
Significant weight gain benefits of nanocrystalline megestrol acetate for patients with cancer anorexia-cachexia syndrome
Highlight box
Key findings
• In hormone-insensitive patients with cancer anorexia-cachexia syndrome (CACS), nanocrystalline megestrol acetate (MA-ES) administered at 625 mg/day for three cycles (4 weeks each) may provide greater weight gain benefits as compared to the conventional megestrol acetate (MA) tablets at 800 mg/day. Additionally, MA-ES may confer superior improvements in appetite and quality of life (QoL).
What is known and what is new?
• CACS is a multifactorial syndrome characterized by weight loss and muscle wasting that leads to impaired physical function and decreased survival rates. MA is an important pharmacological treatment for CACS, with its therapeutic efficacy achieved through multifaceted mechanisms of action, including direct and indirect appetite stimulation pathways, as well as modulation of key catabolic cytokines. The inherent hydrophobicity of MA presents significant pharmacokinetic challenges, necessitating co-administration with high-calorie, lipid-rich meals (800–1,000 calories) to achieve adequate bioavailability. MA-ES combined with nanocrystal technology enhances bioavailability and absorption rates.
• MA-ES administered at 625 mg/day for three cycles (each cycle lasting 4 weeks) may provide more significant weight gain benefits as compared to the conventional MA tablets at 800 mg/day in Chinese hormone-insensitive CACS patients. Furthermore, MA-ES demonstrated significantly greater improvements in appetite and overall QoL as compared to conventional MA tablets.
What is the implication, and what should change now?
• Among Chinese patients with CACS, MA-ES (625 mg/day) may offer superior benefits in weight gain, appetite improvement, and quality-of-life enhancement as compared to MA tablets (800 mg/day). The advantages of MA-ES in clinical practice warrant further research.
Introduction
Cancer anorexia-cachexia syndrome (CACS) is a complex metabolic disorder characterized by progressive skeletal muscle depletion, with or without concomitant adipose tissue loss, that persists despite conventional nutritional interventions, ultimately resulting in significant functional decline (1,2). This syndrome is fundamentally driven by a dual imbalance in nitrogen and energy homeostasis, primarily attributable to reduced caloric intake and increased metabolic demands (3). CACS is highly prevalent among patients with cancer, with recent epidemiological data revealing a cachexia prevalence rate of 37.0% in Chinese patients with cancer, affecting individuals across all disease stages (I–IV) (4). The clinical course of CACS is closely linked to cancer progression, particularly in cases of metastatic disease, where escalating severity significantly undermines therapeutic tolerance, severely diminishes quality of life (QoL), and negatively impacts survival outcomes (5-7).
The main drugs for treating CACS include progestogens, corticosteroids, and anti-inflammatory drugs (such as thalidomide) (8). Among them, megestrol acetate (MA) is one of a mainstay pharmacological treatment for CACS (9-13). The guidelines on cancer cachexia from both American Society of Clinical Oncology (ASCO) and European Society for Medical Oncology (ESMO) have indicated that MA can bring benefits in improving appetite and increasing weight (9,11). MA therapeutic efficacy is achieved through a multifaceted mechanism of action, involving both direct and indirect appetite stimulation pathways, as well as modulation of key catabolic cytokines (14-16). MA’s hydrophobicity poses pharmacokinetic challenges, requiring co-administration with high-calorie, lipid-rich meals (800–1,000 calories) to ensure adequate bioavailability (17-19). A pharmacokinetic study has revealed that fasting results in suboptimal peak plasma concentrations (Cmax =187 ng/mL), failing to reach the minimum effective concentration threshold of 300 ng/mL (20). This pharmacokinetic limitation leads to substantial interpatient variability in therapeutic response and inconsistent clinical outcomes.
Previous randomized controlled study has demonstrated that, compared with placebo, MA could increase body weight, improve appetite, and reduce adverse reactions such as nausea and vomiting (21). However, meta-analyses that include different doses of MA still have controversies regarding weight gain benefits (22-24). Clinical evidence indicates a dose-response relationship, suggesting that a daily dose of 800 mg may represent the optimal therapeutic dose (25,26). The development of nanocrystalline megestrol acetate (MA-ES) formulations represents a significant advancement in drug delivery technology, with preclinical and clinical studies demonstrating its superior therapeutic efficacy as compared to conventional formulations (20,27). A previous randomized controlled trial has shown that MA-ES results in more significant weight improvement compared to non-MA-ES (27). Nevertheless, as an innovative pharmaceutical preparation, its clinical utility and real-world efficacy in Chinese patients with cancer remain inadequately characterized, necessitating further investigation.
Consequently, we conducted a real-world study to systematically evaluate the therapeutic efficacy and safety of MA-ES and MA tablets in patients diagnosed with CACS. We present this article in accordance with the STROBE reporting checklist (available at https://tcr.amegroups.com/article/view/10.21037/tcr-2025-866/rc).
Methods
Study design
This study leveraged data from a prospective, multi-cohort, multicenter, real-world clinical study designed to comprehensively assess the clinical characteristics and therapeutic outcomes in patients diagnosed with CACS.
The demographic and clinicopathological characteristics included age, sex, Eastern Cooperative Oncology Group performance status (ECOG-PS) score, body weight, body mass index (BMI), percentage of weight loss within the 6 months preceding screening, cancer type, lines of systemic antitumor therapy, current treatment regimens, and key laboratory parameters [including serum albumin, C-reactive protein (CRP), interleukin-6 (IL-6), and tumor necrosis factor-α (TNF-α)]. Patient-reported outcomes were systematically collected, including appetite scores assessed using the 12-Item Functional Assessment of Anorexia/Cachexia Therapy-Anorexia/Cachexia Subscale (FAACT-ACS-12) and health-related QoL measured by the European Organization for Research and Treatment of Cancer Quality of Life Questionnaire Core 30 (EORTC QLQ-C30) (28,29). Additionally, adverse events (AEs) were recorded throughout the treatment period.
