
Sphingosine kinase 1 facilitates gastric cancer progression via STAT1 gene methylation-mediated mechanisms
Highlight box
Key findings
• Bioinformatics analysis of The Cancer Genome Atlas (n=375) and GEPIA2 (n=562) datasets revealed a significant positive correlation between sphingosine kinase 1 (SPHK1) expression and STAT1 methylation, as well as an association between high SPHK1 levels and poor GC survival. Our research found that SPHK1 expression in gastric cancer (GC) tissues was significantly higher than that in adjacent tissues.
• We found that SPHK1 expression levels in GC tissues were significantly higher than those in adjacent tissues. We also created models for SPHK1 overexpression and silencing. The results indicated that SPHK1 overexpression promoted the proliferation and migration of GC cells while inhibiting apoptosis.
• Our study revealed that SPHK1 influences GC development through the regulation of STAT1 methylation.
What is known and what is new?
• SPHK1 is upregulated in GC and plays a role in its malignant progression.
• Our study aimed to determine whether SPHK1 promotes the progression of GC by modulating DNA methylation of STAT1.
What is the implication, and what should change now?
• The study’s findings of the high expression of SPHK1 in GC tissues support its potential value as a diagnostic marker for GC.
• Given its role in promoting the proliferation and migration of GC cells, SPHK1 and its signaling pathway are promising therapeutic targets for the development of novel drugs. Monitoring SPHK1 expression can aid in assessing treatment efficacy and optimizing personalized therapy.
Introduction
Gastric cancer (GC) remains the fifth most diagnosed cancer in men and women and is the third leading cause of cancer-related death worldwide. It affects a large portion of the global population, particularly in East Asia and South America. The development of GC is the result of complex interactions between epigenetic, genetic, and environmental factors. The occurrence and development of GC is due to the dysregulation of apoptosis genes and DNA repair genes, as well as the regulation signaling pathways, transcription, and cell cycle (1).
DNA methylation is one of the earliest discovered forms of epigenetic change. It is involved in numerous physiological and pathological processes across all organisms. DNA methylation is a chemical modification that results from the transfer of a methyl group from the cofactor S-adenosylmethionine to the C5 position of the pyrimidine ring of the cytosine residue on DNA to form 5-methylcytosine. Methylation of promoter regions determines the expression of tissue-specific genes, the inactivation of the X chromosome, and the silencing of retroviral elements, and it alters the condensed structure of chromatin by affecting either histone DNA or histone contacts. Deregulation of methylation patterns may give rise to serious diseases, including cancer.
Gastrointestinal tumors and inflammatory cells contain activated sphingolipid-metabolizing enzymes, including sphingosine kinase 1 (SPHK1) and sphingosine kinase 2 (SPHK2). Studies have found that elevated SPHK2, as an independent prognostic factor, is predictive of poor survival in patients with GC. SPHK2 promotes the phosphorylation of KLF2, thereby triggering the ubiquitination and degradation of the KLF2 protein in GC (2,3). SPHK1 plays an important role in the proliferation, migration, invasion, and tumor angiogenesis of cancer cells. Its overexpression and activation promote the occurrence and development of esophageal cancer, GC, and colon cancer (4). SPHK1 messenger RNA (mRNA) levels are higher in GC cells and lesions than they are in normal gastric epithelial cells. In addition, hypermethylation of STAT1 has been observed in GC tumor tissues and peripheral blood (5). Researchers have identified STAT1 and the IFN signaling pathway as key regulatory targets of SPHK1, and this represents a key mechanism by which SPHK1 promotes tumor cell survival via the inhibition STAT1 (6,7). Targeting the SPHK1-STAT1 signaling pathway can trigger multifaceted antitumor responses (8). However, there are no relevant reports on the effect of SPHK1 methylation on STAT1. We thus hypothesized that SPHK1 promotes the progression of GC through STAT1 methylation (9). In this study, we clarified the mechanism by which SPHK1 regulates STAT1 methylation from a clinical perspective through cell and mouse experiments and assessed the effect of this mechanism on GC development. We present this article in accordance with the ARRIVE and MDAR reporting checklists (available at https://tcr.amegroups.com/article/view/10.21037/tcr-2025-948/rc).
