
Plasma-derived exosomal human epidermal growth factor receptor 2 (HER2) protein for distinguishing breast cancer from benign breast disease and assessing the efficacy of neoadjuvant therapy
Highlight box
Key findings
• The concentration of exosomal human epidermal growth factor receptor 2 (HER2) concentration in patients with breast cancer was significantly higher than that in patients with benign breast disease.
• The concordance between exosomal HER2 levels and HER2 immunohistochemistry (IHC) was 74.51%.
What is known and what is new?
• HER2 overexpression may lead to the carcinogenic transformation of human cells. Overexpression of HER2 in tumors is associated with a poor prognosis, and its expression level can inform decision-making regarding the clinical treatment of breast cancer.
• This study evaluated the diagnostic value of exosomal HER2 in distinguishing breast cancer from benign breast disease and analyzed the concordance between exosomal HER2 and clinical HER2 IHC. Finally, the role of exosomal HER2 in monitoring the efficacy of neoadjuvant therapy for breast cancer treatment was also be examined.
What is the implication, and what should change now?
• A plasma-derived exosomal HER2 test could be used to distinguish breast cancer from benign breast disease, screening potential patients who could benefit from HER2-targeted therapy, and monitoring neoadjuvant therapy. However, prospective studies with larger sample sizes need to be further conducted, and the optimal cutoff value still needs to be determined.
Introduction
Global Cancer Statistics 2020 reported that about 19.3 million new cancer cases occurred in 2020, with female breast cancer being the most commonly diagnosed cancer in the world, accounting for 11.7% of cases (1). Additionally, in 2016, female breast cancer ranked first and fourth in incidence and mortality, respectively, among female malignant tumors in China, seriously threatening women’s health (2,3).
With the developments in the biomedical research, many biomarkers have been found to be associated with diagnosis, prognosis and even treatment options. Lower Cachexia Index levels were significantly associated with shorter overall survival (OS) (4). Non-coding RNAs act as potential mediators of immunotherapy and chemotherapy resistance in lung cancer (5). Molecular profiling including ESR1/PIK3CA mutations, HER2-low and intrinsic subtypes is key for personalizing post-CDK4/6 inhibitors therapy in hormone receptor-positive and HER2-negative metastatic breast cancer (6). Elevated pretreatment neutrophil-to-eosinophil ratio levels indicated poorer OS and progression-free survival (PFS) (7). The identification of reliable prognostic biomarkers is important for refining cancer treatment strategies and decreasing death rate, particularly in the era of precision medicine.
Benign breast diseases are generally divided into three histological categories, including nonproliferative disease (NP), proliferative disease without atypia (PDWA), and proliferative disease with atypia (AH) (8), with an incidence of approximately 66%, 30%, and 4% (9), respectively. AH and PDWA are thought to be associated with an increased risk of breast cancer (10). Overlaps of symptoms between malignant breast tumors and benign breast lesions greatly hinder diagnosis. Breast magnetic resonance imaging (MRI) is an important mammographic imaging modality for the screening, diagnosis, and preoperative staging of breast cancer. Many benign lesions also exhibit strong contrast enhancement, which may lead to a false-positive diagnosis, unnecessary biopsies, or overtreatment. The accurate differentiation between benign and malignant breast tumors can enhance the diagnostic precision of breast cancer, alleviate the medical burden, and ensure that patients receive appropriate treatment.
Only about 15% of women diagnosed with breast cancer are positive for the human epidermal growth factor receptor 2 (HER2) and are eligible for HER2-targeted therapy (11). A series of HER2-targeted therapies including trastuzumab (12) and pertuzumab (13) have significantly improved the clinical outcomes for patients with HER2-positive breast cancer. Approximately 55% of patients with breast cancer typically categorized as HER2 negative show low HER2 expression [HER2 immunohistochemistry (IHC) 1+ or IHC 2+/ in situ hybridization (ISH) −] (14-16) and do not have effective HER2-targeted treatment options (17,18). However, the paradigm of therapy efficacy being closely reliant on HER2 amplification is changing. The activity of trastuzumab deruxtecan (T-DXd), a novel HER2-targeted antibody drug conjugate (ADC), has been observed in patients with HER2 IHC scores of 1+ and 2+ (not overexpressed) (19,20). In the DESTINY-Breast 04 phase III trial, compared to chemotherapy, patients treated with T-DXd showed a 36% reduction in death risk (21). Based on the promising results for T-DXd treatment, clinicians are beginning to differentiate levels of HER2 expression and redefine HER2-low patients who may be eligible for T-DXd treatment (22).
HER2 overexpression, usually caused by gene overexpression (23), may lead to the carcinogenic transformation of human cells, resulting in breast, ovarian, bladder, or other cancers. The HER2 signaling pathway is involved in key processes such as cell proliferation, migration, invasion, and angiogenesis, and its overexpression in tumors is associated with a poor prognosis (24,25). Although IHC and fluorescence or silver in situ hybridization (FISH/SISH) are the standard methods used to assess HER2 overexpression in tumor tissues with significant levels of agreement, there remain a few limitations in the tissue detection of HER2. Population differences, tissue sample processing, and tumor heterogeneity may contribute to variability in HER2 expression results. Moreover, breast cancer is highly heterogeneous, and the sizes of tissue samples greatly influence HER2 expression. In a study of 923 patients, the concordance between biopsy and surgical specimens for HER2 was 68%, and amongst the results for each category, the 1+ group was the least concordant group (37% vs. 83%, 79%, and 97% for the 0, 2+, and 3+ groups respectively) (26). In addition, surveys by the College of American Pathologists from 1,391 to 1,452 laboratories around the world found an only 26% concordance between the 0 and 1+ groups compared with a 58% concordance between the 2+ and 3+ groups (27). Moreover, one-third of the patients had a different HER2 status between resection specimens obtained before and after neoadjuvant chemotherapy, which could influence clinical decision-making should T-DXd be used in early breast cancer treatment (28).
