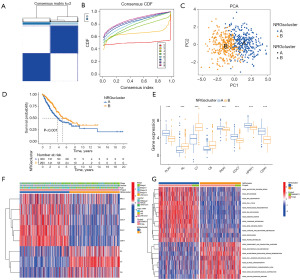
Construction of prognostic models for NRGs
Genes with significant impact on prognosis were screened by regularization method in these 8 DENRGs, which reduced the complexity of the model as well as avoided overfitting (Figure 3A,3B). This process ultimately selected two genes, PLK1 and KL, as the key genes for constructing a prognostic model for LUAD (Figure 3C). The RiskScore formula was defined as:
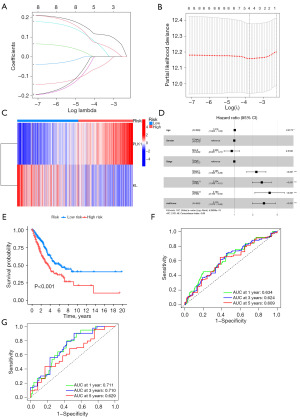
For each patient, gene expression levels were multiplied by their corresponding coefficients, and the sum yielded an individualized risk score. Multivariate Cox regression analysis confirmed that the RiskScore was an independent prognostic factor, unaffected by other clinical variables, and emerged as the strongest predictor of survival outcomes (Figure 3D). The K-M survival curves showed that patients in the low-risk group had a longer OS (Figure 3E). Significant differences in OS were observed, with the low-risk group demonstrating a longer OS. ROC curves revealed area under the curve (AUC) values for 1-, 3-, and 5-year OS of 0.634, 0.624, and 0.609, respectively, in the training group (Figure 3F). Validation of the model in the test cohort produced AUC values of 0.711, 0.710, and 0.629 for 1-, 3-, and 5-year OS, respectively (Figure 3G), closely aligning with the predictions from the training group.
Creation of nomograms
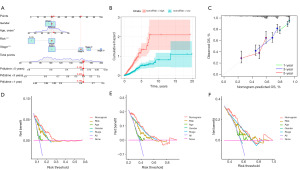
A novel nomogram was developed by integrating age, clinical staging, and RiskScores to predict 1-, 3-, 5-year OS probabilities for individual LUAD patients, offering a valuable tool for guiding clinical decision-making (Figure 4A-4C). Additionally, the reliability of the model was assessed using DCA, which showed (Figure 4D-4F) that the nomogram performed better in predicting OS than other clinical traits, including the RiskScore alone. Based on the findings, this predictive model exhibits reliable prognostic accuracy in LUAD.
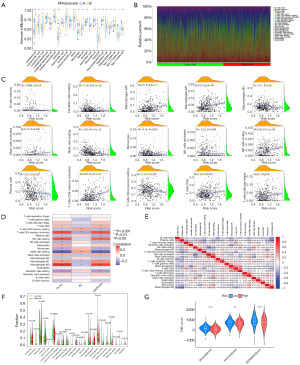
Model-based immunological characterization
The single-sample gene set enrichment analysis (ssGSEA) analysis revealed that several adaptive immune response-associated lymphocyte populations—including activated B cells, immature B cells, bone marrow-derived suppressor cells, macrophages, mast cells, monocytes, natural killer cells, plasma cell-like dendritic cells, regulatory T cells, follicular helper T cells, and type 1 helper T cells—were significantly more abundant in the tissues of patients with subtype B compared to those with subtype A. Conversely, CD56dim natural killer cells and type 2 helper T cells were enriched in group A patients (Figure 5A). Further analysis of ICI between high- and low-risk groups was conducted (Figure 5B). We examined the correlation between immune cell proportions and risk scores. The results (Figure 5C,5D) showed that 15 immune cell types were significantly associated with the risk score, with 7 positively correlated and 8 negatively correlated. Additional analyses examined immune cell infiltration patterns across samples (Figure 5E). The violin plot (Figure 5F) highlighted significant differences in the infiltration levels of 22 immune cell types between the high- and low-risk groups. NRGs were found to be closely associated with ICI, which in turn was linked to tumor progression and prognosis. The comparison of ICI levels revealed that the high-risk group had higher overall infiltration, suggesting that NRGs may influence LUAD prognosis by modulating the tumor immune microenvironment. Consequently, we explored the relationship between the risk score, immune cells, and stromal component ratios. ESTIMATE analysis showed that the ESTIMATEScore, ImmuneScore, and StromalScore were negatively correlated with the risk score, being lower in the high-risk group compared to the low-risk group. This indicates that tumor immunoreactivity was elevated in low-risk patients (Figure 5G). Collectively, these findings indicate an immune-activated state and a more favorable prognosis in low-risk patients, consistent with the characteristics of subtype B.