Methods
Acquisition of GC tissue and paracancerous tissue
Twenty cases of GC tissues (experimental group) and adjacent gastric paracancerous tissues (control group) were collected from Henan Provincial People’s Hospital. All patients with GC were independently diagnosed by two experienced pathologists before subsequent experiments were performed. This study was conducted in accordance with the Declaration of Helsinki and its subsequent amendments. This study was approved by the Ethics Committee of Henan Provincial People’s Hospital (No. 2023047). Written informed consent was obtained from all patients included in this study.
Cell culture and transfection
Mouse forestomach carcinoma (MFC) cells and human GC MKN74 cells were cultured in RPMI-1640 medium supplemented with 10% fetal calf serum and 1% penicillin-streptomycin. The plasmid was transfected into cells using Lipofectamine 3000 (Thermo Fisher Scientific, Waltham, MA, USA), and the transfection was completed 24 hours later.
Quantitative real-time polymerase chain reaction (PCR)
Total DNA from tissues and cells was extracted using TRIzol (Thermo Fisher Scientific), and complement DNA (cDNA) was prepared using the BeyoRT II cDNA first-strand synthesis kit (Beyotime, Haimen, China). cDNA was amplified using TB Green Advantage qPCR premixes (Takara Bio, Kusatsu, Japan), and fluorescence was detected using the CFX Opus Deepwell Real-Time PCR System (Bio-Rad Laboratories, Hercules, CA, USA).
Western blotting
RIPA buffer was used to extract total protein from GC cells and GC tissues. The extracted proteins were separated using sodium dodecyl sulfate-polyacrylamide gel electrophoresis and then electrophoretically transferred to polyvinylidene fluoride (PVDF) membranes (MilliporeSigma, Burlington, MA, USA). After the membrane was blocked with 5% skimmed milk powder for 1 hour at room temperature (23–25 ℃), the corresponding antibody was added, and incubation was conducted at 4 ℃ overnight.
Cell Counting Kit-8 (CCK8) assay
Cell proliferation was assessed using the CCK8 (Beyotime Biotech Inc., Shanghai, China) according to the manufacturer’s instructions. Subsequently, 10 µL of CCK8 solution was to each culture well, which was followed by incubation at 37 ℃ for 2 hours. The absorbance of cells at 450 nm was then detected with a ReadMax 1200 microplate meter (Thermo Fisher Scientific).
Flow cytometry
Cells were collected in centrifuge tubes and incubated in the dark with propidium iodide (Thermo Fisher Scientific) and membrane-bound protein annexin v-fluorescein isothiocyanate (BD Biosciences, Franklin Lakes, NJ, USA) for 30 minutes, and fluorescence was analyzed to identify apoptotic cells via the FACSCanto II flow cytometry measurement system (BD Biosciences).
DNA isolation and methylation-specific PCR
Genomic DNA was extracted from cells and tissues using the Genomic Extraction Kit, and unmethylated cytosine was converted to uracil using the DNA Bisulfite Conversion Kit (Qiagen, USA). The STAT1 gene fragment was then amplified using methylation-specific PCR primers. Finally, the amplified products were detected via agarose gel electrophoresis.
Establishment and verification of a GC mouse model
Five million cells in the logarithmic growth phase were digested into 0.2 mL of culture medium and inoculated subcutaneously into male Balb/c mice (Guangdong Yaokang Biotechnology Co., Ltd.; Guangzhou, China). All mice were maintained in a specific pathogen-free environment. After 4 weeks, the animal body weight changes, tumor volume, and weight were recorded. Animal care and euthanasia were approved by the Ethics Committee of School of Life Sciences, Zhengzhou University (No. 2023027), in compliance with institutional guidelines for the care and use of animals. A study protocol was prepared before experimentation but not registered on a public platform.
