
Effect of periplocin on malignant behavior of oral squamous cell carcinoma cells
Highlight box
Key findings
• This study indicates that periplocin exerts anti- oral squamous cell carcinoma (OSCC) effects by modulating key cellular processes in OSCC cells, offering a promising therapeutic avenue for OSCC management.
What is known and what is new?
• Previous studies have demonstrated that periplocin exhibits therapeutic potential by inhibiting cancer cell proliferation and inducing apoptosis across various cancer types.
• This study revealed the anticancer effects of periplocin on OSCC cells.
What is the implication, and what should change now?
• This study provides a promising therapeutic pathway for OSCC management.
Introduction
Study indicates that in 2020, there were 377,713 new cases of oral cancer and 177,757 associated deaths worldwide (1). Oral squamous cell carcinoma (OSCC), originating from epithelial cells, is the most prevalent malignant tumor in the oral and maxillofacial regions. Risk factors for OSCC include betel nut chewing, alcohol consumption, tobacco use, malnutrition, smoking, free radicals, and human papilloma virus (HPV) infection (2,3). The prognosis of patients with OSCC is poor, with high rates of recurrence and metastasis (4,5). Current treatments for OSCC involve surgery, radiotherapy, and combined chemotherapy (6). Unfortunately, many OSCC cases are diagnosed at advanced stages, limiting treatment options to chemotherapy. However, chemotherapy often induces rapid drug resistance, reducing patient compliance and therapeutic efficacy. Thus, there is an urgent need to identify more effective chemotherapeutic agents.
Research on the anticancer effects of traditional Chinese medicine extracts has increased significantly in recent years. Yang et al. (7) reported that oridonin inhibits OSCC cell growth by suppressing the PI3K/Akt signaling pathway. Peng et al. (8) demonstrated that pomegranate extract (POMx) promotes apoptosis in OSCC cells and inhibits tumor growth through mitochondrial damage mechanisms. Collectively, traditional Chinese medicine extracts have shown potential value in anticancer therapies.
Cortex periplocae is a traditional Chinese medicinal herb derived from the dried root of Periploca sepium Bge. Periplocin, a group of cardiac glycosides found in cortex periplocae, has demonstrated anticancer properties in various cancer cells (9-13). However, the detailed mechanisms underlying the anticancer effects of periplocin in OSCC remain unclear.
In this study, we investigated the inhibitory effects of periplocin on the malignant behaviors of OSCC cells. Additionally, we explored the relevant mechanisms using proteomics and bioinformatics technologies. We present this article in accordance with the MDAR reporting checklist (available at https://tcr.amegroups.com/article/view/10.21037/tcr-24-2025/rc).
Methods
Cell culture
We used OSCC cell lines SCC-15 (Saibaikang Biotechnology Company, Shanghai, China) and CAL-27 (Wuhan Punosai Life Technology Company, Wuhan, China) in this study. SCC-15 and CAL-27 cells were cultured in Dulbecco’s Modified Eagle Medium (DMEM) supplemented with 10% fetal bovine serum (FBS). The cells were maintained as monolayers at 37 ℃ in a humidified atmosphere containing 5% CO2. In this study, the OSCC cell lines SCC-15 (Saibaikang Biotechnology Company) and CAL-27 (Wuhan Punosai Life Technology Company) were without mycoplasma contamination in this study
Reagents and antibodies
Periplocin (C36H56O13, purity ≥96%) was dissolved in normal saline and stored at 4 ℃.
Antibodies for western blotting: Bax (50599-2-lg, Proteintech, Wuhan, China), Bcl-2 (12789-1-AP, Proteintech), Bad (WL02140, Wanleibio, Shenyang, China), Caspase3 (T40044F, Ab-mart), Cleaved-caspase3 (WL01992, Wanleibio), CDK1 (WL02373, Wanleibio), CDK2 (WL01543, Wanleibio), E-cadherin (WL01482, Wanleibio), N-cadherin (WL01047, Wanleibio), Syndecan 1 (SDC1) (DF12066, Affinity, Jiangsu, China), coiled-coil-helix-coiled-coil-helix domain containing 2 (CHCHD2) (DF6367, Affinity), β-actin (GB15003, Servicebio, Wuhan, China), Goat anti-Rabbit IgG (H + L) HRP conjugate (A23920, Abbkine, Wuhan, China).
MTS [3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2-H-tetrazolium bromide] assay
SCC-15 and CAL-27 cells were seeded in 96-well plates at approximately 6×103 cells per well. The cells were divided into control and periplocin groups. The control group was treated with normal saline, while the periplocin groups were treated with periplocin at concentrations of 50, 100, 200, and 400 ng/mL, respectively.