The study protocol was approved by the Institutional Review Board of the Main Research Center at Xuzhou Central Hospital, China (No. XZXY-LK-20241014-0154, Data: October 14, 2024, protocol Version: V1.0) and was conducted in accordance with the Declaration of Helsinki and its subsequent amendments. All participants provided written informed consent, including permission for data disclosure and publication. Miaoshou Internet Hospital was informed and agreed with this study.
The patient consent procedure included providing participants with a detailed introduction to the study, obtaining their voluntary consent through the signing of an informed consent form, and maintaining continuous communication. Meanwhile, the data monitoring procedure ensured data integrity and participant safety through systematic data collection, validation, cleaning, and regulatory compliance.
The analytical dataset comprised patients with CACS who received MA-ES and MA tablets were enrolled between October 15, 2024 and February 28, 2025 from Xuzhou Central Hospital and Miaoshou Internet hospital.
Patients
The inclusion criteria for this study were as follows: (I) diagnosis of CACS [defined as unintentional weight loss >5% over the preceding 6 months or >2% with a BMI <20 kg/m2 in accordance with the international consensus definition of cachexia (1)]; (II) hormone-insensitive tumors (excluding breast cancer, endometrial cancer, and prostate cancer); (III) completion of three cycles of MA at a dose of 800 mg/day (one cycle defined as 28 days, with ≥21 days considered as completion of one cycle); (IV) age ≥18 years; and (V) life expectancy ≥2 months. Meanwhile, the exclusion criteria were as follows: (I) failure to complete three cycles of MA; (II) duration of 800 mg/day administration within each cycle ≤21 days; and (III) incomplete data or loss to follow-up.
Interventions and procedures
The MA-ES group received 5 mL/day (625 mg/day) [a previous study indicated that 625 mg of MA-ES is bioequivalent to 800 mg of non-MA-ES (20)], while the MA tablet group received 800 mg/day. All patients were treated for three consecutive cycles, with each cycle lasting 4 weeks.
End points and assessments
The primary endpoint of this study was the change in body weight between baseline and 12 weeks. The key secondary endpoint was the proportion of patients demonstrating clinically significant appetite improvement at week 12 according to the FAACT-ACS-12. The FAACT-ACS-12 is a validated instrument with scores ranging from 0 to 48, in which higher scores indicate better outcomes; a clinically meaningful improvement is defined as an increase of ≥4 points from baseline (2).
Additional secondary endpoints included changes in laboratory parameters (serum albumin, CRP, IL-6, and TNF-α) from baseline to week 12, as well as changes in the EORTC QLQ-C30 scores between baseline and week 12. Safety assessments included the number of incidences, severity, and type of AEs recorded during the treatment period. Efficacy and safety evaluations were conducted at 28-day intervals (±4 days), resulting in a total of three assessments over the 12-week treatment period. No conclusions can be made regarding differences for secondary outcomes because of the lack of planned adjustment of P value for multiple comparisons.
Statistical analysis
Patients who received MA-ES at 5 mL/day or MA tablet at 800 mg/day, completed three treatment cycles, and had complete data with no loss to follow-up were included in the propensity score matching (PSM) analysis. To ensure comparability between the MA-ES and MA tablet groups, 1:2 PSM was performed using logistic regression. A caliper width of 0.1 (maximum allowable difference in propensity scores) was applied to minimize potential bias. The matching process was based upon several key variables, including sex, ECOG PS, BMI, percentage of weight loss in the 6 months prior to screening, cancer type, and cancer stage. The standardized mean differences (SMDs) before and after PSM were calculated to measure balance between groups. Additionally, to further explore the robustness of our results after PSM, we conducted sensitivity analyses using a caliper width of 0.08 for 1:2 matching.
The baseline characteristics were summarized using descriptive statistics. Continuous variables were expressed as the mean ± standard deviation (SD) or median [interquartile range (IQR)], while categorical variables were presented as frequencies and percentages. For between-group comparisons, categorical variables were analyzed using the χ2 test or Fisher exact test, and continuous variables were assessed using the independent samples t-test. A mixed-effect model with patient as a random effect and treatment, time, and their interaction as fixed effects was used to analyze changes from baseline over time.
Statistical significance was defined as a two-sided P value <0.05. All analyses were conducted using R software version 3.6.3 (The R Foundation for Statistical Computing, Vienna, Austria) and SPSS version 25.0 (IBM Corp., Armonk, NY, USA).
Results
Patients
From October 15, 2024 to February 28, 2025, a total of 898 patients with CACS receiving MA were screened. Among them, 160 patients met the screening criteria and were enrolled in the pre-matching cohort (Figure 1). Of these, 107 patients were allocated to the MA-ES group and 53 to the MA tablet group. The baseline characteristics are summarized in Table 1. Patients in the MA-ES group exhibited a significantly poorer appetite but higher albumin levels as compared to the MA tablet group, but no other significant differences in baseline characteristics were observed.