Hematoxylin and eosin staining
After deparaffinization and rehydration, 5 µm longitudinal sections were stained with hematoxylin solution for 5 minutes and then immersed 5 times in 1% acid ethanol (1% HCl in 70% ethanol) and rinsed in distilled water. The sections were then stained with eosin solution for 3 minutes, which was followed by dehydration with graded ethanol and washing in xylene. The mounted slides were then examined and photographed using a fluorescence microscope.
Immunohistochemistry
Sections of 5 µm paraffin-embedded tumor tissue were kept in an oven at 60 ℃ for 24 hours and then dewaxed with xylene and hydrated with an ethanol gradient (70–100%). After continuous incubation with antigen retrieval solution and 3% H2O2, the slides were rinsed for 30 minutes and incubated with primary antibodies overnight at 4 ℃. For negative controls, the primary antibody was replaced with unimmunized serum. The next day, the slides were rinsed and stained with the corresponding secondary antibodies for 30 minutes, which was followed by 3,3’-diaminobenzidine (DAB) and hematoxylin staining, respectively. The slides were then examined using a fluorescence microscope and photographed.
Statistics analysis
GraphPad Prism (GraphPad Software, San Diego, CA, USA) was used to create statistical maps. Bioinformatics analysis: The Cancer Genome Atlas (TCGA) GC methylation and expression data were downloaded from the UCSC Xena platform (https://xenabrowser.net/). Survival analyses were performed using GEPIA2 (http://gepia2.cancer-pku.cn/), with statistical significance defined as P<0.05. Western blots were analyzed in gray-scale with ImageJ software (National Institutes of Health, Bethesda, MD, USA), and three independent replications of each experiment were conducted. Comparisons between groups were made using the independent samples t-test, and differences were considered statistically significant at P<0.05. All data are expressed as the mean ± standard deviation. All in vitro and in vivo experiments were performed at least three times with biological replicates to ensure reproducibility. Representative results are shown in the figures.
Results
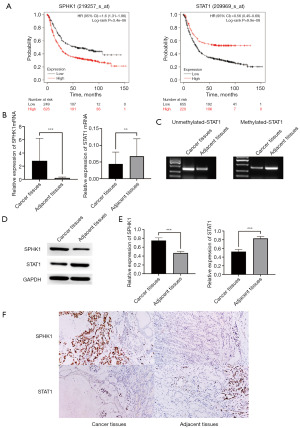
SPHK1 and STAT1 expression change in patients with GC
The prognostic significance of SPHK1 and STAT1 expression in patients with GC was evaluated through the Kaplan-Meier plotter online database, and it was found that the survival time of patients with GC was significantly correlated with the expression differences of SPHK1 and STAT1. A high expression of SPHK1 and a low expression of STAT1 tended to predict lower survival (Figure 1A). To further validate the clinical relevance of SPHK1 and STAT1 methylation, we analyzed The Cancer Genome Atlas (TCGA) GC dataset (n=375). Spearman’s correlation analysis revealed a significant positive association between SPHK1 mRNA expression and STAT1 promoter methylation (r=0.68, P<0.001). Additionally, survival analysis using GEPIA2 (562 GC patients) demonstrated that high SPHK1 expression was strongly correlated with shorter overall survival [hazard ratio (HR) =1.82, 95% confidence interval (CI): 1.23–2.71, P<0.001]. These bioinformatics findings were consistent with our experimental results in clinical samples and cell lines. Next, by detecting the expression of SPHK1 and STAT1 in cancer tissues and paracancerous tissues of 20 pairs of patients with GC, we found that the expression of SPHK1 mRNA in cancer tissues was significantly higher than that in paracancerous tissues, while the expression of STAT1 in cancer tissues was significantly lower than that in paracancerous tissues (Figure 1B). Through methylation-specific PCR, we found that STAT1 methylation in cancer tissues was significantly higher than that in paracancerous tissues, which may be the reason for the decrease in STAT1 expression in cancer tissues (Figure 1C). Simultaneously, through Western blot method, we found that compared with the paracancerous tissues, the expression of SPHK1 was enhanced in the cancer tissues, while the expression of STAT1 protein decreased (Figure 1D,1E). Immunohistochemistry corroborated these results (Figure 1F).