After 24, 48, and 72 h of treatment, 10 µL of MTS solution was added to each well and incubated in the dark for 2 h. The absorbance value (OD value) at 492 nm was measured using an enzyme-labelling instrument. The inhibition rate was calculated as follows: [1 − (OD periplocin group − OD blank control group)/(OD normal saline group OD − blank control group)] × 100%.
Colony formation assay
After treatment of OSCC cells with 0, 50 and 100 ng/mL periplocin for 48 h, the cells were digested with trypsin and seeded into 6-well plates at 5×103 cells per well. The cells were incubated in DMEM medium for 10–14 days, with the medium replaced every 3–5 days. Upon visible colony formation, the cells were fixed with 700 µL of 4% paraformaldehyde for 20 minutes and stained with 700 µL of 1% crystal violet solution (Solarbio, Beijing) for 15 minutes. The crystal violet solution was removed by gently rinsing with running tap water, and the plates were allowed to dry naturally before imaging and analysis.
Flow cytometry assay
SCC-15 and CAL-27 cells were seeded in 6-well plates at approximately 3×105 cells per well. After cell attachment, the cells were treated with different concentrations of periplocin (0, 50, and 100 ng/mL) for 48 h.
For the apoptosis assay, cells were resuspended in 500 µL of 1× binding buffer and incubated with 5 µL of Annexin V-PE and 5 µL of 7-AAD reagent for 15 min at room temperature. For the cell cycle assay, after washing the cells with phosphate-buffered saline (PBS), 700 µL of propidium iodide staining solution was added and incubated for 30 min at room temperature in the dark. Flow cytometry analysis was then performed using FACSCalibur (USA).
Transwell migration assay
SCC-15 and CAL-27 cells were seeded in 6-well plates at approximately 3×105 cells per well. After cell attachment, the cells were treated with different concentrations of periplocin (0, 50, and 100 ng/mL) for 48 h.
A Boyden chamber with a pore size of 8 µm was used, and 1×105 cells were added to the upper chamber, which was supplemented to 200 µL with serum-free DMEM. The lower chamber was filled with 600 µL of DMEM containing 10% FBS. After 24 h incubation in a 5%CO2 incubator at 37 ℃, the cells were fixed with 4% paraformaldehyde for 20 min and stained with 1% crystal violet solution at room temperature for 15 min. After washing the Boyden chamber with PBS, the cells on the upper surface of the membrane were gently removed with a cotton ball, and the migrated cells were imaged using a microscope.
Proteomic analysis
Tandem mass tag (TMT) quantitative proteomics technology was used to analyze the changes in protein expression of OSCC cells treated with periplocin. CAL-27 cells treated with periplocin at concentrations of 0 and 100 ng/mL were used for proteomic analysis, following previously established methods (14).
Western blotting analysis of apoptosis, cell cycle, migration, and key differentially expressed proteins (DEPs)
Total proteins extracted with RIPA lysis buffer (RIPA:cocktail =100:10) were separated by 10% SDS-PAGE and transferred to PVDF membranes. The membranes were blocked with 5% skim milk for 2 h, and primary antibodies were incubated overnight at 4 ℃. The membranes were then washed with 1×TBST and incubated with a Goat anti-Rabbit IgG (H + L) HRP conjugate secondary antibody. Antibody for western blotting: Bax (50599-2-lg, Proteintech, 1:2,000), Bcl-2 (12789-1-AP, Proteintech, 1:1,500), Bad (WL02140, Wanleibio, 1:750), Caspase3 (T40044F, Ab-mart, 1:1,000), Cleaved-caspase3 (WL01992, Wanleibio, 1:750), CDK1 (WL02373, Wanleibio, 1:1,000), CDK2 (WL01543, Wanleibio, 1:1,000), E-cadherin (WL01482, Wanleibio, 1:1,500), N-cadherin (WL01047, Wanleibio, 1:1,500), SDC1 (DF12066, Affinity, 1:1,000), CHCHD2 (DF6367, Affinity, 1:1,000), β-actin (GB15003, Servicebio, 1:2,000), Goat anti Rabbit IgG (H + L) HRP conjugate (A23920, Abbkine, 1:5,000). Following this step, the membranes were washed again with 1×TBST and scanned using an Odyssey Dual-Color Infrared Fluorescence Imaging System (LI-COR, USA). The expression levels of each protein were normalized to β-actin.
Bioinformatic analysis of DEPs
We screened proteins with a ratio of protein expression levels ≥1.5 or ≤0.667 between the two groups as the final differential proteins. After DEPs were identified, heatmaps and gene ontology (GO) enrichment analyses were generated using https://www.bioinformatics.com.cn, an online platform for data analysis and visualization (15). Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway enrichment analysis was conducted using the WebGestalt database (www.webgestalt.org/).