Table 1
| Characteristic | Before matching | After matching | |||||||
|---|---|---|---|---|---|---|---|---|---|
| MA-ES group (n=107) | MA tablet group (n=53) | P value | SMD | MA-ES group (n=76) | MA tablet group (n=50) | P value | SMD | ||
| Age (years) | 62.9±12.4 | 59.8±11.2 | 0.12 | NA | 63.4±12.2 | 60.5±11.1 | 0.17 | NA | |
| ≤65 | 62 (57.9) | 32 (60.4) | 0.76 | 41 (53.9) | 29 (58.0) | 0.65 | |||
| >65 | 45 (42.1) | 21 (39.6) | 35 (46.1) | 21 (42.0) | |||||
| Sex | 0.69 | 0.064 | 0.66 | 0.060 | |||||
| Male | 68 (63.6) | 32 (60.4) | 50 (65.8) | 31 (62.0) | |||||
| Female | 39 (36.4) | 21 (39.6) | 26 (34.2) | 19 (38.0) | |||||
| ECOG-PS | 0.89 | 0.081 | 0.93 | 0.080 | |||||
| 0 | 5 (4.6) | 2 (3.8) | 2 (2.6) | 2 (4.0) | |||||
| 1 | 34 (31.8) | 20 (37.8) | 24 (31.6) | 17 (34.0) | |||||
| 2 | 54 (50.5) | 25 (47.1) | 42 (55.3) | 25 (50.0) | |||||
| ≥3 | 14 (13.1) | 6 (11.3) | 8 (10.5) | 6 (12.0) | |||||
| Appetite (FAACT-ACS) | 23 [21–26] | 26 [21–28] | 0.02 | NA | 23 [21–27] | 25 [21–27] | 0.06 | NA | |
| Weight (kg) | 52.9 [50.0–54.7] | 53.6 [49.7–55.7] | 0.19 | NA | 53.5 [50.3–54.7] | 53.5 [49.7–55.6] | 0.54 | NA | |
| Body mass index (kg/m2) | 0.46 | 0.132 | 0.91 | 0.062 | |||||
| ≥18.5 | 66 (61.7) | 36 (67.9) | 51 (67.1) | 34 (68.0) | |||||
| <18.5 | 41 (38.3) | 17 (32.1) | 25 (32.9) | 16 (32.0) | |||||
| Percent weight loss within 6 months before screening | 0.77 | 0.049 | 0.55 | 0.020 | |||||
| <10% | 60 (56.1) | 31 (58.5) | 40 (52.6) | 29 (58.0) | |||||
| ≥10% | 47 (43.9) | 22 (41.5) | 36 (47.4) | 21 (42.0) | |||||
| Cancer type | 0.65 | 0.033 | 0.59 | 0.024 | |||||
| Lung cancer | 29 (27.1) | 17 (32.1) | 21 (27.6) | 15 (30.0) | |||||
| Gastrointestinal tumors | 42 (39.3) | 17 (32.1) | 31 (40.8) | 16 (32.0) | |||||
| Others | 36 (33.6) | 19 (35.8) | 24 (31.6) | 19 (38.0) | |||||
| Cancer stage | 0.96 | 0.009 | 0.90 | 0.091 | |||||
| I | 3 (2.8) | 2 (3.8) | 2 (2.6) | 2 (4.0) | |||||
| II | 5 (4.7) | 2 (3.8) | 2 (2.6) | 2 (4.0) | |||||
| III | 12 (11.2) | 5 (9.4) | 6 (7.9) | 5 (10.0) | |||||
| IV | 87 (81.3) | 44 (83.0) | 66 (86.9) | 41 (82.0) | |||||
| Line of systemic anticancer therapy | 0.80 | NA | 0.26 | NA | |||||
| 1 | 2 (1.9) | 1 (1.9) | 2 (2.6) | 1 (2.0) | |||||
| 2 | 6 (5.6) | 5 (9.4) | 3 (3.9) | 5 (10.0) | |||||
| ≥3 | 79 (73.8) | 36 (67.9) | 61 (80.3) | 33 (66.0) | |||||
| Other | 20 (18.7) | 11 (20.8) | 10 (13.2) | 11 (22.0) | |||||
| Present cancer treatment | |||||||||
| Any treatment | 88 (82.2) | 45 (84.9) | 0.67 | NA | 64 (84.2) | 43 (86.0) | 0.78 | NA | |
| Chemotherapy | 46 (43.0) | 27 (50.9) | 0.34 | NA | 32 (42.1) | 27 (54.0) | 0.19 | NA | |
| Targeted therapy | 20 (18.7) | 8 (15.1) | 0.57 | NA | 15 (19.7) | 7 (14.0) | 0.40 | NA | |
| Immunotherapy | 19 (17.8) | 15 (28.3) | 0.12 | NA | 15 (19.7) | 14 (28.0) | 0.28 | NA | |
| Any radiotherapy | 19 (17.8) | 12 (22.6) | 0.46 | NA | 13 (17.1) | 12 (24.0) | 0.34 | NA | |
| Laboratory examinations | |||||||||
| Albumin (g/dL) | 2.7±0.2 | 2.5±0.2 | <0.001 | NA | 2.7±0.2 | 2.5±0.2 | <0.001 | NA | |
| CRP (μg/mL) | 29.0±7.9 | 31.4±9.4 | 0.08 | NA | 28.4±8.0 | 31.3±9.3 | 0.058 | NA | |
| IL-6 (pg/mL) | 27.6±12.6 | 29.2±11.4 | 0.43 | NA | 28.6±13.4 | 29.3±11.7 | 0.75 | NA | |
| TNF-α (pg/mL) | 32.3±13.2 | 35.5±11.9 | 0.13 | NA | 33.0±13.5 | 36.1±11.8 | 0.19 | NA | |
Data are presented as n (%), mean ± standard deviation or median [interquartile range]. MA-ES group, nanocrystalline megestrol acetate group; MA tablet group, megestrol acetate tablet group; SMD, standardized mean differences; CRP, C-reactive protein; ECOG-PS, Eastern Cooperative Oncology Group performance status; FAACT-ACS, functional assessment of anorexia cachexia treatment-anorexia cachexia subscale; IL-6, interleukin-6; TNF-α, tumor necrosis factor-α.