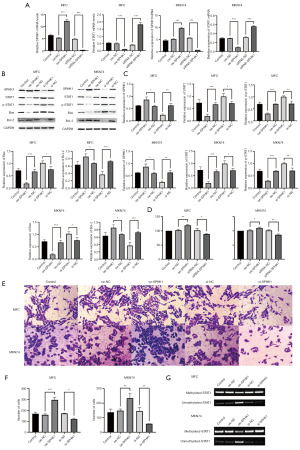
STAT1 expression in GC cells was affected by SPHK1
In order to determine the role of SPHK1 in GC, we constructed an overexpression vector and interference vector of SPHK1 and transfected them into two GC cell lines, MFC and MKN74. Through qPCR, it was found that the overexpression of SPHK1 caused a decrease in the expression of STAT1 in two GC cell line, while knockdown caused an increase in the expression of STAT1 (Figure 2A). Western blot experiments showed the same expression changes of STAT1 and phosphorylated STAT1. Meanwhile, the apoptosis-related proteins Bax and Bcl-2 demonstrated reduced apoptosis when SPHK1 was overexpressed, while the opposite was true when SPHK1 was knocked down (Figure 2B,2C). The CCK8 assay confirmed these results (Figure 2D). Through Transwell experiments, it was found that two types of GC cells showed stronger invasive ability when overexpressing SPHK1 but showed lower invasive ability after SPHK1 expression was interfered with (Figure 2E,2F). Methylation-specific PCR demonstrated that SPHK1 influenced the methylation of the STAT1 gene (Figure 2G).
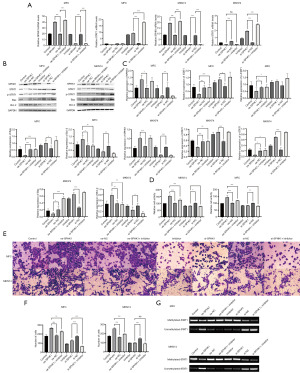
The methylation regulation of the STAT1 gene via SPHK1 was affected by methyltransferase inhibitors
To determine whether SPHKK1 regulates STAT1 expression through methylation, we treated two types of GC cells with a methyltransferase inhibitor and found that the reduced STAT1 expression level, decreased apoptosis, and increased invasive capacity caused by SPHK1 overexpression were blocked by the methyltransferase inhibitor. Conversely, interfering with the expression of SPHK1 and adding methyltransferase inhibitors caused these two cancer cells to have a higher expression of STAT1 and tended to reduce their invasion ability and induce apoptosis. These effects may represent an effective approach for treating GC (Figure 3).
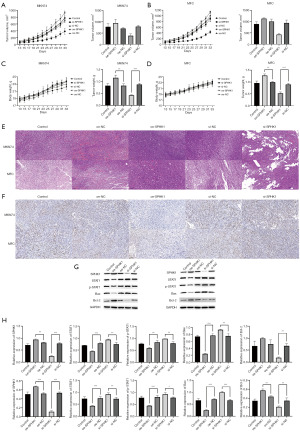
SPHK1 regulated STAT1 methylation and affected tumor development in mice
In order to further determine whether the regulation of STAT1 gene methylation SPHK1, we subcutaneously injected MFC and MKN74 GC cells into mice. By overexpressing and interfering with the expression of SPHK1 in mice, we found that regardless of whether MFC or MKN74 was loaded, the overexpression of SPHK1 accelerated the development of tumors and the death of mice, while knocking down SPHK1 could effectively slow down the progression of tumors and death of cancer-affected mice. Hematoxylin and eosin staining and immunohistochemistry indicated that SPHK1 reduced the expression of STAT1, and its reduced expression accelerated the apoptosis of GC cells (Figure 4).