HER2 status is a critical factor to consider in the selection of clinical strategy. There is thus an urgent need to establish a novel, accurate, and sensitive method to assess HER2 expression in patients with breast cancer. Exosomes are extracellular vesicles that are 30–150 nm in size and are widely distributed in various body fluids such as the blood, milk, urine, and saliva (29-31). Exosomes secreted by tumor cells carry tumor-specific proteins and nucleic acids and have been shown to promote the migration and proliferation of tumors (32). The membrane of exosomes has a characteristic lipid bilayer composition, which ensures its integrity and stability, and therefore possesses considerable potential in tumor diagnosis (29,33). HER2 has been detected in exosomes from patients with breast (34), gastric (35), or ovarian cancer (36). Furthermore, it was reported that HER2 is present in serum-derived exosomes from patients with advanced gastric cancer, with the levels being consistent with those in tumor tissues (35).
A good detection method can not only help doctors quickly diagnose the condition and guide medication, but also monitor the patient’s physical state in real time, reflect the prognosis and adjust the treatment plan in time. In the study, we isolated exosomes from patients with benign breast disease or breast cancer and detected the HER2 protein level using magnetic particle chemiluminescence. In order to investigate the potential of exosomal HER2 in breast cancer diagnosis, explore the significance in guiding clinicians in the selection of treatment options, and find out whether changes in exosomal HER2 levels could be used to evaluate the efficacy of neoadjuvant chemotherapy. We compared the exosomal HER2 levels to serum HER2 levels from these samples and determined the diagnostic value of exosomal HER2 in distinguishing breast cancer from benign breast disease. The concordance between exosomal HER2 and clinical HER2 IHC was further probed. Finally, we observed the concentration changes of exosomal HER2 during neoadjuvant therapy and assessed its potential in predicting the efficacy of neoadjuvant therapy for breast cancer. We present this article in accordance with the STARD reporting checklist (available at https://tcr.amegroups.com/article/view/10.21037/tcr-2025-825/rc).
Methods
Patients and samples
Patients with breast cancer and benign breast disease admitted to the First Affiliated Hospital of Soochow University from June 2022 to April 2023 were enrolled in this study. All patients were diagnosed for the first time and were untreated. The primary exclusion criteria were as follows: unavailable IHC or FISH testing for HER2, diagnosis of other malignant tumors within 5 years, and serious infection. For the analysis of concordance between exosomal HER2 levels and HER2 IHC, we enrolled HER2-positive cases (HER2 IHC 3+) and negative controls (HER2 IHC 0, 1+, or IHC 2+). For the evaluation of neoadjuvant therapy efficacy, the following additional inclusion criteria were applied: administration of more than five cycles of neoadjuvant therapy and measurable lesions according to the Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1. The clinical and pathological characteristics of all enrolled patients were recorded. Researchers were blinded to the participants’ information.
Study recruitment was determined by HER2 IHC status, and about 15% of women diagnosed with breast cancer are positive for HER2 (11). We sought to recruit approximately 60 participants, of whom approximately 9 would have undergone neoadjuvant therapy.
The study was conducted in accordance with the Declaration of Helsinki and its subsequent amendments. The study was approved by the ethics committee of the First Affiliated Hospital of Soochow University (No. 2023-357) and informed consent was taken from all the patients.
Exosome enrichment from plasma
Plasma was centrifuged at 1,300 ×g for 10 min followed by 13,000 ×g for 10 min and filtered using a 0.22-µm filter (MilliporeSigma, Burlington, MA, USA). Exosomes were isolated with size exclusion chromatography (SEC). Each SEC column was packed with 5 mL of Capto Core 700 multimodal chromatography resin (GE HealthCare, Chicago, IL, USA) and subsequently loaded with 1 mL of plasma. Exosomes were eluted with phosphate-buffered saline (PBS) according to the 280-nm UV absorbance chromatogram and concentrated using an Amicon Ultra-15 100 kDa molecular weight cutoff spin filter (MilliporeSigma).
Nanoparticle tracking analysis
The size distribution and concentration of exosomes in plasma were measured via nanoparticle tracking analysis (NTA) with a NanoSight NS300 device (Malvern Panalytical, Malvern, UK). Three videos typically 60 s in duration were obtained. Data were analyzed using the NTA 3.0 software (Malvern Panalytical), which was optimized to first identify and then track each particle on a frame-by-frame basis. These experiments were carried out in the nano-biochemical platform at the Suzhou Institute of Nano-Tech and Nano-Bionics (SINANO), Chinese Academy of Sciences.
Western blotting
Following the reported method in the literature (37), we analyzed exosomal proteins using Western blotting to identify exosomes. Enriched exosomes were lysed in RIPA buffer (89900; Thermo Scientific, Waltham, MA, USA) for 20 min on ice, followed by centrifugation at 12,000 ×g at 4 ℃ for 10 min. The samples were subjected to 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and then electrotransferred onto polyvinylidene difluoride (PVDF) membranes for 2 h. The membranes were incubated with 5% bovine serum albumin (BSA) for 1 h to exclude the binding of nonspecific antibodies and then incubated overnight with specific antibodies, including CD63 antibody (133586; Abcam, Cambridge, UK), ALIX antibody (2171s; Cell Signaling Technology, Danvers, MA, USA), and TSG101 antibody (133586; Abcam). Finally, the protein-specific horseradish peroxidase (HRP)-conjugated secondary antibodies were added. The bound antibodies were detected with a Beyo ECL Plus kit (P0018S; Beyotime Biotechnology, Nantong, China) and quantified using the Amersham Imager 600 fluorescence imaging system (Thermo Fisher Scientific).
Detection of HER2
HER2 magnetic particle-based chemiluminescence immunoassay was used to assess the exosomal HER2 and free HER2 levels in the plasma of patients with breast cancer.