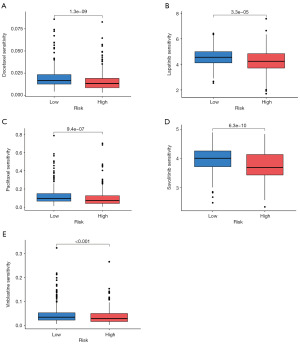
Drug sensitivity analysis
To evaluate the differential chemotherapy responses between the high- and low-risk groups, we conducted a drug sensitivity analysis of commonly used antitumor agents. The analysis revealed significant differences in the sensitivity to five anticancer drugs between the two groups (P<0.001), specifically doxorubicin, lapatinib, paclitaxel, savolitinib, and vincristine (Figure 6A-6E). These agents appear to be more effective in high-risk patients, making them potential therapeutic candidates for individuals within this subgroup. Notably, doxorubicin and paclitaxel, both frontline therapies for NSCLC, exhibited lower half-maximal inhibitory concentration (IC50) values in patients with high NRG scores, suggesting that these patients may derive greater benefit from conventional chemotherapy.
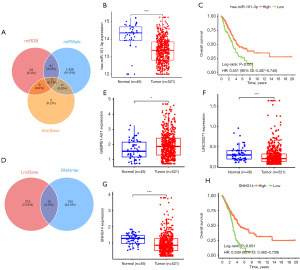
Construction of necroptosis-related regulatory axis
We performed lncRNA-miRNA-mRNA regulatory axis analysis to elucidate the potential molecular mechanisms of KL and PLK1 in LUAD. The miRNA targets of KL and PLK1 were predicted using miRDB, miRWalk and StarBase. Through the database, we identified hsa-miR-101-3p and hsa-miR-296-5p as miRNA targets of KL and PLK1 (Figure 7A). Interestingly, further analysis showed that hsa-miR-101-3p expression was down-regulated in LUAD, and hsa-miR-101-3p high-expressing LUAD patients had better OS rates (Figure 7B,7C). Therefore, hsa-miR-101-3p may be the most promising miRNA target for KL and PLK1. Subsequently, we explored their upstream lncRNA targets using LncBase and RNAInter databases, which showed that 14 lncRNA targets interacted with miR-582-5p, and further analyses showed that SNHG14, GABPB1-AS1, and LINC00271 were upregulated in LUAD compared to lung tissues (Figure 7D-7G). were upregulated, however, only lncRNA SNHG14 was associated with OS rate in LUAD (Figure 7H), suggesting that C5orf64 is the most promising lncRNA target. Thus, the lncRNA SNHG14 /hsa-miR-101-3p/KL/PLK1 regulatory axis may play an important role in the progression of LUAD.
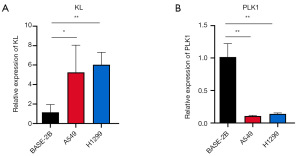
Experimental validation of bioinformatics model reliability
To confirm the reliability of the bioinformatics prediction model, we measured the mRNA expression levels of KL and PLK1 in BEAS-2B, A549, and H1299 cell lines using RT-qPCR. The results revealed a significant increase in KL expression in the LUAD cell lines A549 and H1299 compared to the normal lung epithelial cell line BEAS-2B (Figure 8A). Conversely, PLK1 expression was markedly reduced in the LUAD cell lines (Figure 8B).