Discussion
Cell membrane sphingomyelin derivatives play an extremely critical role in regulating the dynamic balance of cell proliferation and apoptosis. A high expression of SPHK1 can not only stimulate cell growth but can also lead to the malignant transformation of cells, and thus SPHK1 itself also has the characteristics of an oncogene (10). Recently, in a study of clinical case data, it was found that the expression level of SPHK1 is related to clinical stage and TNM tumor stage (11). People with overexpressed SPHK1 have a short overall survival time, while those with a low expression of SPHK1 are conversely affected by lysophosphatidic acid (LPA) and endothelial growth factor. The tumor microenvironment mediates the upregulation of SPHK1 expression during GC cell invasion and migration. In GC MKN1 cells, LPA significantly increases the mRNA and protein levels of SPHK1. Downregulating the expression of SPHK1 can weaken the migration and invasion of MKN1 cells stimulated by LPA (12). Therefore, SPHK1 is expected to become an independent predictor and therapeutic target of GC. In addition, SPHK1 can induce autophagy in peritoneal mesothelial cells and enhance the peritoneal spread of GC. The cytokine TGF-β1 induces autophagy in human peritoneal mesothelial cells (HPMCs) and promotes gastric cancer peritoneal dissemination (GCPD) through SPHK1. Overexpression of SPHK1 induces HPMC fibrosis, while inhibition of autophagy attenuates HPMC fibrosis. Moreover, SPHK1-mediated autophagy may be a regulator of HPMC fibrosis (13). These facts may constitute the mechanism underlying GCPD and suggest SPHK1 as a new target for controlling GCPD. In our study, both the survival analysis of network data and the examination of clinical patients indicated that SphK1 is closely related to GC. We also found similar findings for STAT1, and its gene methylation demonstrated a certain correlation with GC. These findings are consistent with the above description of SPHK1 and suggest that STAT1 methylation may also be involved.
Tumor epigenetics is a rapidly evolving field in oncology, with growing evidence that tumors arise from the interplay of genetic and epigenetic alterations (14). In GC, DNA methylation of tumor-suppressor genes is a critical epigenetic event, often leading to transcriptional silencing and promoting carcinogenesis, progression, and metastasis (15,16). The bioinformatics analyses from TCGA and GEPIA2 strengthened the clinical significance of our findings. In a large GC cohort (n=375), the positive correlation between SPHK1 expression and STAT1 methylation validated the epigenetic mechanism observed in our experimental models. Moreover, the independent prognostic value of SPHK1 (562 patients) supports its potential as a robust biomarker for GC prognosis. These data collectively suggest that the SPHK1-STAT1 methylation axis is not only mechanistically relevant but also clinically actionable, providing a rationale for targeting this pathway in GC therapy. In our study, we identified a significant correlation between STAT1 methylation and GC progression. Reduced STAT1 expression, linked to promoter hypermethylation, was associated with enhanced cell proliferation and invasion, while increased STAT1 levels correlated with apoptosis induction. These findings suggest STAT1 functions as a tumor suppressor in GC, and its methylation represents a potential therapeutic target.