The measurement of HER2 calibrators was performed on a Keysmile 500S automatic chemiluminescence immune analyzer (Keysmile Biological Technology, Chongqing, China). The protein sample, detection antibody (10004-R205; Sino Biological, Beijing, China), and capture antibody (10004-RP03; Sino Biological) were added into each reaction tube. After incubation at 30 ℃ for 25 min, streptavidin magnetic particles (11636502103; Roche, Basel, Switzerland) were added into the reaction tubes. Finally, the chemiluminescence substrate solution was added, and the total relative luminous unit (RLU) was measured. Logistic four-parameter fitting was carried out using GraphPad Prism 9.0 (Dotmatics, Boston, MA, USA), and the calibration curve was plotted based on the concentration of HER2 calibrators and the obtained RLUs.
For exosomal HER2 detection, the exosomes concentrated to 100 µL were lysed in 700 µL of M-PER mammalian protein extraction reagent (78501; Thermo Fisher Scientific) and mixed on a vertical mixer at 4 ℃ for 40 min, followed by centrifugation at 12,000 ×g at 4 ℃ for 10 min. The supernatant was used to detect the exosomal HER2 protein. For free HER2, plasma was directly diluted 20-fold with PBS, and HER2 was detected using the same system.
Statistical analysis
Clinicopathological parameters are described as frequencies and percentages. The groups were compared using chi-squared tests. A t-test was used to compare the difference in exosomal HER2 levels between the breast cancer and benign breast disease groups. The sensitivity, specificity, and area under the receiver operator characteristic (ROC) curve (AUC) were calculated to assess the diagnostic value of the exosomal HER2. To analyze the concordance between exosomal HER2 and HER2 IHC, ROC analysis was performed, and the cutoff point of exosomal HER2 was determined using the Youden index. For neoadjuvant efficacy analysis, the clinical effect of neoadjuvant therapy was evaluated with reference to the changes in tumor size according to Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1. The changes in exosomal HER2 and clinical outcomes were compared to evaluate the applicability of exosomal HER2 as a biomarker in neoadjuvant efficacy analysis. GraphPad Prism 9.0 was used for statistical analysis, and a two-sided P value <0.05 was considered to be statistically significant. Indeterminate results were excluded from the final analysis.
Results
Patient characteristics
In this study, 59 patients with untreated breast cancer who were diagnosed for the first time were registered. Of these, 51 patients were eligible for evaluation of the diagnostic value of plasma-derived exosomal HER2, and an additional 36 patients with benign breast disease were also enrolled. Additionally, the exosomal HER2 levels of these 51 patients with breast cancer were compared with the clinical HER2 IHC findings to analyze the concordance. According to the principle of sample and data integrity, 8 patients were selected to evaluate the applicability of exosomal HER2 as a biomarker in neoadjuvant therapy (Figure 1). The baseline characteristics of patients are shown in Table 1. The mean time between neoadjuvant therapy was 21 days. No significant adverse events occurred during the study.
Table 1
| Characteristic | Overall (n=51) | HER2 IHC | P value | |
|---|---|---|---|---|
| 2+/1+/0 (n=35) | 3+ (n=16) | |||
| Age (years), n (%) | 0.20 | |||
| ≤40 | 16 (31.4) | 9 (25.7) | 7 (43.8) | |
| >40 | 35 (68.6) | 26 (74.3) | 9 (56.3) | |
| Tumor stage, n (%) | 0.51 | |||
| I | 4 (7.8) | 4 (11.4) | 0 (0.0) | |
| II | 34 (66.7) | 23 (65.7) | 11 (68.8) | |
| III | 8 (15.7) | 4 (11.4) | 4 (25.0) | |
| IV | 5 (9.8) | 4 (11.4) | 1 (6.3) | |
| ER status, n (%) | <0.001 | |||
| Positive | 35 (68.6) | 29 (82.9) | 6 (37.5) | |
| Negative | 15 (29.4) | 6 (17.1) | 9 (56.3) | |
| Missing | 1 (2.0) | 0 (0.0) | 1 (6.3) | |
| PR status, n (%) | 0.001 | |||
| Positive | 25 (49.0) | 23 (65.7) | 2 (12.5) | |
| Negative | 24 (47.1) | 11 (31.4) | 13 (81.3) | |
| Missing | 2 (3.9) | 1 (2.9) | 1 (6.3) | |
| Ki-67 index, n (%) | 0.46 | |||
| ≥14% | 41 (80.4) | 28 (80.0) | 13 (81.3) | |
| <14% | 8 (15.7) | 7 (20.0) | 1 (6.3) | |
| Missing | 2 (3.9) | 0 (0.0) | 2 (12.5) | |
ER, estrogen receptor; HER2, human epidermal growth factor receptor 2; IHC, immunohistochemistry; PR, progesterone receptor.
Identification and detection of exosomes
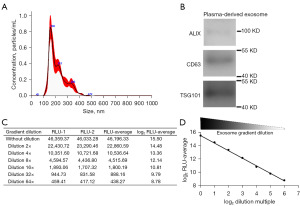
Plasma-derived exosomes from enrolled patients were collected and examined via transmission electron microscopy (TEM) and Western blot analysis. TEM revealed structurally intact exosomes with a probable diameter of 100–200 nm, which is consistent with the reported size of exosomes (Figure 2A). We further conducted Western blot analysis to confirm the presence of several exosome markers, including TSG101, ALIX and CD63. The results showed that ALIX, CD63, and TSG101 were all detected (Figure 2B). In addition, we also constructed a sandwich chemiluminescence detection system to detect exosome markers CD63–CD81. Gradient-diluted exosomes were added to the CD63-coated reaction well, and after incubation and washing, CD81 biotin antibody and streptavidin HRP were added in order. The result showed that the RLU was inversely proportional to the exosome dilution factor, and the higher the dilution factor was, the lower the RLU, indicating simultaneous expression of CD63 and CD81 on the surface of exosomes (Figure 2C,2D). These results confirmed the successful enrichment of exosomes, which could then be further tested.