Discussion
Necroptosis, a regulated form of cell death that shares features with both necrosis and apoptosis, is distinguished by its independence from caspase activity. Necroptosis in non-transformed cells within the TME may facilitate metastasis by triggering an inflammatory response (17). Given that tumor cells often evade apoptosis, the induction of necroptosis has emerged as a promising therapeutic strategy (18). However, necroptosis exhibits dual effects in various cancers. On one hand, the release of cellular contents from necroptotic tumor cells can activate surrounding immune cells, eliciting inflammatory and immune responses (19,20). Necroptosis can damage normal peri-tumor cells, leading to inflammation that drives tumor progression and metastasis (21).
Moreover, necroptosis contributes tumor sensitivity to immune checkpoint inhibitors by fostering an inflammatory microenvironment (22). All of these suggest a complex interplay between necroptosis and LUAD, although the precise mechanisms remain poorly understood.
The classification of LUAD patients according to different biological features is seen as a promising approach, and it has been widely applied in fields such as ICI (23), tumor mutation burden (TMB) (24), pyroptosis (25) and DNA methylation (26). In this study, we propose a novel approach for defining LUAD subtypes associated with necroptosis by clustering NRGs according to their prognostic and immune features, including TME, ICI, GSVA, and drug sensitivity. Notably, patients classified in subtype B exhibited a longer median survival time compared to those in subtype A.
In addition, TME analysis revealed that both the ImmuneScore and StromalScore were higher in subtype B compared to subtype A. GSVA also identified the activation of immune-related pathways in subtype B, indicating a significant activation of anticancer immune responses in patients with this subtype. Taken together, these findings suggest that subtype B is an immune-activated subtype, associated with a more favorable prognosis for LUAD patients.
The prognostic model we developed incorporates two NRGs: KL and PLK1. The KL gene, first identified in 1997, is located on chromosome 13q12 and spans 50 kb (27). As an anti-aging gene, KL has been shown to inhibit tumor progression (28) and is implicated in various cancers, including breast cancer (29), lung cancer (30), and prostate cancer (31,32). Primarily expressed in the kidney, parathyroid, and brain tissues, KL proteins exert multiple biological functions, including antioxidative stress, inflammation suppression, and regulation of autophagy. The gene plays a key role in diseases such as kidney disorders, cardiovascular diseases, and cancer (33,34). PLK1, located on chromosome 16p12, encodes PLK1, a protein with a molecular weight of approximately 68 kDa (35). It plays a pivotal role in cell mitosis and is one of the central regulators of eukaryotic cell division (36). Overexpression of PLK1 has been implicated in the progression of various cancers (37). Inhibiting PLK1 expression has been shown to effectively suppress cancer cell proliferation, thereby hindering tumor progression (38). For example, elevated PLK1 levels have been associated with poor prognosis in patients with pancreatic ductal adenocarcinoma (PDAC) (39). In LUAD, PLK1 has been found to influence metastasis and immune evasion (40). Additionally, Yan et al. demonstrated that miR-593-5p directly targets PLK1, inhibiting cell proliferation in NSCLC (41). These findings underscore the critical role of PLK1 in multiple tumor types, including LUAD, further supporting the relevance of its inclusion in our prognostic model.
In addition, we developed an innovative nomogram system for predicting the prognosis of LUAD patients. The system combines the risk score with other key clinical characteristics (age and clinical stage) to predict 1-, 3-, and 5-year survival. This tool integrates multiple prognostic factors, which has valuable implications for clinical practice.
Our study identified significant differences in immune function between high- and low-risk groups, suggesting that the poor prognosis in high-risk LUAD patients may be driven by the immunosuppressive nature of the TME. Drug sensitivity analysis revealed that several chemotherapeutic agents, including doxorubicin, lapatinib, paclitaxel, savolitinib, and trametinib, were more effective in high-risk patients, as indicated by significantly lower IC50 values compared to those in the low-risk group. These findings suggest that prognostic risk scores in high-risk patients are correlated with enhanced chemotherapy response, making chemotherapy a more suitable option for this subgroup. Conversely, low-risk patients may derive greater benefit from immunotherapy. Of course, this needs to be explored in follow-up studies.