Differential expression of the key proteins SDC1 and CHCHD2 in OSCC tissues and para-carcinoma tissue
The mRNA expression levels of SDC1 and CHCHD2 in OSCC tissues and para-carcinoma tissue from patients with OSCC were obtained from the Xiantao academic database (https://www.xiantaozi.com/).
Predictive value of SDC1 and CHCHD2 in patients with OSCC and their relationships with clinicopathological characteristics
RNA-seq data from The Cancer Genome Atlas-head and neck squamous cell carcinoma (TCGA-HNSC) cohort were downloaded and organized from The Cancer Genome Atlas (TCGA) database (https://portal.gdc.cancer.gov). Clinical information specific to oral cancer sites (alveolar ridge, base) was retained. The pROC package (1.18.0) was used to perform diagnostic receiver operating characteristic (ROC) curve analysis, and the results were visualized using ggplot2 (3.3.6) to evaluate the predictive value of SDC1 and CHCHD2 in patients with OSCC. Additionally, we analyzed the relationship between these two proteins and the clinicopathological features of OSCC.
Statistical analysis
GraphPad Prism 8.3.0 software was used for statistical analysis and graphical presentation. Data were expressed as the mean ± standard deviation (SD). One-way analysis of variance (ANOVA) was performed to analyze differences among multiple groups, while Dunnett’s test was used to compare differences between two groups. A P value <0.05 was considered statistically significant. All experiments were repeated three times.
Results
Periplocin inhibited the proliferation of OSCC cells
As shown in Figure 1A,1B, the two-dimensional and three-dimensional molecular structures of periplocin were obtained from the PubChem database (https://pubchem.ncbi.nlm.nih.gov/).
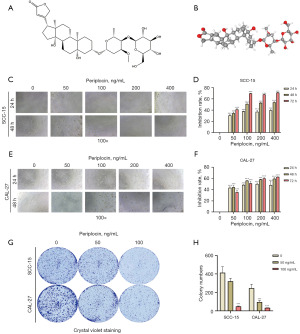
To evaluate the anticancer potential of periplocin in OSCC, we studied its effect on the viability and proliferation of SCC-15 and CAL-27 cells using the MTS assay (Figure 1C-1F). We found that cell proliferation was significantly reduced in a time- and dose-dependent manner after 24, 48, and 72 h of treatment with periplocin (Figure 1D,1F). Moreover, at 24 and 48 h, the sensitivity of CAL-27 cells was higher than that of SCC-15 cells, except for the 24 h 400 ng/mL group. At 72 h, except for the 50 ng/mL group, SCC-15 cells showed higher sensitivity than CAL-27 cells (Figure S1). In terms of cell morphology, cells in the control group exhibited epithelial-like adherent growth with smooth cell walls. However, after periplocin treatment, the number of both cell types decreased to varying degrees, and the number of cell vacuoles and cell debris increased significantly (Figure 1C,1E).
We then performed colony formation assays on both cell lines, and the results showed that the colony formation ability of both cell lines was inhibited after treatment with periplocin (Figure 1G,1H).
Periplocin promoted the apoptosis of OSCC cells
To clarify whether the inhibitory effect of periplocin on OSCC cells is related to cell apoptosis, we treated cells with different concentrations of periplocin (0, 50 and 100 ng/mL) for 48 h. The apoptosis rate of SCC-15 cells treated with 50 and 100 ng/mL periplocin was 7.85% and 27.57%, respectively, while the apoptosis rate of CAL-27 cells was 4.23% and 22.28%, respectively (Figure 2A,2B). We found that the apoptosis rate of OSCC cells significantly increased after periplocin treatment.
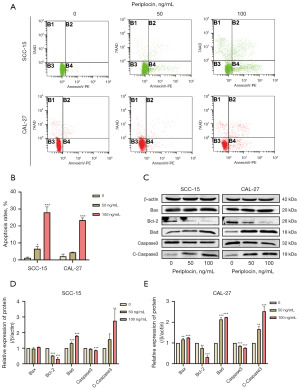
In addition, western blotting results revealed that, after treatment with periplocin, the relative protein expression levels of cleaved caspase 3, Bax, and Bad increased in OSCC cells, while total caspase 3 and the anti-apoptotic protein Bcl2 decreased in a dose-dependent manner (Figure 2C-2E).