Diagnostic performance of exosomal HER2
Plasma-derived exosomes were enriched, lysed, and then detected with chemiluminescence detection systems (Figure 3A). In all, 36 samples from patients with benign breast diseases and 51 samples from patients with breast cancer were tested. Unpaired t-test analysis showed that the exosomal HER2 concentration in patients with breast cancer was significantly higher than that in patients with benign breast disease (P=0.001) (Figure 3B) while there was no statistical difference in free HER2 levels (Figure 3C). The ROC analysis of exosomal HER2 was conducted (Figure 3D). The sensitivity was 45.1% (95% CI: 32.27–58.62%) and the specificity was 97.22% (95% CI: 85.83–99.86%), with an optimal cutoff value of 772.7 pg/mL.
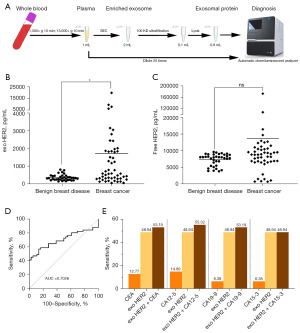
Carcinoembryonic antigen (CEA), carbohydrate antigen (CA)15-3, and CA12-5 have been widely used in the clinical detection of breast cancer, with elevated levels being associated with higher breast cancer stage, tumor size, and worse prognosis (38-41). In this study, due to missing data in some patients, only 47 patients with breast cancer were included in the performance assessment of serum markers. The detection values were obtained from patient medical records. The sensitivity of CEA and CA12-5 was 12.77% (6/47) and 14.89% (7/47), respectively. The sensitivity for each of CA19-9 and CA15-3 was 6.38% (3/47). Due to the decrease in the number of patients, the sensitivity of the exosomal HER2 concentration was updated to 48.94% (23/47). We found that the detection sensitivity would be improved by combining exosomal HER2 with one of the serum markers. The detection sensitivity of exosomal HER2 combined with CA12-5 was the highest, with a sensitivity of 55.32%. There was no sensitivity difference between exosomal HER2 alone and combined with CA15-3 (Figure 3E). These findings indicate that exosomal HER2 levels can significantly distinguish between patients with benign breast disease and breast cancer; furthermore, the clinical detection rate of breast cancer can be improved if CA12-5 levels are considered.
Concordance between exosomal HER2 and HER2 IHC
To analyze concordance between exosomal HER2 and HER2 IHC, patients with IHC 3+ were considered as positive samples and those with IHC –/1+/2+ as negative samples, and their respective exosomal HER2 levels were scored. Figure 4A shows a histogram of 51 patients colored according to pathologist score (red = 3+, orange = 2+, green = 1+, and blue = –). The Y-axis shows the levels of exosomal HER2 in 51 patients with breast cancer (Figure 4A). Exosomal HER2 in patients with HER2 IHC 3+ was significantly higher than that in patients with IHC –/1+/2+ (Figure 4B). The ROC curve of exosomal HER2 in analyzing the concordance with HER2 IHC was plotted, with an optimal cutoff of 743 pg/mL (indicated by the black arrow in Figure 4A) (AUC =0.7393; 95% CI: 0.5936–0.8850) (Figure 4A,4C). The sensitivity of exosome HER2 detection was 81.25% (95% CI: 56.99–93.41%), and the specificity was 71.43% (95% CI: 54.95–83.67%). The concordance between exosomal HER2 and HER2 IHC was 74.51% (38/51). It is worth noting that some HER2 IHC-negative patients showed positive exosomal HER2 results and that the exosomal HER2 detection rates in IHC 0, 1+, and 2+ patients were 35.71% (5/14), 20% (2/10), and 27.27% (3/11), respectively (Table 2).
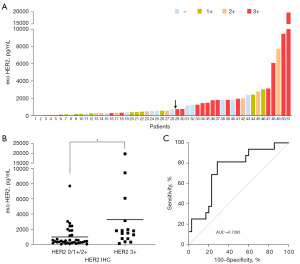
Table 2
| Group analyzed | Cross-table | P value | Concordance rate |
Prediction performance | ||||
|---|---|---|---|---|---|---|---|---|
| Exosomal HER2 (≤743 pg/mL) |
Exosomal HER2 (>743 pg/mL) | Sensitivity | Specificity | PPV | NPV | |||
| HER2 IHC (n=51) | 0.005 | 74.51% | 81.25% | 71.43% | 56.52% | 89.28% | ||
| 0 (n=14) | 9 | 5 | ||||||
| 1+ (n=10) | 8 | 2 | ||||||
| 2+ (n=11) | 8 | 3 | ||||||
| 3+ (n=16) | 3 | 13 | ||||||
HER2, human epidermal growth factor receptor 2; IHC, immunohistochemistry; NPV, negative predictive value; PPV, positive predictive value.
The applicability of exosomal HER2 in neoadjuvant therapy
According to the principles of data integrity, we enrolled eight patients who received neoadjuvant therapy to evaluate the applicability of exosomal HER2 concentration as a biomarker in assessing the efficacy of neoadjuvant therapy. MRI scans were performed to determine the initial tumor size before neoadjuvant therapy, and scans were performed after as needed. The effect of the neoadjuvant therapy on the tumors was assessed according to the tumor size and the clinicopathology, which are regarded as the gold standard. Samples were collected from patients before each cycle of neoadjuvant therapy, and the exosomal HER2 concentration was measured.