Through 1:2 PSM, a final cohort of 126 patients was established, with 76 patients in the MA-ES group and 50 in the MA tablet group (Figure 1). The baseline characteristics of the matched cohort are detailed in Table 1. After matching, no significant differences in appetite were noted between the two groups; however, the MA-ES group continued to demonstrate significantly higher albumin levels. No other clinical characteristics showed statistically significant differences between the groups. SMD of all variables included in PSM reduced to less than 0.1, demonstrating a good balance between two groups. Additionally, a sensitivity analysis conducted with a caliper width of 0.08 for 1:2 PSM revealed that, at Week 12, the average weight gain was 5.46 kg in the MA-ES group and 2.94 kg in the MA tablet group, resulting in a mean difference of 2.51 kg [95% confidence interval (CI): 1.08–3.94; P<0.001].
Change in body weight
We analyzed the weight data using a linear mixed-effect model, with patients included as random effects and treatment group, time, and their interaction as fixed effects. The results revealed a significant interaction between time and treatment group (P<0.01), suggesting that the trajectory of weight change differed between the treatment groups over time. To further explore this interaction, we conducted between-group comparisons at each time point.
At week 12, the average weight gain was 4.49 kg in the MA-ES group and 2.10 kg in the MA tablet group. Compared to the MA tablet group, the MA-ES group showed a significantly greater increase in body weight from baseline to week 12, with a mean difference of 2.39 kg (95% CI: 1.33–3.45; P<0.001) (Figure 2A). The proportion of patients achieving a weight gain of ≥5% was 78.9% in the MA-ES group compared to 24% in the MA tablet group. At weeks 4 and 8, the increase in body weight was significantly higher in the MA-ES group as compared to that in the MA tablet group (week 4: difference of 1.75 kg, 95% CI: 0.92–2.59, P<0.001; week 8: difference of 1.93 kg, 95% CI: 0.99–2.88, P<0.001) (Figure 2A).
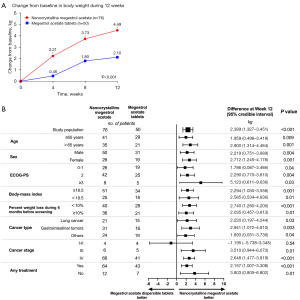
Subgroup analyses revealed that the MA-ES group experienced a more substantial weight gain benefit across most subgroups, including age (≤65 years: P=0.009; >65 years: P=0.001), sex (male: P=0.004; female: P=0.001), ECOG PS (0–1: P=0.04; 2: P=0.004; ≥3: P=0.03), body mass index (≥18.5 kg/m2: P=0.001; <18.5 kg/m2: P=0.01), percentage of weight loss in the 6 months prior to screening (<10%: P<0.001; ≥10%: P=0.01), cancer type (lung cancer: P=0.03; gastrointestinal tumors: P=0.003; others: P=0.04), and administration of anticancer treatment (yes: P<0.001; no: P=0.01). Regarding cancer stage, patients with stage III and IV disease exhibited a more pronounced benefit from MA-ES (stage III, P=0.01; stage IV: P<0.001), while those with stage I–II disease tended to benefit from MA tables, but not significantly so (P=0.54) (Figure 2B).
Appetite improvement
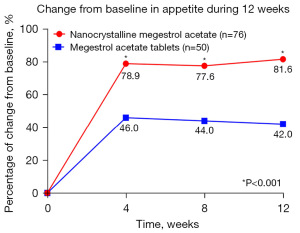
At week 4, a significant difference in the proportion of patients with improved appetite was observed between the MA-ES group and the MA tablet group (78.9% vs. 46.0%; P<0.001). This significant disparity persisted throughout the study period, with notable differences maintained at week 8 (77.6% vs. 44.0%; P<0.001) and week 12 (81.6% vs. 42.0%; P<0.001) (Figure 3).
Laboratory examinations
The laboratory examination indicators at baseline and week 12 are presented in the Table S1. Between baseline and week 12, the MA-ES group demonstrated significant improvements in serum albumin levels (P<0.001) and notable reductions in inflammatory markers, including CRP (P<0.001), IL-6 (P<0.001), and TNF-α (P<0.001). Similarly, the MA tablet group exhibited significant increases in albumin (P=0.002) and decreases in CRP (P=0.04) and IL-6 (P=0.02), although the reduction in TNF-α did not reach statistical significance (P=0.10). Furthermore, at week 12, the MA-ES group, as compared to the MA tablet group, demonstrated significantly greater improvements in albumin (P<0.001), CRP (P<0.001), IL-6 (P=0.02), and TNF-α (P=0.005).
Safety
The overall incidence of AEs was 59.2% in the MA-ES group and 66.0% in the MA tablet group. The incidence of grade ≥3 AEs was 18.4% in the MA-ES group and 20.0% in the MA tablet group (Table 2). In the MA-ES group, AEs with an incidence exceeding 5% included rash, diarrhea, abdominal distension, constipation, hyperglycemia, insomnia, fatigue, pain, and nausea. In the MA tablet group, AEs with an incidence exceeding 5% included rash, pain, diarrhea, constipation, fatigue, nausea, vomiting, abdominal distension, and muscular weakness.