Another important finding of our study is that we identified the lncRNA SNHG14/hsa-miR-101-3p/KL/PLK1 regulatory axis, which may play a crucial role in the progression of LUAD. Accumulating evidence indicates that aberrant expression of miR-101-3p exerts crucial regulatory functions in the pathogenesis of multiple malignancies (42-45), with its potential as a predictive biomarker for metastatic cancers being supported by numerous studies (46,47). In the field of lung cancer, previous research has established the regulatory role of the GSEC/TYMSOS-miR-101-3p axis in LUAD progression (48-50). Our study systematically expands the molecular mechanisms underlying this regulatory network. Notably, the oncogenic effects of long non-coding RNA SNHG14 in tumor biology have been extensively validated (51). It regulates downstream targets through competitive endogenous RNA (ceRNA) mechanisms, such as driving breast cancer progression via miR-543 sponging to upregulate KLF7 expression (52), promoting colorectal cancer cell proliferation through the miR-519b-3p/DDX5 axis (53), and accelerating hepatocellular carcinoma metastasis via the miR-876-5p/SSR2 signaling axis (54).
Nevertheless, our study has several limitations. While the correlation between necroptosis and LUAD prognosis was validated using data from the TCGA and GEO databases, the relatively small sample size of the LUAD cohort has not yet been corroborated with tissue microarray data. Model construction relies on retrospective data from public databases such as TCGA and GEO, lacks prospective clinical cohort validation, and may be biased. Additional in vivo and in vitro experiments are needed to further substantiate these findings. Moreover, the risk scores require further validation and refinement through more extensive basic research and clinical trials.
In conclusion, the LUAD prognostic model developed in this study, based on NRGs, accurately predicts patient outcomes and sheds light on the tumor immune microenvironment. Compared with previous clinical indicator models, the model’s multiple levels of biological information reflect tumor biological heterogeneity more comprehensively. The model demonstrated relatively stable predictive efficacy in both TCGA and GEO independent cohorts, and significantly improved the accuracy of 1-, 3-, 5-year survival prediction by integrating the risk score with traditional indicators such as clinical staging and age through the column-line diagram tool. This helps clinicians to quickly identify high-risk patients.
Conclusions
This study successfully constructed a prognostic model for LUAD based on NRGs, particularly KL and PLK1. In addition, the model provided a novel regulatory axis involving SNHG14/hsa-miR-101-3p/KL/PLK1, which may affect the progression of LUAD. In summary, the model could be an important tool for improving prognostic prediction and guiding individualized treatment regimens in LUAD, but further validation through larger clinical cohorts and in vivo and in vitro experimental studies are needed to consolidate its clinical applicability.
Acknowledgments
None.
Footnote
Reporting Checklist: The authors have completed the TRIPOD reporting checklist. Available at https://tcr.amegroups.com/article/view/10.21037/tcr-24-2165/rc
Data Sharing Statement: Available at https://tcr.amegroups.com/article/view/10.21037/tcr-24-2165/dss
Peer Review File: Available at https://tcr.amegroups.com/article/view/10.21037/tcr-24-2165/prf