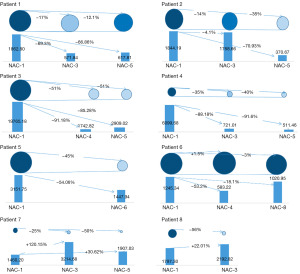
Figure 5 shows the tumor size in eight patients and their corresponding exosomal HER2 levels. For each patient, the upper circles indicate tumor size, and the lower diagrams show exosomal HER2 levels at different time points. Exosomal HER2 levels of patients 1 to 6 decreased to varying degrees after they received the first cycle of neoadjuvant therapy, indicating that the body had an obvious response to the treatment and that the tumor microenvironment was changing. Accordingly, the tumor size in patients 1 to 6 was also reduced. The tumor size in patient 1 before the third and the fifth cycles of neoadjuvant therapy decreased by 17% and 12.1%, respectively, as compared to the initial tumor size; the corresponding exosomal HER2 concentration were also reduced by 69.3% and 66.86%, respectively. Exosomal HER2 concentration in patient 2 before the third cycle was only 4.1% lower than the initial measure, while tumor size decreased by 14%. The biopsy of patient 2 before neoadjuvant therapy showed lymph node metastasis, which might have been the cause of the relatively slight decline in exosomal HER2. The exosomal HER2 concentration of patient 2 was reduced by 70.93%, and the corresponding tumor size was reduced by 35% before the fifth cycle. Compared to that in the initial stage, the tumor size in patients 3 and 4 before the fifth cycle was significantly reduced by 51% and 40%, respectively, while the corresponding exosomal HER2 concentration was decreased by 85.28% and 91.6%, respectively. The exosomal HER2 concentration of patients 3 and 4 after the third cycle was stable, indicating that the patients responded quickly to the drug and that the tumor growth in vivo was significantly inhibited. The tumor size in patient 5 before the sixth cycle was reduced by 45%. Moreover, the clinicopathological outcome was Miller-Payne 5 grade, which indicated that infiltrating cancer cells were invisible, and pathologic complete response (pCR) was achieved, corresponding to a 54% reduction in exosomal HER2. Biopsy before neoadjuvant therapy of both patients 7 and 8 showed lymph node metastases, which affected the correspondence of exosomal HER2 levels to changes in tumor size. After patient 6 received neoadjuvant therapy, the size of solid tumors increased slightly with exosomal HER2 levels fluctuating. Compared to initial levels before neoadjuvant therapy, exosomal HER2 was reduced by 53.2% before the fourth cycle and by 18.1% before the eighth cycle. These results demonstrate that exosomal HER2 concentration reflects the systemic condition of the patient, and other indicators need to be considered in the evaluation of the patient drug response and the efficacy of neoadjuvant therapy.
Discussion
The importance of distinguishing breast cancer from benign breast disease
Benign breast disease is recognized as one of the risk factors for breast cancer (42,43), and might increase the risk of breast cancer by 30% (44). Benign breast lesions may show similar symptoms as malignant breast tumors and interfere with the diagnosis of breast cancer (45,46).
A study conducted by the Dartmouth College, the University of Vermont, and the Fred Hutchinson Cancer Research Center provided data on the consistency of pathological diagnoses of breast cancer. Three pathologists observed the same breast biopsy samples and compared their interpretation of the results with the final consensus diagnosis. The results showed that the agreement rate of independent diagnosis of the pathologists was 75.3% and that ductal carcinoma in situ failed to be diagnosed in 13% of the patients, whose condition was underestimated. The consistency of breast dysplasia diagnosis was only 48%, and dysplasia failed to be diagnosed in 35% of the patients. A further 13% of patients with nonatypic benign breast disease were overdiagnosed (47). These would cause patients to miss out on treatment and even turn otherwise curable diseases into incurable diseases due to receiving the wrong treatment plan.
It is therefore essential to develop other methods to help pathologists accurately distinguish breast cancer from benign breast disease. In this study, patients with breast cancer and patients with benign breast diseases from the same hospital were enrolled, which ensured the reliability of the sample source and sample consistency. The automatic chemiluminescence immune analyzer was used to quantify the HER2 levels in the exosomes of patients. The exosomal HER2 levels of patients with breast cancer were found to be significantly higher than those of patients with benign breast diseases. With 772.7 pg/mL as the cutoff value, the sensitivity and specificity of exosomal HER2 were 45.1% and 97.22%, respectively. Serum markers including CA15-3, CEA, and CA12-5 have been associated with breast cancer stage, tumor size, positive axillary lymph nodes, OS, and disease-free survival (34-37). However, the diagnostic sensitivity in breast cancer is low. It was reported that only 8.3% to 25% of patients with breast cancer showed elevated CA15-3 levels and 7% of patients showed elevated CA12-5 levels (48). We found that the detection sensitivity could be improved by combining exosomal HER2 with CA12-5, with a sensitivity of 55.32%.
Applying HER2-targeted therapy in suitable populations
HER2-targeted drugs have been shown to significantly improve the clinical outcome of HER2-positive patients with breast cancer. The clinical trial of T-DXd (21) has given a reason for optimism for patients with low HER2 expression (HER2 IHC 1+ or IHC 2+/ISH−). T-DXd was found to reduce the risk of disease progression or death by 50% as compared to chemotherapy. A median OS of 23.4 months was observed in patients treated with T-DXd and that of 16.8 months in those treated with chemotherapy.
At present, serum HER2, whose consistency with tissue HER2 is low, has not been widely applied to guide clinical therapy. In one study that examined 2,318 patients, only 12% of tissue-HER2-positive patients had elevated serum HER2 levels before surgery (49). In another study of 2,862 patients with stage I–III primary breast cancer, 24% patients with were tissue HER2 positive, while only 15% of these tissue-positive patients showed elevated serum HER2 levels (50). The data above indicate that the concordance between serum HER2 and tissue HER2 results is too low to replace or even supplement traditional HER2 IHC or FISH in medication guidance. However, a study using microfluidic chips combined with immunofluorescence experiments indicated that the HER2 levels in plasma-derived exosomes of patients with breast cancer were found to be basically consistent with tissue HER2 (51). In our study, we quantified exosomal HER2 levels and plotted ROC curves and found that 81.25% of HER2-positive people could be screened out to have HER2-targeted therapy. Moreover, exosomal HER2 had a high concordance (74.51%) with clinical HER2 IHC and could therefore be used to assist clinicians in therapeutic decisions. In addition, we determined specific HER2 detection rates (35.71%, 20%, and 27.27% for HER2 IHC 0, 1+, and 2+, respectively), which may be explained as follows: (I) the linear range of clinical tissue HER2 detection is narrow, and the sensitivity for HER2 IHC 1+ and 2+ breast cancer is low, which may lead to missed diagnoses. Patients with low HER2 expression (HER2 IHC 1+ or IHC 2+/ISH−) but increased exosomal HER2 expression may also benefit from HER2-targeted therapy; (II) breast cancer is highly heterogeneous, and the difference in sampling locations and periods may substantially influence the results.