Table 2
| Event | MA-ES group (n=76), n (%) | MA tablet group (n=50), n (%) |
|---|---|---|
| Any adverse event | 45 (59.2) | 33 (66.0) |
| Adverse events grades 3, 4, or 5 | 14 (18.4) | 10 (20.0) |
| Occurred in ≥3% of patients in either group | ||
| Rash | 9 (11.8) | 6 (12.0) |
| Diarrhea | 7 (9.2) | 5 (10.0) |
| Abdominal distension | 6 (7.9) | 3 (6.0) |
| Constipation | 6 (7.9) | 5 (10.0) |
| Hyperglycemia | 5 (6.6) | 2 (4.0) |
| Insomnia | 5 (6.6) | 2 (4.0) |
| Fatigue | 4 (5.3) | 5 (10.0) |
| Pain | 4 (5.3) | 6 (12.0) |
| Nausea | 4 (5.3) | 4 (8.0) |
| Venous thrombosis limb | 3 (3.9) | 2 (4.0) |
| Hypertension | 3 (3.9) | 1 (2.0) |
| Vomiting | 3 (3.9) | 4 (8.0) |
| Blood cholesterol increased | 3 (3.9) | 0 (0.0) |
| Libido decreased | 2 (2.6) | 2 (4.0) |
| Muscular weakness | 2 (2.6) | 3 (6.0) |
| Anemia | 2 (2.6) | 2 (4.0) |
| Hyperkalemia | 1 (1.3) | 2 (4.0) |
| Fever | 0 | 2 (4.0) |
MA-ES group, nanocrystalline megestrol acetate group; MA tablet group, megestrol acetate tablet group.
QoL
The mean scores for the EORTC QLQ-C30 measures at baseline and week 12 are presented in Table 3. In the MA-ES group, significant improvements were observed in appetite loss (P<0.001), nausea and vomiting (P=0.002), and global health status (P<0.001) by week 12. In contrast, the MA tablet group showed significant improvement only in appetite loss (P=0.006), although global health status and nausea and vomiting improved to a degree, these changes did not reach statistical significance.
Table 3
| Domain | MA-ES group (n=76) | MA tablet group (n=50) | |||||
|---|---|---|---|---|---|---|---|
| Baseline | Week 12 | P value | Baseline | Week 12 | P value | ||
| Functional scale | |||||||
| Physical functioning | 54.1±15.4 | 57.4±12.8 | 0.15 | 51.1±16.4 | 50.0±15.0 | 0.73 | |
| Role functioning | 60.4±16.0 | 62.1±16.6 | 0.52 | 54.5±11.1 | 55.8±10.6 | 0.55 | |
| Emotional functioning | 66.5±15.1 | 69.7±13.0 | 0.16 | 61.5±10.8 | 62.3±11.9 | 0.72 | |
| Cognitive functioning | 72.2±12.1 | 71.3±12.9 | 0.67 | 74.4±8.3 | 75.3±11.1 | 0.62 | |
| Social functioning | 66.6±14.6 | 65.8±14.2 | 0.70 | 66.4±13.8 | 70.3±12.9 | 0.14 | |
| Symptom scale | |||||||
| Fatigue | 57.4±20.0 | 52.0±15.4 | 0.06 | 51.6±12.9 | 49.4±12.7 | 0.38 | |
| Nausea and vomiting | 29.5±13.3 | 22.7±13.7 | 0.002 | 26.0±10.1 | 23.2±8.2 | 0.12 | |
| Pain | 39.6±19.5 | 34.7±16.6 | 0.09 | 32.0±13.2 | 30.2±10.6 | 0.46 | |
| Dyspnea | 42.5±18.9 | 39.1±17.3 | 0.25 | 31.6±14.8 | 36.3±15.1 | 0.11 | |
| Insomnia | 34.95±20.2 | 31.54±18.7 | 0.28 | 34.4±16.0 | 33.5±15.9 | 0.77 | |
| Appetite loss | 56.7±15.4 | 36.1±18.6 | <0.001 | 57.3±12.6 | 48.4±18.2 | 0.006 | |
| Constipation | 27.9±14.0 | 28.1±15.5 | 0.91 | 24.9±13.9 | 23.7±13.0 | 0.65 | |
| Diarrhea | 17.1±7.4 | 18.0±8.3 | 0.50 | 16.6±8.2 | 15.0±8.2 | 0.35 | |
| Financial difficulties | 15.0±10.3 | 15.4±10.6 | 0.78 | 14.3±9.7 | 13.8±8.2 | 0.77 | |
| Global health status | 47.4±11.9 | 55.8±12.1 | <0.001 | 48.4±11.6 | 51.2±12.6 | 0.25 | |
Data are presented as mean ± standard deviation. MA-ES group, nanocrystalline megestrol acetate group; MA tablet group, megestrol acetate tables group. EORTC QLQ-C30, European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire Core 30.
Discussion
In this study, we enrolled hormone-insensitive patients with CACS who were treated with three cycles of MA at a daily dose of 800 mg (MA-ES administered at 625 mg/day). A total of 126 participants were included after 1:2 PSM analysis, with 76 in the MA-ES group and 50 in the MA tablet group. Our findings revealed that MA-ES could provide significantly greater weight gain benefits as compared to MA tablets. The patients treated with MA-ES appeared to experience significant improvements in appetite and overall QoL. Moreover, the safety profile was equivalent between two groups.