Effect of periplocin on the cell cycle of OSCC cells
To explore the relationship between the inhibitory effects of periplocin on OSCC cell proliferation and the cell cycle, SCC-15 and CAL-27 cells were treated with varying concentrations of periplocin (0, 50, and 100 ng/mL) for 48 hours, followed by an analysis of cell cycle distribution. The findings indicated that periplocin induced second gap (G2)/mitosis (M) phase arrest in OSCC cells (Figure 3A-3C).

Subsequent analysis of the relative protein expression levels of cell cycle-associated proteins using western blotting demonstrated a significant, dose-dependent reduction in CDK1 and CDK2 levels (Figure 3D-3F).
Periplocin reduced the migration ability of OSCC cells
We performed a transwell assay to evaluate whether periplocin influences OSCC cell migration.
In the transwell migration assay, the number of OSCC cells passing through the Boyden chamber membrane after periplocin treatment was significantly reduced, indicating a substantial weakening of cell migration ability (Figure 4A,4B).
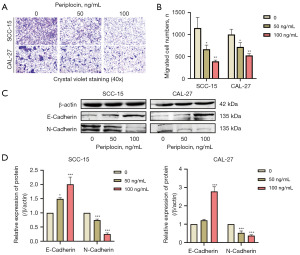
Western blotting results revealed that periplocin treatment led to a dose-dependent increase in the relative protein expression of E-cadherin and a decrease in N-cadherin in OSCC cells (Figure 4C,4D), corroborating the findings of the migration assay.
Proteomic changes in OSCC cells induced by periplocin treatment
Changes in protein expression in CAL-27 cells following periplocin treatment were assessed using TMT-based quantitative proteomics. Principal component analysis (PCA) demonstrated that all samples were distinctly separated in alignment with experimental requirements (Figure 5A).
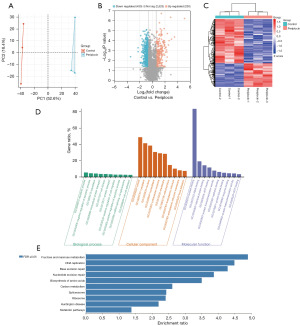
Comparison between the two groups identified 660 significant DEPs, comprising 230 upregulated and 430 downregulated proteins (Figure 5B,5C). Further analysis of these 660 proteins using GO/KEGG enrichment methods revealed the top 10 GO-enriched and KEGG-enriched pathways, as illustrated in the figure. GO enrichment analysis showed that the primary biological processes enriched included apoptosis and its negative regulation. For cellular components, enrichment was observed in the cytoplasm and nucleus, while molecular functions were predominantly enriched for protein binding and RNA binding (Figure 5D). KEGG pathway enrichment analysis demonstrated that these proteins were primarily involved in the fructose and mannose metabolism pathways, DNA replication pathways, and nucleotide excision repair pathways (Figure 5E). Collectively, these findings highlight that the proteomic changes induced by periplocin in OSCC cells are intricately linked to cancer formation and progression.
Analysis, predictive value and verification of key proteins
We selected two key proteins (SDC1 and CHCHD2) from the 660 DEPs identified in the proteomic analysis. These proteins were significantly downregulated in CAL-27 cells treated with periplocin (Figure 6A). The relative protein expression levels of SDC1 and CHCHD2 in SCC-15 and CAL-27 cells after periplocin treatment were validated by western blotting, which demonstrated that the relative protein expression levels of both proteins were significantly downregulated (Figure 6B,6C). These findings corroborated the results of the proteomic analysis.
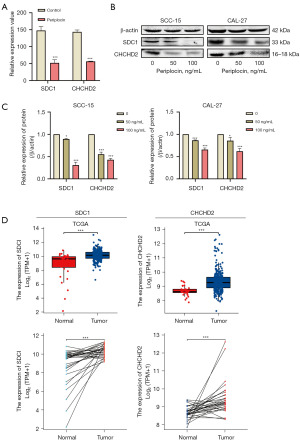
Using the TCGA database, we performed a bioinformatics analysis of these two proteins. Compared with para-carcinoma tissues, SDC1 and CHCHD2 expression were significantly elevated in OSCC tissues (Figure 6D).