Application of exosomal HER2 in assessing the efficacy of neoadjuvant therapy
The clinical methods to monitor the efficacy of neoadjuvant treatment primarily include imaging detection, serum marker detection, and postoperative pathology. Among them, postoperative pathology is considered the ultimate evaluation of the therapy but is not suitable for monitoring the treatment process. Both serum and imaging tests have distinct limitations, and more reliable methods are needed to administer neoadjuvant therapy. In 1987, Slamon et al. reported a correlation between HER2 levels and the prognosis of patients with breast cancer (52); since then, numerous studies have consistently found that overexpression of HER2 in breast cancer to be associated with poor prognosis (53,54). In addition, HER2 content is closely related to the patients’ response to chemotherapy (28,55,56). Compared with the baseline level before treatment, serum HER2 content is significantly increased in patients receiving nontrastuzumab neoadjuvant therapy but decreased in patients treated with trastuzumab (57).
Research also indicates that the content of tumor-derived exosomes is related to tumor metastasis and patient condition (33,58). In our study, we collected and enriched exosomes from patients with breast cancer receiving neoadjuvant therapy and detected exosomal HER2 concentration. In patients 1 to 5 after medication, the posttreatment changes in exosomal HER2 levels correlated with the changes in tumor size. The exosomal HER2 concentration in patients 6, 7, and 8 was not consistent with the changes in tumor size; we speculate this discrepancy can be attributed to the following: The lymph node metastasis before neoadjuvant therapy of patients 7 and 8 could account for the increased exosomal HER2 levels, although the sizes of solid tumors were reduced. It is essential to combine different detection methods and pay close attention to the prognosis of the patients. For patient 6, there was no significant change in tumor size, but the exosomal HER2 level decreased to varying degrees after treatment. The fluctuation in exosomal HER2 might be due to the emerging resistance to drugs provoked by the therapy. More time might be needed to observe the effects of therapy and to detect malignant tumors via mammography.
There are some limitations in this study. First, the sensitivity of exosomal HER2 in distinguishing breast cancer from benign breast disease was 45.1%, which should be increased. Second, much more time should be spent in assessing the correlation between exosomal HER2 and the efficacy of neoadjuvant therapy, and even in conjunction with other monitoring indicators.
Exosomal HER2 plays an important role in cancer’s diagnosis, prognosis and even treatment options. Complex and uncertain relationship between exosomal HER2 levels in patients and the treatment strategies of breast cancer impeded the practical implementation. It is necessary to further combine the technologies of different disciplines to optimize the detection methods, and then carry out large-scale precision clinical trials on this basis. We believe that with the cross-penetration of various disciplines in the future, there will be a qualitative breakthrough in the diagnosis of breast cancer, and the treatment methods will be greatly improved.
Conclusions
HER2 in plasma-derived exosomes might be a highly promising biomarker for assessing the tissue HER2 status with a stable diagnostic effect in patients with breast cancer. Moreover, exosomal HER2 was a potential predictive marker to screen the population who would benefit from receiving HER2-targeted therapy. Further studies in clinical settings are needed to validate the eligibility of exosomal HER2 to monitor and evaluate the efficacy of neoadjuvant therapy for breast cancer treatment.
Acknowledgments
None.
Footnote
Reporting Checklist: The authors have completed the STARD reporting checklist. Available at https://tcr.amegroups.com/article/view/10.21037/tcr-2025-825/rc
Data Sharing Statement: Available at https://tcr.amegroups.com/article/view/10.21037/tcr-2025-825/dss
Peer Review File: Available at https://tcr.amegroups.com/article/view/10.21037/tcr-2025-825/prf
Funding: This work was supported by
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tcr.amegroups.com/article/view/10.21037/tcr-2025-825/coif). X.Y., Z.B., C.H., and Yipu Wang are from Jiangsu MicroDiag Biomedical Technology Co., Ltd. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki and its subsequent amendments. The study was approved by the ethics committee of the First Affiliated Hospital of Soochow University (No. 2023-357) and informed consent was taken from all the patients.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Sung H, Ferlay J, Siegel RL, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin 2021;71:209-49. [Crossref] [PubMed]
- Zheng R, Zhang S, Zeng H, et al. Cancer incidence and mortality in China, 2016. J Natl Cancer Cent 2022;2:1-9. [Crossref] [PubMed]
- Zhang S, Sun K, Zheng R, et al. Cancer incidence and mortality in China, 2015. J Natl Cancer Cent 2021;1:2-11. [Crossref] [PubMed]