CACS is a multifaceted clinical syndrome characterized by malnutrition, weight loss, muscle wasting, anorexia, disrupted fat metabolism, inflammation, gut microbiota imbalance, and frailty (1,30). Tumor cells secrete various factors, including cytokines such as IL-1, IL-6, and TNF-α, which induce muscle atrophy and impact tissues in the brain, myocardium, gut, and adipocytes. This process not only triggers CACS but also exacerbates its progression (8,31). Cytokines can activate pro-opiomelanocortin (POMC)/cocaine amphetamine-regulated transcript (CART) neurons in the hypothalamic arcuate nucleus, which primarily function to suppress appetite and reduce food intake. Simultaneously, they inhibit neuropeptide Y (NPY)/agouti-related protein (AgRP) neurons, which promote appetite and increase food intake. This dual action leads to anorexia (14,32-35). Previous search has reported that MA can inhibit the secretion of IL-1β, IL-6, and TNF-α, thereby alleviating or reversing CACS through both humoral and neural regulatory mechanisms (15,16,36-39). Our study further corroborates these findings. From baseline to week 12, the levels of CRP, IL-6, and TNF-α were significantly reduced in the MA-ES group. In the MA tablet group, the CRP and IL-6 levels were also significantly lowered, while the TNF-α level decreased, but not significantly so.
The therapeutic impact of MA on CACS is closely tied to its dosing regimen. In a randomized controlled trial, patients with CACS were divided into four groups receiving MA at 160, 480, 800, and 1,280 mg/day, respectively, for 12 weeks. The findings indicated that the most substantial improvements in appetite (P=0.02) and food intake (P=0.003) were achieved at a dose of 800 mg/day. The corresponding average weight gains across these four groups were 0.31, 0.91, 3.6, and 2.6 kg, respectively (25). In a separate study, patients with cachexia were randomized to receive MA at 100, 400, or 800 mg/day for 3 months, with results consistently indicating 800 mg/day as the most effective dose for enhancing appetite (26). Collectively, these clinical findings support 800 mg/day as the optimal dose of MA for treating CACS. Our study further demonstrated that, after 12 weeks of treatment with 800 mg/day MA (625 mg of MA-ES is bioequivalent to 800 mg of non-MA-ES), patients in the MA-ES group experienced a significantly greater weight gain compared to those in the MA tablet group, with respective increases of 4.49 and 2.10 kg (P<0.001).
The superior efficacy of MA-ES compared to the MA tablet may primarily be attributed to the optimization achieved through nanocrystalline technology. MA is a Biopharmaceutics Classification System class II drug, which is poorly soluble in water and typically requires co-administration with a high-fat, high-calorie meal to ensure adequate absorption. In its traditional tablet form, MA achieves a Cmax of only 187 ng/mL under fasting conditions, which falls short of the minimum effective concentration threshold of 300 ng/mL (17,18,20). MA nanocrystal dispersion optimized by nanocrystalline technology is a highly concentrated suspension with enhanced solubility (40). As a result, MA-ES significantly improves bioavailability, with a Cmax of up to 1,133 ng/mL under fasting conditions, ensuring efficacy both in the fasted and fed states (20,41).
Previous research has consistently demonstrated the superior therapeutic efficacy of MA-ES. A randomized controlled trial conducted among patients with cachexia revealed that those receiving MA-ES achieved an average weight gain of 5.4 kg from baseline. Specifically, 69.1% of these patients experienced a weight increase of at least 5% from baseline, and 72% achieved an absolute weight gain exceeding 5 pounds (Ibs). In comparison, the traditional MA group exhibited a more modest average weight gain of 3.5 kg from baseline, with 57.4% of patients achieving a 5% weight increase from baseline and 64.2% realizing an absolute weight gain of more than 5 Ibs (27). In our study, the MA-ES group demonstrated an average weight gain of 4.49 kg by week 12. Notably, 78.9% of these patients achieved a weight gain of ≥5%, and 81.6% realized an absolute weight increase of more than 5 Ibs. Conversely, for those receiving MA tablets, the average weight gain was 2.10 kg, with only 24% of patients achieving a weight gain of ≥5% and 26% achieving an absolute weight increase of more than 5 Ibs. Furthermore, subgroup analyses demonstrated that the MA-ES group provided a more substantial weight gain benefit across most subgroups. Patients with stage I–II disease tended to benefit from MA tablets but not significantly so. Due to the limited sample size of the stage I–II patients, further exploration and validation with larger cohorts are needed in the future.
In this study, using the EORTC QLQ-C30 questionnaire, we observed that the MA-ES group demonstrated significant improvements in global health status, appetite, and nausea/vomiting. Conversely, these parameters in the MA tablet group tended to worsen, but not significantly so. The notable reduction in nausea and vomiting in the MA-ES group may be attributed to MA’s ability to suppress the synthesis and release 5-hydroxytryptamine (5-HT) (39). Additionally, both fatigue and pain were decreased in the MA-ES group, which may be potentially linked to the observed reductions in cytokine levels (42,43). Additionally, the incidence of safety events remained comparable to those reported in previous study (44).
Newer agents (such as anamorelin and ghrelin receptor agonists) have shown a trend of efficacy. However, two randomized controlled trials showed that anamorelin can increase lean body mass but does not improve grip strength, so it is only approved in Japan and has not been approved by the European Medical Agency (45,46). Additionally, a Phase II study demonstrated that in patients with cancer cachexia and elevated GDF-15 levels, ponsegromab treatment to inhibit GDF-15 resulted in substantial weight gain and significant alleviation of cachexia symptoms (2). We look forward to further validation through subsequent studies.
Our study involved certain limitations that should be addressed. First, we did not conduct a randomized controlled trial, which limits the strength of our conclusions. Secondly, while PSM was employed to adjust for confounding factors, unrecognized biases and residual confounding may still be present. Thirdly, all exploratory analyses were conducted without multiplicity adjustment and should be interpreted with caution. Additionally, supportive care measures were not standardized. Finally, the relatively small sample size underscores the need for larger-scale investigations to further validate our findings.