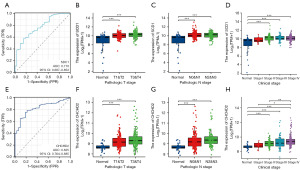
We performed ROC curve analysis to assess the diagnostic value of SDC1 and CHCHD2. The area under curve (AUC) of SDC1 was 0.770 (Figure 7A). We further explored the associations between SDC1 expression and clinicopathological characteristics, including clinical stages I-IV and pathological tumor (T) and node(N) stages, in patients with OSCC based on the TCGA-HNSC database. The analysis revealed that SDC1 expression was elevated in pathological T1&T2, T3&T4, and pathological N0&N1, N2&N3 stages in OSCC tissues (Figure 7B,7C) (P<0.001). Furthermore, the expression of SDC1 was also increased in patients with OSCC with clinical stage II-IV disease (Figure 7D) (P<0.001). The AUC of CHCHD2 was 0.825 (Figure 7E). And the analysis revealed that CHCHD2 expression was elevated in pathological T1&T2, T3&T4, and pathological N0&N1, N2&N3 stages in OSCC tissues (Figure 7F,7G). Furthermore, the expression of CHCHD2 was also increased in patients with OSCC with clinical stage II–IV disease (Figure 7H). Our finding indicating that the expression levels of SDC1 and CHCHD2 effectively distinguished OSCC tissues from normal tissues. Moreover, these findings demonstrate that SDC1 and CHCHD2 possess high diagnostic value in differentiating OSCC tissues from para-carcinoma tissues.
Discussion
The role of periplocin in oral cancer has not been previously reported. However, studies have investigated its effects on other tumor types. In earlier research, periplocin was shown to induce apoptosis via the ERK1/2-EGR1 pathway and enhance the sensitivity of gastric cancer cells to TRAIL, thereby inhibiting their activity (16,17). Periplocin has also been found to suppress pancreatic cancer cell proliferation by inducing autophagy through the AMPK/mTOR pathway (18). These studies suggest that periplocin possesses antitumor effects, which align with our findings. In addition, periplocin functions as a cardiac glycoside, increasing the contractility and calcium transients of cardiomyocytes (19).
Our study demonstrated that periplocin inhibits OSCC cell proliferation and migration, promotes apoptosis, and induces cell cycle arrest at the G2/M phase. Western blotting results revealed increased relative protein expressions of Bad, E-cadherin, Bax/Bcl-2, and cleaved-caspase3/caspase3, alongside decreased expressions of N-cadherin, CDK1, and CDK2.
Bcl-2 influences apoptosis by interacting with pro-apoptotic members of the Bcl-2 family and plays a pivotal role in the apoptotic process (20). Caspase-3 acts as the primary execution protein responsible for proteolytic degradation during apoptosis (21); while total caspase-3 expression generally remains stable or slightly decreases as apoptosis progresses, the expression of cleaved caspase-3 typically increases. Based on these findings, we speculate that periplocin induces apoptosis in OSCC cells through an endogenous mitochondrial pathway.
CDK1 is essential for the G2/M phase transitions of the eukaryotic cell cycle, while CDK2 is a core regulatory factor that governs cell cycle progression and DNA replication. Furthermore, study has demonstrated that CDK1 and CDK2 play pivotal roles in regulating the transition from the G2 phase to the M phase (22), suggesting that periplocin may inhibit OSCC cell growth by affecting the cell cycle through CDK1 and CDK2.
After OSCC cells were treated with periplocin, their migration ability significantly decreased, accompanied by a reduction in N-cadherin protein expression and an increase in E-cadherin expression. Abnormal N-cadherin expression has been observed in various cancers and is closely associated with the transformation, metastasis, and adhesion of human malignant tumors (23). E-cadherin, strongly expressed in normal epithelial cells of most organs, plays a crucial role in maintaining cell adhesion and polarity (24,25). The loss of E-cadherin is often linked to poor cancer prognosis and resistance to chemotherapy drugs (26).
To investigate the potential mechanisms by which periplocin affects OSCC cells, CAL-27 cells were divided into a blank control group and a high-concentration periplocin group (100 ng/mL) for proteomic analysis. GO/KEGG enrichment analysis revealed that the associated proteins were primarily enriched in the apoptosis pathway, fructose and mannose metabolism pathways, and DNA replication pathways. Fructose can be metabolized via multiple pathways, influencing viability and proliferation in cancer cells (27). Study has indicated that fructose metabolism enhances mitochondrial respiration in liver cancer endothelial cells, stimulating tumor angiogenesis (28). Additionally, glucose transporter 5 (GLUT5) plays a critical role in fructose absorption and metabolism and is closely linked to colon cancer, inflammatory bowel disease, intestinal microbiota dysbiosis, and intestinal barrier damage (29).
We conducted western blotting and bioinformatics analyses based on the TCGA-HNSC database to further validate key proteins. SDC1 and CHCHD2 were identified as potential downstream targets of periplocin in OSCC.