- Bas O, Sahin TK, Karahan L, et al. Prognostic significance of the cachexia index (CXI) in patients with cancer: A systematic review and meta-analysis. Clin Nutr ESPEN 2025; Epub ahead of print. [Crossref] [PubMed]
- Wang J, Ge H, Yu Z, et al. Non-coding RNAs as potential mediators of resistance to lung cancer immunotherapy and chemotherapy. Oncol Res 2025;33:1033-54. [PubMed]
- Sahin TK, Rizzo A, Guven DC, et al. Post-progression treatment options after CDK4/6 inhibitors in hormone receptor-positive, HER2-negative metastatic breast cancer. Cancer Treat Rev 2025;135:102924. [Crossref] [PubMed]
- Sahin TK, Ayasun R, Rizzo A, et al. Prognostic Value of Neutrophil-to-Eosinophil Ratio (NER) in Cancer: A Systematic Review and Meta-Analysis. Cancers (Basel) 2024;16:3689. [Crossref] [PubMed]
- Dupont WD, Page DL. Risk factors for breast cancer in women with proliferative breast disease. N Engl J Med 1985;312:146-51. [Crossref] [PubMed]
- Hartmann LC, Sellers TA, Frost MH, et al. Benign breast disease and the risk of breast cancer. N Engl J Med 2005;353:229-37. [Crossref] [PubMed]
- Dyrstad SW, Yan Y, Fowler AM, et al. Breast cancer risk associated with benign breast disease: systematic review and meta-analysis. Breast Cancer Res Treat 2015;149:569-75. [Crossref] [PubMed]
- Wolff AC, Hammond MEH, Allison KH, et al. Human Epidermal Growth Factor Receptor 2 Testing in Breast Cancer: American Society of Clinical Oncology/College of American Pathologists Clinical Practice Guideline Focused Update. J Clin Oncol 2018;36:2105-22. [Crossref] [PubMed]
- Pegram MD, Lipton A, Hayes DF, et al. Phase II study of receptor-enhanced chemosensitivity using recombinant humanized anti-p185HER2/neu monoclonal antibody plus cisplatin in patients with HER2/neu-overexpressing metastatic breast cancer refractory to chemotherapy treatment. J Clin Oncol 1998;16:2659-71. [Crossref] [PubMed]
- von Minckwitz G, Huang CS, Mano MS, et al. Trastuzumab Emtansine for Residual Invasive HER2-Positive Breast Cancer. N Engl J Med 2019;380:617-28. [Crossref] [PubMed]
- Denkert C, Seither F, Schneeweiss A, et al. Clinical and molecular characteristics of HER2-low-positive breast cancer: pooled analysis of individual patient data from four prospective, neoadjuvant clinical trials. Lancet Oncol 2021;22:1151-61. [Crossref] [PubMed]
- Schettini F, Chic N, Brasó-Maristany F, et al. Clinical, pathological, and PAM50 gene expression features of HER2-low breast cancer. NPJ Breast Cancer 2021;7:1. [Crossref] [PubMed]
- Schalper KA, Kumar S, Hui P, et al. A retrospective population-based comparison of HER2 immunohistochemistry and fluorescence in situ hybridization in breast carcinomas: impact of 2007 American Society of Clinical Oncology/College of American Pathologists criteria. Arch Pathol Lab Med 2014;138:213-9. [Crossref] [PubMed]
- Miglietta F, Griguolo G, Bottosso M, et al. Evolution of HER2-low expression from primary to recurrent breast cancer. NPJ Breast Cancer 2021;7:137. [Crossref] [PubMed]
- Matutino A, Joy AA, Brezden-Masley C, et al. Hormone receptor-positive, HER2-negative metastatic breast cancer: redrawing the lines. Curr Oncol 2018;25:S131-41. [Crossref] [PubMed]
- Modi S, Park H, Murthy RK, et al. Antitumor Activity and Safety of Trastuzumab Deruxtecan in Patients With HER2-Low-Expressing Advanced Breast Cancer: Results From a Phase Ib Study. J Clin Oncol 2020;38:1887-96. [Crossref] [PubMed]
- Nakada T, Sugihara K, Jikoh T, et al. The Latest Research and Development into the Antibody-Drug Conjugate, [fam-] Trastuzumab Deruxtecan (DS-8201a), for HER2 Cancer Therapy. Chem Pharm Bull (Tokyo) 2019;67:173-85. [Crossref] [PubMed]
- Modi S, Jacot W, Yamashita T, et al. Trastuzumab Deruxtecan in Previously Treated HER2-Low Advanced Breast Cancer. N Engl J Med 2022;387:9-20. [Crossref] [PubMed]
- Pous A, Bernat-Peguera A, López-Paradís A, et al. Deciphering HER2-low breast cancer (BC): insights from real-world data in early stage breast cancer. Ther Adv Med Oncol 2024;16:17588359241290720. [Crossref] [PubMed]
- Yan M, Schwaederle M, Arguello D, et al. HER2 expression status in diverse cancers: review of results from 37,992 patients. Cancer Metastasis Rev 2015;34:157-64. [Crossref] [PubMed]
- Loibl S, Gianni L. HER2-positive breast cancer. Lancet 2017;389:2415-29. [Crossref] [PubMed]
- Hayes DF. HER2 and Breast Cancer - A Phenomenal Success Story. N Engl J Med 2019;381:1284-6. [Crossref] [PubMed]
- Rossi C, Fraticelli S, Fanizza M, et al. Concordance of immunohistochemistry for predictive and prognostic factors in breast cancer between biopsy and surgical excision: a single-centre experience and review of the literature. Breast Cancer Res Treat 2023;198:573-82. [Crossref] [PubMed]
- Fernandez AI, Liu M, Bellizzi A, et al. Examination of Low ERBB2 Protein Expression in Breast Cancer Tissue. JAMA Oncol 2022;8:1-4. [Crossref] [PubMed]
- Baez-Navarro X, van Bockstal MR, Jager A, et al. HER2-low breast cancer and response to neoadjuvant chemotherapy: a population-based cohort study. Pathology 2024;56:334-42. [Crossref] [PubMed]
- Logozzi M, Mizzoni D, Di Raimo R, et al. Exosomes: A Source for New and Old Biomarkers in Cancer. Cancers (Basel) 2020;12:2566. [Crossref] [PubMed]