Funding: This work was supported by
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tcr.amegroups.com/article/view/10.21037/tcr-24-2165/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki and its subsequent amendments.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Bray F, Laversanne M, Sung H, et al. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2024;74:229-63. [Crossref] [PubMed]
- Zhang L, Zhang Y, Wang C, et al. Integrated single-cell RNA sequencing analysis reveals distinct cellular and transcriptional modules associated with survival in lung cancer. Signal Transduct Target Ther 2022;7:9. [Crossref] [PubMed]
- Fang H, Sun Q, Zhou J, et al. m(6)A methylation reader IGF2BP2 activates endothelial cells to promote angiogenesis and metastasis of lung adenocarcinoma. Mol Cancer 2023;22:99. [Crossref] [PubMed]
- Zhang Y, Vaccarella S, Morgan E, et al. Global variations in lung cancer incidence by histological subtype in 2020: a population-based study. Lancet Oncol 2023;24:1206-18. [Crossref] [PubMed]
- Yuan C, Chen H, Tu S, et al. A systematic dissection of the epigenomic heterogeneity of lung adenocarcinoma reveals two different subclasses with distinct prognosis and core regulatory networks. Genome Biol 2021;22:156. [Crossref] [PubMed]
- Chen J, Wen Y, Su H, et al. Deciphering Prognostic Value of TTN and Its Correlation With Immune Infiltration in Lung Adenocarcinoma. Front Oncol 2022;12:877878. [Crossref] [PubMed]
- Sprooten J, De Wijngaert P, Vanmeerbeerk I, et al. Necroptosis in Immuno-Oncology and Cancer Immunotherapy. Cells 2020;9:1823. [Crossref] [PubMed]
- Imai T, Lin J, Kaya GG, et al. The RIPK1 death domain restrains ZBP1- and TRIF-mediated cell death and inflammation. Immunity 2024;57:1497-1513.e6. [Crossref] [PubMed]
- Jun W, Benjanuwattra J, Chattipakorn SC, et al. Necroptosis in renal ischemia/reperfusion injury: A major mode of cell death? Arch Biochem Biophys 2020;689:108433. [Crossref] [PubMed]
- Du Y, Zhong Y, Ding R, et al. New insights of necroptosis and immune infiltration in sepsis-induced myocardial dysfunction from bioinformatics analysis through RNA-seq in mice. Front Cell Infect Microbiol 2022;12:1068324. [Crossref] [PubMed]
- Gong Y, Fan Z, Luo G, et al. The role of necroptosis in cancer biology and therapy. Mol Cancer 2019;18:100. [Crossref] [PubMed]
- Lin C, Lin K, Lin X, et al. Necroptosis-related lncRNAs: biomarkers for predicting prognosis and immune response in lung adenocarcinoma. Transl Lung Cancer Res 2024;13:2713-28. [Crossref] [PubMed]
- Zhao X, Zhang X, Li F, et al. Exploration of the prognostic prediction value of the PANoptosis-based risk score and its correlation with tumor immunity in lung adenocarcinoma. J Gene Med 2024;26:e3682. [Crossref] [PubMed]
- Yu Y, Li L, Luo B, et al. Predicting potential therapeutic targets and small molecule drugs for early-stage lung adenocarcinoma. Biomed Pharmacother 2024;174:116528. [Crossref] [PubMed]
- Cho MG, Kumar RJ, Lin CC, et al. MRE11 liberates cGAS from nucleosome sequestration during tumorigenesis. Nature 2024;625:585-92. [Crossref] [PubMed]
- Wu Z, Huang X, Cai M, et al. Novel necroptosis-related gene signature for predicting the prognosis of pancreatic adenocarcinoma. Aging (Albany NY) 2022;14:869-91. [Crossref] [PubMed]
- Najafov A, Chen H, Yuan J. Necroptosis and Cancer. Trends Cancer 2017;3:294-301. [Crossref] [PubMed]
- Zhang Z, Zhang F, Xie W, et al. Induced Necroptosis and Its Role in Cancer Immunotherapy. Int J Mol Sci 2024;25:10760. [Crossref] [PubMed]