Conclusions
MA-ES at a dosage of 625 mg/day may offer more substantial weight gain benefits compared to MA tablets at 800 mg/day in hormone-insensitive patients with CACS. Additionally, MA-ES appears to provide more pronounced benefits in improving appetite and overall QoL.
Acknowledgments
The authors greatly appreciate all patients who contributed to this study.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://tcr.amegroups.com/article/view/10.21037/tcr-2025-866/rc
Data Sharing Statement: Available at https://tcr.amegroups.com/article/view/10.21037/tcr-2025-866/dss
Peer Review File: Available at https://tcr.amegroups.com/article/view/10.21037/tcr-2025-866/prf
Funding: This study was financially supported by
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tcr.amegroups.com/article/view/10.21037/tcr-2025-866/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study protocol was approved by the Institutional Review Board of the Main Research Center at Xuzhou Central Hospital, China (No. XZXY-LK-20241014-0154, data: October 14, 2024, protocol version: V1.0) and was conducted in accordance with the Declaration of Helsinki and its subsequent amendments. All participants provided written informed consent, including permission for data disclosure and publication. Miaoshou Internet Hospital was informed and agreed with this study.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Fearon K, Strasser F, Anker SD, et al. Definition and classification of cancer cachexia: an international consensus. Lancet Oncol 2011;12:489-95. [Crossref] [PubMed]
- Groarke JD, Crawford J, Collins SM, et al. Ponsegromab for the Treatment of Cancer Cachexia. N Engl J Med 2024;391:2291-303. [Crossref] [PubMed]
- Ni J, Zhang L. Cancer Cachexia: Definition, Staging, and Emerging Treatments. Cancer Manag Res 2020;12:5597-605. [Crossref] [PubMed]
- Li X, Hu C, Zhang Q, et al. Cancer cachexia statistics in China. Precision Nutrition 2022;1: [Crossref]
- Bruera E, Sweeney C. Cachexia and asthenia in cancer patients. Lancet Oncol 2000;1:138-47. [Crossref] [PubMed]
- Dewys WD, Begg C, Lavin PT, et al. Prognostic effect of weight loss prior to chemotherapy in cancer patients. Eastern Cooperative Oncology Group. Am J Med 1980;69:491-7. [Crossref] [PubMed]
- You Y, Wang Y, Zhang G, et al. The Molecular Mechanisms and Treatment of Cancer-Related Cachexia. J Nutr Sci Vitaminol (Tokyo) 2025;71:1-15. [Crossref] [PubMed]
- Setiawan T, Sari IN, Wijaya YT, et al. Cancer cachexia: molecular mechanisms and treatment strategies. J Hematol Oncol 2023;16:54. [Crossref] [PubMed]
- Roeland EJ, Bohlke K, Baracos VE, et al. Management of Cancer Cachexia: ASCO Guideline. J Clin Oncol 2020;38:2438-53. [Crossref] [PubMed]
- NCCN Clinical Practice Guidelines in Oncology: Palliative Care. Version 1.2023 J, 2023.
- Arends J, Strasser F, Gonella S, et al. Cancer cachexia in adult patients: ESMO Clinical Practice Guidelines ESMO Open 2021;6:100092. [Crossref] [PubMed]
- Arends J, Baracos V, Bertz H, et al. ESPEN expert group recommendations for action against cancer-related malnutrition. Clin Nutr 2017;36:1187-96. [Crossref] [PubMed]
- Chowdhury IH, Rahman MS, Chowdhury MNK, et al. Mirtazapine versus megestrol acetate in treatment of anorexia-cachexia in advanced cancer patients: a randomized, double-blind trial. Jpn J Clin Oncol 2024;54:530-6. [Crossref] [PubMed]
- Hariyanto TI, Kurniawan A. Appetite problem in cancer patients: Pathophysiology, diagnosis, and treatment. Cancer Treat Res Commun 2021;27:100336. [Crossref] [PubMed]
- Inui A. Cancer anorexia-cachexia syndrome: current issues in research and management. CA Cancer J Clin 2002;52:72-91. [Crossref] [PubMed]
- Fedotcheva TA. Clinical Use of Progestins and Their Mechanisms of Action: Present and Future Sovrem Tekhnologii Med 2021;13:93-106. (Review). [Crossref] [PubMed]
- Galia E, Nicolaides E, Hörter D, et al. Evaluation of various dissolution media for predicting in vivo performance of class I and II drugs. Pharm Res 1998;15:698-705. [Crossref] [PubMed]
- Amidon GL, Lennernäs H, Shah VP, et al. A theoretical basis for a biopharmaceutic drug classification: the correlation of in vitro drug product dissolution and in vivo bioavailability. Pharm Res 1995;12:413-20. [Crossref] [PubMed]
- Food Effect Working Group of the Biopharmaceutics Coordinating Committee, Office of Pharmaceutical Science Guidance for industry: food-effect bioavailability and fed bioequivalence studies. Available online: http://www.fda.gov/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/ucm064964.htm. Published December 2002. Accessed June 1, 2009.