CHCHD2 is a mitochondrial protein encoded by the CHCHD2 gene on human chromosome 7p11.2 (30), containing a conserved C-terminal CHCH domain and an N-terminal mitochondrial localization sequence (31). Study has confirmed that mutation in CHCHD2 can lead to Alzheimer’s and Parkinson’s diseases (32). Additionally, Ma et al. (33) reported that CHCHD2 expression levels in breast cancer tissues were significantly higher than in adjacent normal tissues, and the protein inhibited breast cancer cell proliferation and migration. Furthermore, multiple studies have shown that knocking down CHCHD2 reduces the viability and migration of adrenal tumors, liver cancer, glioblastoma, non-small cell lung cancer, and other cancer cells while increasing apoptosis rates (34-40).
SDC1 is a proteoglycan family member primarily responsible for maintaining cell morphology and facilitating cell adhesion (41,42). Previous studies have highlighted the clinical significance of SDC1 in prostate, colorectal, and breast cancers (43-46). Yu et al. (47) demonstrated that knocking down SDC1 resensitized liver cancer cells to cisplatin, suggesting its potential role in overcoming drug resistance. Moreover, research indicates that breast cancer cell migration is influenced by a correlation between SDC1 and E-cadherin-dependent mechanisms (48). However, the potential relationship between E-cadherin and SDC1 in OSCC cells requires further investigation.
In conclusion, the morphology of oral squamous cells changed following treatment with periplocin, proliferation decreased, and apoptosis may have been promoted through the endogenous mitochondrial pathway. Additionally, periplocin reduced the migratory ability of OSCC cells and blocked cell cycle progression, presenting a novel potential therapeutic approach for OSCC.
However, there are some limitations to our study. Firstly, periplocin has only been evaluated in vitro assays and animal experiments, and it has not yet been tested in clinical settings. Further in vivo studies and clinical data collection are required to assess the feasibility of periplocin as a treatment for OSCC. Secondly, periplocin exhibits cardiotoxicity; thus, future research should focus on minimizing its cardiotoxic effects while maintaining its anticancer efficacy.
Conclusions
This study revealed the anticancer effects of periplocin on OSCC cells. However, further investigations are required through in vivo experiments and clinical applications.
Acknowledgments
None.
Footnote
Reporting Checklist: The authors have completed the MDAR reporting checklist. Available at https://tcr.amegroups.com/article/view/10.21037/tcr-24-2025/rc
Data Sharing Statement: Available at https://tcr.amegroups.com/article/view/10.21037/tcr-24-2025/dss
Peer Review File: Available at https://tcr.amegroups.com/article/view/10.21037/tcr-24-2025/prf
Funding: This work was supported by
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tcr.amegroups.com/article/view/10.21037/tcr-24-2025/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Sung H, Ferlay J, Siegel RL, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin 2021;71:209-49. [Crossref] [PubMed]
- Hsiao JR, Chang CC, Lee WT, et al. The interplay between oral microbiome, lifestyle factors and genetic polymorphisms in the risk of oral squamous cell carcinoma. Carcinogenesis 2018;39:778-87. [Crossref] [PubMed]
- Prostakishina EA, Sidenko EA, Kolegova ES, et al. Premalignant lesions of the oral cavity: a narrative review of factors and mechanisms of transformation into cancer. Int J Oral Maxillofac Surg 2024;S0901-5027(24)00472-7.
- Rao SK, Pavicevic Z, Du Z, et al. Pro-inflammatory genes as biomarkers and therapeutic targets in oral squamous cell carcinoma. J Biol Chem 2010;285:32512-21. [Crossref] [PubMed]
- Jagadeesan D, Sathasivam KV, Fuloria NK, et al. Comprehensive insights into oral squamous cell carcinoma: Diagnosis, pathogenesis, and therapeutic advances. Pathol Res Pract 2024;261:155489. [Crossref] [PubMed]