- Krysko O, Aaes TL, Kagan VE, et al. Necroptotic cell death in anti-cancer therapy. Immunol Rev 2017;280:207-19. [Crossref] [PubMed]
- Wang KJ, Wang KY, Zhang HZ, et al. Up-Regulation of RIP3 Alleviates Prostate Cancer Progression by Activation of RIP3/MLKL Signaling Pathway and Induction of Necroptosis. Front Oncol 2020;10:1720. [Crossref] [PubMed]
- Zhou Y, Gao W, Xu Y, et al. Implications of different cell death patterns for prognosis and immunity in lung adenocarcinoma. NPJ Precis Oncol 2023;7:121. [Crossref] [PubMed]
- Workenhe ST, Nguyen A, Bakhshinyan D, et al. De novo necroptosis creates an inflammatory environment mediating tumor susceptibility to immune checkpoint inhibitors. Commun Biol 2020;3:645. [Crossref] [PubMed]
- Li C, Pan J, Luo J, et al. Prognostic characterization of immune molecular subtypes in non-small cell lung cancer to immunotherapy. BMC Pulm Med 2021;21:389. [Crossref] [PubMed]
- Zhao Z, He B, Cai Q, et al. Combination of tumor mutation burden and immune infiltrates for the prognosis of lung adenocarcinoma. Int Immunopharmacol 2021;98:107807. [Crossref] [PubMed]
- Li H, Chang X, Wang H, et al. Identification of a prognostic index system and tumor immune infiltration characterization for lung adenocarcinoma based on mRNA molecular of pyroptosis. Front Med (Lausanne) 2022;9:934835. [Crossref] [PubMed]
- Pan X, Zhang C, Wang J, et al. Epigenome signature as an immunophenotype indicator prompts durable clinical immunotherapy benefits in lung adenocarcinoma. Brief Bioinform 2022;23:bbab481. [Crossref] [PubMed]
- Kuro-o M, Matsumura Y, Aizawa H, et al. Mutation of the mouse klotho gene leads to a syndrome resembling ageing. Nature 1997;390:45-51. [Crossref] [PubMed]
- Ligumsky H, Merenbakh-Lamin K, Keren-Khadmy N, et al. The role of α-klotho in human cancer: molecular and clinical aspects. Oncogene 2022;41:4487-97. [Crossref] [PubMed]
- Shmulevich R, Nissim TB, Wolf I, et al. Klotho rewires cellular metabolism of breast cancer cells through alteration of calcium shuttling and mitochondrial activity. Oncogene 2020;39:4636-49. [Crossref] [PubMed]
- Chen B, Huang S, Pisanic Ii TR, et al. Rab8 GTPase regulates Klotho-mediated inhibition of cell growth and progression by directly modulating its surface expression in human non-small cell lung cancer. EBioMedicine 2019;49:118-32. [Crossref] [PubMed]
- Kim HJ, Lee J, Lee SY, et al. The association between KL polymorphism and prostate cancer risk in Korean patients. Mol Biol Rep 2014;41:7595-606. [Crossref] [PubMed]
- Skrajnowska D, Bobrowska-Korczak B, Tokarz A. Disorders of Mechanisms of Calcium Metabolism Control as Potential Risk Factors of Prostate Cancer. Curr Med Chem 2017;24:4229-44. [Crossref] [PubMed]
- Kuro-O M. The Klotho proteins in health and disease. Nat Rev Nephrol 2019;15:27-44. [Crossref] [PubMed]
- Lim K, Halim A, Lu TS, et al. Klotho: A Major Shareholder in Vascular Aging Enterprises. Int J Mol Sci 2019;20:4637. [Crossref] [PubMed]
- Golsteyn RM, Schultz SJ, Bartek J, et al. Cell cycle analysis and chromosomal localization of human Plk1, a putative homologue of the mitotic kinases Drosophila polo and Saccharomyces cerevisiae Cdc5. J Cell Sci 1994;107:1509-17. [Crossref] [PubMed]
- Gelot C, Kovacs MT, Miron S, et al. Polθ is phosphorylated by PLK1 to repair double-strand breaks in mitosis. Nature 2023;621:415-22. [Crossref] [PubMed]