- Deschamps B, Musaji N, Gillespie JA. Food effect on the bioavailability of two distinct formulations of megestrol acetate oral suspension. Int J Nanomedicine 2009;4:185-92. [Crossref] [PubMed]
- Loprinzi CL, Ellison NM, Schaid DJ, et al. Controlled trial of megestrol acetate for the treatment of cancer anorexia and cachexia. J Natl Cancer Inst 1990;82:1127-32. [Crossref] [PubMed]
- Lim YL, Teoh SE, Yaow CYL, et al. A Systematic Review and Meta-Analysis of the Clinical Use of Megestrol Acetate for Cancer-Related Anorexia/Cachexia. J Clin Med 2022;11:3756. [Crossref] [PubMed]
- Berenstein EG, Ortiz Z. Megestrol acetate for the treatment of anorexia-cachexia syndrome. Cochrane Database Syst Rev 2005;CD004310. [Crossref] [PubMed]
- Ruiz Garcia V, López-Briz E, Carbonell Sanchis R, et al. Megestrol acetate for treatment of anorexia-cachexia syndrome. Cochrane Database Syst Rev 2013;2013:CD004310. [Crossref] [PubMed]
- Loprinzi CL, Michalak JC, Schaid DJ, et al. Phase III evaluation of four doses of megestrol acetate as therapy for patients with cancer anorexia and/or cachexia. J Clin Oncol 1993;11:762-7. [Crossref] [PubMed]
- Von Roenn JH. Randomized trials of megestrol acetate for AIDS-associated anorexia and cachexia. Oncology 1994;51:19-24. [Crossref] [PubMed]
- Cilla DD, Gutierrez JL, Femia RA, et al. A Pilot Study Comparing Megestrol Acetate Concentrated Suspension (MA-CS) to Megestrol Acetate Oral Suspension (MA-OS) on Weight Gain and Body Composition in Patients with HIV-Associated Unintended Weight Loss (UWL). Blood 2005;106:1433.
- Ribaudo JM, Cella D, Hahn EA, et al. Re-validation and shortening of the Functional Assessment of Anorexia/Cachexia Therapy (FAACT) questionnaire. Qual Life Res 2000;9:1137-46. [Crossref] [PubMed]
- Aaronson NK, Ahmedzai S, Bergman B, et al. The European Organization for Research and Treatment of Cancer QLQ-C30: a quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst 1993;85:365-76. [Crossref] [PubMed]
- Rausch V, Sala V, Penna F, et al. Understanding the common mechanisms of heart and skeletal muscle wasting in cancer cachexia. Oncogenesis 2021;10:1. [Crossref] [PubMed]
- Zhao B, Shi G, Shi J, et al. Research progress on the mechanism and treatment of cachexia based on tumor microenvironment. Nutrition 2025;133:112697. [Crossref] [PubMed]
- Laviano A, Meguid MM, Inui A, et al. Therapy insight: Cancer anorexia-cachexia syndrome--when all you can eat is yourself. Nat Clin Pract Oncol 2005;2:158-65. [Crossref] [PubMed]
- Currie PJ, Mirza A, Fuld R, et al. Ghrelin is an orexigenic and metabolic signaling peptide in the arcuate and paraventricular nuclei. Am J Physiol Regul Integr Comp Physiol 2005;289:R353-8. [Crossref] [PubMed]
- Garfield AS, Li C, Madara JC, et al. A neural basis for melanocortin-4 receptor-regulated appetite. Nat Neurosci 2015;18:863-71. [Crossref] [PubMed]
- Morton GJ, Cummings DE, Baskin DG, et al. Central nervous system control of food intake and body weight. Nature 2006;443:289-95. [Crossref] [PubMed]
- Mantovani G, Macciò A, Esu S, et al. Medroxyprogesterone acetate reduces the in vitro production of cytokines and serotonin involved in anorexia/cachexia and emesis by peripheral blood mononuclear cells of cancer patients. Eur J Cancer 1997;33:602-7. [Crossref] [PubMed]
- Goddard LM, Ton AN, Org T, et al. Selective suppression of endothelial cytokine production by progesterone receptor. Vascul Pharmacol 2013;59:36-43. [Crossref] [PubMed]
- McCarthy HD, Crowder RE, Dryden S, et al. Megestrol acetate stimulates food and water intake in the rat: effects on regional hypothalamic neuropeptide Y concentrations. Eur J Pharmacol 1994;265:99-102. [Crossref] [PubMed]
- Mantovani G, Macciò A, Lai P, et al. Cytokine involvement in cancer anorexia/cachexia: role of megestrol acetate and medroxyprogesterone acetate on cytokine downregulation and improvement of clinical symptoms. Crit Rev Oncog 1998;9:99-106. [Crossref] [PubMed]
- Femia RA, Goyette RE. The science of megestrol acetate delivery: potential to improve outcomes in cachexia. BioDrugs 2005;19:179-87. [Crossref] [PubMed]
- Tian Y, Peng YF, Zhang ZW, et al. Research progress on preparation technology of nanocrystal drugs. Acta Pharmaceutica Sinica 2021;56:1902-10.
- Ruivo J, Tavares I, Pozza DH. Molecular targets in bone cancer pain: a systematic review of inflammatory cytokines. J Mol Med (Berl) 2024;102:1063-88. [Crossref] [PubMed]
- Saligan LN, Kim HS. A systematic review of the association between immunogenomic markers and cancer-related fatigue. Brain Behav Immun 2012;26:830-48. [Crossref] [PubMed]
- Currow DC, Glare P, Louw S, et al. A randomised, double blind, placebo-controlled trial of megestrol acetate or dexamethasone in treating symptomatic anorexia in people with advanced cancer. Sci Rep 2021;11:2421. [Crossref] [PubMed]
- Temel JS, Abernethy AP, Currow DC, et al. Anamorelin in patients with non-small-cell lung cancer and cachexia (ROMANA 1 and ROMANA 2): results from two randomised, double-blind, phase 3 trials. Lancet Oncol 2016;17:519-31. [Crossref] [PubMed]
- Dev R, Amano K, Naito T, et al. Anamorelin for the Treatment of Cancer Anorexia-Cachexia Syndrome. Curr Oncol Rep 2024;26:762-72. [Crossref] [PubMed]