- Chatzistefanou I, Lubek J, Markou K, et al. The role of neck dissection and postoperative adjuvant radiotherapy in cN0 patients with PNI-positive squamous cell carcinoma of the oral cavity. Oral Oncol 2014;50:753-8. [Crossref] [PubMed]
- Yang J, Ren X, Zhang L, et al. Oridonin inhibits oral cancer growth and PI3K/Akt signaling pathway. Biomed Pharmacother 2018;100:226-32. [Crossref] [PubMed]
- Peng SY, Lin LC, Chen SR, et al. Pomegranate Extract (POMx) Induces Mitochondrial Dysfunction and Apoptosis of Oral Cancer Cells. Antioxidants (Basel) 2021;10:1117. [Crossref] [PubMed]
- Zhao R, Han C, Dai S, et al. Inhibitory Effects of Periplocin on Lymphoma Cells: A Network Pharmacology Approach and Experimental Validation. Drug Des Devel Ther 2021;15:1333-44. [Crossref] [PubMed]
- Lin JP, Huang MH, Sun ZT, et al. Periplocin inhibits hepatocellular carcinoma progression and reduces the recruitment of MDSCs through AKT/NF-κB pathway. Life Sci 2023;324:121715. [Crossref] [PubMed]
- Liu X, Liu J, Yan B, et al. Study of the PI3K/Akt/mTOR signaling pathway in vitro and molecular docking analysis of periplocin inhibits cell cycle progression and induces apoptosis in MDA-MB-231. Environ Toxicol 2024;39:444-56. [Crossref] [PubMed]
- Bae ES, Byun WS, Ock CW, et al. Periplocin exerts antitumor activity by regulating Nrf2-mediated signaling pathway in gemcitabine-resistant pancreatic cancer cells. Biomed Pharmacother 2023;157:114039. [Crossref] [PubMed]
- Weng J, Liu H, Wu Z, et al. Periplocin improves the sensitivity of oxaliplatin-resistant hepatocellular carcinoma cells by inhibiting M2 macrophage polarization. Biomol Biomed 2025;25:857-68. [Crossref] [PubMed]
- Cheng Y, Wang G, Zhao L, et al. Periplocymarin Induced Colorectal Cancer Cells Apoptosis Via Impairing PI3K/AKT Pathway. Front Oncol 2021;11:753598. [Crossref] [PubMed]
- Tang D, Chen M, Huang X, et al. SRplot: A free online platform for data visualization and graphing. PLoS One 2023;18:e0294236. [Crossref] [PubMed]
- Li L, Zhao LM, Dai SL, et al. Periplocin Extracted from Cortex Periplocae Induced Apoptosis of Gastric Cancer Cells via the ERK1/2-EGR1 Pathway. Cell Physiol Biochem 2016;38:1939-51. [Crossref] [PubMed]
- Zhao LM, Li L, Huang Y, et al. Antitumor Effect of Periplocin in TRAIL-Resistant gastric cancer cells via upregulation of death receptor through activating ERK1/2-EGR1 pathway. Mol Carcinog 2019;58:1033-45. [Crossref] [PubMed]
- Xie G, Sun L, Li Y, et al. Periplocin inhibits the growth of pancreatic cancer by inducing apoptosis via AMPK-mTOR signaling. Cancer Med 2021;10:325-36. [Crossref] [PubMed]
- Hao J, Chang L, Wang D, et al. Periplocin Alleviates Cardiac Remodeling in DOCA-Salt-Induced Heart Failure Rats. J Cardiovasc Transl Res 2023;16:127-40. [Crossref] [PubMed]
- Raghav PK, Kumar R, Kumar V, et al. Docking-based approach for identification of mutations that disrupt binding between Bcl-2 and Bax proteins: Inducing apoptosis in cancer cells. Mol Genet Genomic Med 2019;7:e910. [Crossref] [PubMed]
- Huang KH, Fang WL, Li AF, et al. Caspase-3, a key apoptotic protein, as a prognostic marker in gastric cancer after curative surgery. Int J Surg 2018;52:258-63. [Crossref] [PubMed]
- Sakurikar N, Eastman A. Critical reanalysis of the methods that discriminate the activity of CDK2 from CDK1. Cell Cycle 2016;15:1184-8. [Crossref] [PubMed]
- Cao ZQ, Wang Z, Leng P. Aberrant N-cadherin expression in cancer. Biomed Pharmacother 2019;118:109320. [Crossref] [PubMed]
- Burandt E, Lübbersmeyer F, Gorbokon N, et al. E-Cadherin expression in human tumors: a tissue microarray study on 10,851 tumors. Biomark Res 2021;9:44. [Crossref] [PubMed]
- Xu X, Ge C, Wang S, et al. Polyamine-modified naphthalimide derivative 9C inhibits colorectal cancer through ROS-mediated ER stress, migration and invasion. Toxicol In Vitro 2025;103:105974. [Crossref] [PubMed]
- Kaneta Y, Sato T, Hikiba Y, et al. Loss of Pancreatic E-Cadherin Causes Pancreatitis-Like Changes and Contributes to Carcinogenesis. Cell Mol Gastroenterol Hepatol 2020;9:105-19. [Crossref] [PubMed]