- Jia W, Wu X, Chen Z, et al. Negative regulation of HBG1/2 expression through S6K by long noncoding RNA NR_120526. Transl Pediatr 2023;12:907-17. [Crossref] [PubMed]
- Liang H, Yang C, Zeng R, et al. Targeting CBX3 with a Dual BET/PLK1 Inhibitor Enhances the Antitumor Efficacy of CDK4/6 Inhibitors in Prostate Cancer. Adv Sci (Weinh) 2023;10:e2302368. [Crossref] [PubMed]
- Zhang Z, Cheng L, Li J, et al. Targeting Plk1 Sensitizes Pancreatic Cancer to Immune Checkpoint Therapy. Cancer Res 2022;82:3532-48. [Crossref] [PubMed]
- Jang HR, Shin SB, Kim CH, et al. PLK1/vimentin signaling facilitates immune escape by recruiting Smad2/3 to PD-L1 promoter in metastatic lung adenocarcinoma. Cell Death Differ 2021;28:2745-64. [Crossref] [PubMed]
- Yan L, Zhang Y, Li K, et al. miR-593-5p inhibit cell proliferation by targeting PLK1 in non small cell lung cancer cells. Pathol Res Pract 2020;216:152786. [Crossref] [PubMed]
- Xu Y, Ji T, An N, et al. LINC00943 is correlated with gastric cancer and regulates cancer cell proliferation and chemosensitivity via hsa-miR-101-3p. Int J Clin Oncol 2021;26:1650-60. [Crossref] [PubMed]
- Rao X, Cao H, Yu Q, et al. NEAT1/MALAT1/XIST/PKD--Hsa-Mir-101-3p--DLGAP5 Axis as a Novel Diagnostic and Prognostic Biomarker Associated With Immune Cell Infiltration in Bladder Cancer. Front Genet 2022;13:892535. [Crossref] [PubMed]
- Eun JW, Ahn HR, Baek GO, et al. Aberrantly Expressed MicroRNAs in Cancer-Associated Fibroblasts and Their Target Oncogenic Signatures in Hepatocellular Carcinoma. Int J Mol Sci 2023;24:4272. [Crossref] [PubMed]
- Chakravarthi BV, Goswami MT, Pathi SS, et al. MicroRNA-101 regulated transcriptional modulator SUB1 plays a role in prostate cancer. Oncogene 2016;35:6330-40. [Crossref] [PubMed]
- Lin Y, Chen F, Shen L, et al. Biomarker microRNAs for prostate cancer metastasis: screened with a network vulnerability analysis model. J Transl Med 2018;16:134. [Crossref] [PubMed]
- Duca RB, Massillo C, Dalton GN, et al. MiR-19b-3p and miR-101-3p as potential biomarkers for prostate cancer diagnosis and prognosis. Am J Cancer Res 2021;11:2802-20. [PubMed]
- Su Z, Wang Y, Cao J, et al. Identification and validation of non-coding RNA-mediated high expression of IQGAP3 in poor prognosis of lung adenocarcinoma. J Gene Med 2024;26:e3664. [Crossref] [PubMed]
- Chen X, Guo J, Zhou F, et al. Over-Expression of Long Non-Coding RNA-AC099850.3 Correlates With Tumor Progression and Poor Prognosis in Lung Adenocarcinoma. Front Oncol 2022;12:895708. [Crossref] [PubMed]
- Wang H, Tang F, Tang P, et al. Noncoding RNAs-mediated overexpression of KIF14 is associated with tumor immune infiltration and unfavorable prognosis in lung adenocarcinoma. Aging (Albany NY) 2022;14:8013-31. [Crossref] [PubMed]
- Shen S, Wang Y, Zhang Y, et al. Long Non-coding RNA Small Nucleolar RNA Host Gene 14, a Promising Biomarker and Therapeutic Target in Malignancy. Front Cell Dev Biol 2021;9:746714. [Crossref] [PubMed]
- Zhang D, Ding X, Peng M. LncRNA SNHG14 accelerates breast cancer progression through sponging miR-543 and regulating KLF7 expression. Arch Gynecol Obstet 2022;305:1507-16. [Crossref] [PubMed]
- Wang X, Yang P, Zhang D, et al. LncRNA SNHG14 promotes cell proliferation and invasion in colorectal cancer through modulating miR-519b-3p/DDX5 axis. J Cancer 2021;12:4958-70. [Crossref] [PubMed]
- Liao Z, Zhang H, Su C, et al. Long noncoding RNA SNHG14 promotes hepatocellular carcinoma progression by regulating miR-876-5p/SSR2 axis. J Exp Clin Cancer Res 2021;40:36. [Crossref] [PubMed]