- Théry C, Zitvogel L, Amigorena S. Exosomes: composition, biogenesis and function. Nat Rev Immunol 2002;2:569-79. [Crossref] [PubMed]
- Théry C, Witwer KW, Aikawa E, et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): a position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J Extracell Vesicles 2018;7:1535750. [Crossref] [PubMed]
- Yáñez-Mó M, Siljander PR, Andreu Z, et al. Biological properties of extracellular vesicles and their physiological functions. J Extracell Vesicles 2015;4:27066. [Crossref] [PubMed]
- Jia Y, Chen Y, Wang Q, et al. Exosome: emerging biomarker in breast cancer. Oncotarget 2017;8:41717-33. [Crossref] [PubMed]
- Moutafi M, Robbins CJ, Yaghoobi V, et al. Quantitative measurement of HER2 expression to subclassify ERBB2 unamplified breast cancer. Lab Invest 2022;102:1101-8. [Crossref] [PubMed]
- Li Q, Lv M, Lv L, et al. Identifying HER2 from serum-derived exosomes in advanced gastric cancer as a promising biomarker for assessing tissue HER2 status and predicting the efficacy of trastuzumab-based therapy. Cancer Med 2023;12:4110-24. [Crossref] [PubMed]
- Peng P, Yan Y, Keng S. Exosomes in the ascites of ovarian cancer patients: origin and effects on anti-tumor immunity. Oncol Rep 2011;25:749-62. [PubMed]
- Kowal EJK, Ter-Ovanesyan D, Regev A, et al. Extracellular Vesicle Isolation and Analysis by Western Blotting. Methods Mol Biol 2017;1660:143-52. [Crossref] [PubMed]
- Li J, Liu L, Feng Z, et al. Tumor markers CA15-3, CA125, CEA and breast cancer survival by molecular subtype: a cohort study. Breast Cancer 2020;27:621-30. [Crossref] [PubMed]
- Uehara M, Kinoshita T, Hojo T, et al. Long-term prognostic study of carcinoembryonic antigen (CEA) and carbohydrate antigen 15-3 (CA 15-3) in breast cancer. Int J Clin Oncol 2008;13:447-51. [Crossref] [PubMed]
- Tang Y, Cui Y, Zhang S, et al. The sensitivity and specificity of serum glycan-based biomarkers for cancer detection. Prog Mol Biol Transl Sci 2019;162:121-40. [Crossref] [PubMed]
- Seale KN, Tkaczuk KHR. Circulating Biomarkers in Breast Cancer. Clin Breast Cancer 2022;22:e319-31. [Crossref] [PubMed]
- Bertelsen L, Mellemkjaer L, Balslev E, et al. Benign breast disease among first-degree relatives of young breast cancer patients. Am J Epidemiol 2008;168:261-7. [Crossref] [PubMed]
- Harmer V. Breast cancer. Part 1: Awareness and common benign diseases. Br J Nurs 2008;17:950-5. [Crossref] [PubMed]
- Zeinomar N, Phillips KA, Daly MB, et al. Benign breast disease increases breast cancer risk independent of underlying familial risk profile: Findings from a Prospective Family Study Cohort. Int J Cancer 2019;145:370-9. [Crossref] [PubMed]
- Mann RM, Kuhl CK, Moy L. Contrast-enhanced MRI for breast cancer screening. J Magn Reson Imaging 2019;50:377-90. [Crossref] [PubMed]
- Marino MA, Helbich T, Baltzer P, et al. Multiparametric MRI of the breast: A review. J Magn Reson Imaging 2018;47:301-15. [Crossref] [PubMed]
- Elmore JG, Longton GM, Carney PA, et al. Diagnostic concordance among pathologists interpreting breast biopsy specimens. JAMA 2015;313:1122-32. [Crossref] [PubMed]
- Hou MF, Chen YL, Tseng TF, et al. Evaluation of serum CA27.29, CA15-3 and CEA in patients with breast cancer. Kaohsiung J Med Sci 1999;15:520-8. [PubMed]
- Moreno-Aspitia A, Hillman DW, Dyar SH, et al. Soluble human epidermal growth factor receptor 2 (HER2) levels in patients with HER2-positive breast cancer receiving chemotherapy with or without trastuzumab: results from North Central Cancer Treatment Group adjuvant trial N9831. Cancer 2013;119:2675-82. [Crossref] [PubMed]
- Lee SB, Lee JW, Yu JH, et al. Preoperative serum HER2 extracellular domain levels in primary invasive breast cancer. BMC Cancer 2014;14:929. [Crossref] [PubMed]
- Fang S, Tian H, Li X, et al. Clinical application of a microfluidic chip for immunocapture and quantification of circulating exosomes to assist breast cancer diagnosis and molecular classification. PLoS One 2017;12:e0175050. [Crossref] [PubMed]
- Slamon DJ, Clark GM, Wong SG, et al. Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science 1987;235:177-82. [Crossref] [PubMed]
- Ross JS, Fletcher JA, Linette GP, et al. The Her-2/neu gene and protein in breast cancer 2003: biomarker and target of therapy. Oncologist 2003;8:307-25. [Crossref] [PubMed]
- Schnitt SJ. Breast cancer in the 21st century: neu opportunities and neu challenges. Mod Pathol 2001;14:213-8. [Crossref] [PubMed]
- Xu L, Xie Y, Gou Q, et al. HER2-targeted therapies for HER2-positive early-stage breast cancer: present and future. Front Pharmacol 2024;15:1446414. [Crossref] [PubMed]
- Cantini L, Trapani D, Guidi L, et al. Neoadjuvant therapy in hormone Receptor-Positive/HER2-Negative breast cancer. Cancer Treat Rev 2024;123:102669. [Crossref] [PubMed]
- Bianchini G, Kiermaier A, Bianchi GV, et al. Biomarker analysis of the NeoSphere study: pertuzumab, trastuzumab, and docetaxel versus trastuzumab plus docetaxel, pertuzumab plus trastuzumab, or pertuzumab plus docetaxel for the neoadjuvant treatment of HER2-positive breast cancer. Breast Cancer Res 2017;19:16. [Crossref] [PubMed]
- Zhou Y, Chen F, Xie X, et al. Tumor-derived Exosome Promotes Metastasis via Altering its Phenotype and Inclusions. J Cancer 2021;12:4240-6. [Crossref] [PubMed]