- Krause N, Wegner A. Fructose Metabolism in Cancer. Cells 2020;9:2635. [Crossref] [PubMed]
- Fang JH, Chen JY, Zheng JL, et al. Fructose Metabolism in Tumor Endothelial Cells Promotes Angiogenesis by Activating AMPK Signaling and Mitochondrial Respiration. Cancer Res 2023;83:1249-63. [Crossref] [PubMed]
- Song A, Mao Y, Wei H. GLUT5: structure, functions, diseases and potential applications. Acta Biochim Biophys Sin (Shanghai) 2023;55:1519-38. [Crossref] [PubMed]
- Liu Y, Clegg HV, Leslie PL, et al. CHCHD2 inhibits apoptosis by interacting with Bcl-x L to regulate Bax activation. Cell Death Differ 2015;22:1035-46. [Crossref] [PubMed]
- Liu Y, Zhang Y. CHCHD2 connects mitochondrial metabolism to apoptosis. Mol Cell Oncol 2015;2:e1004964. [Crossref] [PubMed]
- Shi CH, Mao CY, Zhang SY, et al. CHCHD2 gene mutations in familial and sporadic Parkinson's disease. Neurobiol Aging 2016;38:217.e9-217.e13. [Crossref] [PubMed]
- Ma L, Zheng LH, Zhang DG, et al. CHCHD2 decreases docetaxel sensitivity in breast cancer via activating MMP2. Eur Rev Med Pharmacol Sci 2020;24:6426-33. [Crossref] [PubMed]
- Lumibao JC, Haak PL, Kolossov VL, et al. CHCHD2 mediates glioblastoma cell proliferation, mitochondrial metabolism, hypoxia induced invasion and therapeutic resistance. Int J Oncol 2023;63:117. [Crossref] [PubMed]
- Cheng Q, Qu D, Lu Z, et al. Knockdown of CHCHD2 inhibits migration and angiogenesis of human renal cell carcinoma: A potential molecular marker for treatment of RCC. Oncol Lett 2019;17:765-72. [Crossref] [PubMed]
- Wei Y, Vellanki RN, Coyaud É, et al. CHCHD2 Is Coamplified with EGFR in NSCLC and Regulates Mitochondrial Function and Cell Migration. Mol Cancer Res 2015;13:1119-29. [Crossref] [PubMed]
- Xu R, Wang SY, Wang L, et al. Value of CHCHD2 as a potential marker of non-small cell lung cancer: Analysis of 60 cases. Nan Fang Yi Ke Da Xue Xue Bao 2018;38:329-33. [Crossref] [PubMed]
- Yao Y, Su J, Zhao L, et al. CHCHD2 promotes hepatocellular carcinoma and indicates poor prognosis of hepatocellular carcinoma patients. J Cancer 2019;10:6822-8. [Crossref] [PubMed]
- Yin X, Xia J, Sun Y, et al. CHCHD2 is a potential prognostic factor for NSCLC and is associated with HIF-1a expression. BMC Pulm Med 2020;20:40. [Crossref] [PubMed]
- Karapanagioti A, Nasiri-Ansari N, Moustogiannis A, et al. What is the role of CHCHD2 in adrenal tumourigenesis? Endocrine 2023;81:357-67. [Crossref] [PubMed]
- Saunders S, Jalkanen M, O'Farrell S, et al. Molecular cloning of syndecan, an integral membrane proteoglycan. J Cell Biol 1989;108:1547-56. [Crossref] [PubMed]
- Liao S, Liu C, Zhu G, et al. Relationship between SDC1 and cadherin signalling activation in cancer. Pathol Res Pract 2020;216:152756. [Crossref] [PubMed]
- Kim SY, Choi EJ, Yun JA, et al. Syndecan-1 expression is associated with tumor size and EGFR expression in colorectal carcinoma: a clinicopathological study of 230 cases. Int J Med Sci 2015;12:92-9. [Crossref] [PubMed]
- Szarvas T, Reis H, Vom Dorp F, et al. Soluble syndecan-1 (SDC1) serum level as an independent pre-operative predictor of cancer-specific survival in prostate cancer. Prostate 2016;76:977-85. [Crossref] [PubMed]
- Cui X, Jing X, Yi Q, et al. Clinicopathological and prognostic significance of SDC1 overexpression in breast cancer. Oncotarget 2017;8:111444-55. [Crossref] [PubMed]
- Yen HR, Liao WC, Chen CH, et al. Targeting chondroitin sulfate suppresses macropinocytosis of breast cancer cells by modulating syndecan-1 expression. Mol Oncol 2024;18:2569-85. [Crossref] [PubMed]
- Yu L, Xu H, Zhang S, et al. SDC1 promotes cisplatin resistance in hepatic carcinoma cells via PI3K-AKT pathway. Hum Cell 2020;33:721-9. [Crossref] [PubMed]
- Zhong Y, Li F, Zhang S, et al. Syndecan-1 as an immunogene in Triple-negative breast cancer: regulation tumor-infiltrating lymphocyte in the tumor microenviroment and EMT by TGFb1/Smad pathway. Cancer Cell Int 2023;23:76. [Crossref] [PubMed]