Sphingosine-1-phosphate (S1P), produced by SPHK1 and SPHK2, is a highly bioactive compound. Many inflammatory responses, including lymphocyte trafficking, are directed by circulating S1P, which is found in high concentrations in both plasma and lymph in patients with cancer (17). High-fat and high-sugar diets, intestinal dysbiosis, and obesity have recently been implicated in the activation of inflammation and SPHK/S1P/S1P receptor (S1PR) signaling in numerous gastrointestinal pathologies, including cancer (18,19). Dong et al. reported that diffuse histologic type and lymphatic infiltration in patients with GC were independently associated with the S1P produced by SPHK1 expression, suggesting a role for S1P in disease progression in GC (20). Shida Dai et al. found that LPA significantly enhanced SPHK1 in GC MKN1 cells but had no effect on SphK2 (21). Man et al. found that downregulation of miR-124 in MGC-803 and SGC-7901 GC cells induced the upregulation of SPHK1 and that miR-124 plays an important role in gastric carcinogenesis through the modulation of the SPHK1/AKT/FOXO1 signaling pathway (22). In a study of endothelial dysfunction in the heart, ALKBH5 was found to help maintain angiogenesis in endothelial cells after acute ischemic stress by decreasing SPHK1 m6A methylation and the downstream eNOS-AKT signaling pathway (23). In the study by Zhang et al., it was found that the deletion of NF-kB1 and the ensuing aberrant activation of STAT1 collectively lead to sterile inflammation, dysregulation of immune checkpoints, and ultimately to the development of GC (24). In addition, Huang et al. inhibited cell proliferation and induced apoptosis in mammary cancer stem cells (CSCs) and non-CSCs via the downregulation of SPHK1, whereas aberrant expression of SPHK1 enhanced the survival of mammary CSCs and the efficiency of mammary sphere formation (25). STAT1 is critical to the regulation of tumor progression by SPHK1. SPHK1 can promote tumor progression by inhibiting the expression of STAT1, but the specific regulatory mechanism has not been clarified. To clarify this gap, prior studies have demonstrated that SPHK1 modulates STAT signaling through multiple mechanisms. For example, in breast cancer, SPHK1 suppresses STAT1 phosphorylation via the S1P/S1PR1-JAK2 axis, thereby inhibiting IFN-γ-mediated antitumor immunity (9). In colorectal cancer, SPHK1 activates STAT3 through AKT signaling to enhance cell survival (22). These findings highlight the context-dependent crosstalk between SPHK1 and STAT pathways. By overexpressing and knocking down SPHK1 in GC cells and mice, we found that methylation of the STAT1 gene was also affected, and this effect could be reversed by methyltransferase inhibitors. Therefore, we suggest that SPHK1 can regulate GC progression by affecting the methylation of STAT1. However, whether SPHK1 directly or indirectly regulates the methylation process of STAT1 and the specific mechanism need to be further investigated. Although two cell types were selected for our experiments, GC cells from both demonstrated the strong regulatory effect of SPHK1 on STAT1 gene methylation. In the survival analysis of network data, SPHK1 showed a poor prognostic correlation in both GC and other cancers. The phosphorylation and nuclear entry of STAT3, another cancer-promoting gene, regulate gene expression and are closely related to the proliferation of cancer cells. In this study, we found that SPHK1 played a role in regulating the methylation of the STAT1 gene. Therefore, SPHK1 may be a highly suitable parent target for cancer therapy. The interference in SPHK1 expression and inhibition of its activity, as well as the inhibition of methyltransferase, may be novel methods of treating GC. However, this study only demonstrated that SPHK1 can regulate the progression of GC by promoting STAT1 methylation and did not elaborate upon the mechanism by which methylation is regulated. In future studies, we will seek to clarify this mechanism, which may provide a more reproducible theoretical basis for using SPHK1 as a cancer treatment target.
DNA methylation plays an important role in the evolution of GC, and clarifying its role in the pathogenesis of GC may hold considerable significance for the early diagnosis, treatment, and prognosis of GC and of other cancers. However, the exact mechanism underlying the link between DNA methylation and the progression of GC, as well the related methods for early detection, treatment, and prognostic assessment, remain unclear and controversial; therefore, further studies are needed.
Conclusions
This study scrupulously examined the process by which SPHK1 regulates the expression of the STAT1 gene through methylation, thereby affecting the development of GC. SPHK1, as a proto-oncogene, exhibits a strong cancer-promoting effect, and methyltransferase inhibitors effectively limit the regulation of SPHK1. This provides a certain theoretical basis for cancer occurrence and cancer metastasis. Perhaps intervening upon the methylation of SPHK1 may be effective in treating GC.
Acknowledgments
None.
Footnote
Reporting Checklist: The authors have completed the ARRIVE and MDAR reporting checklists. Available at https://tcr.amegroups.com/article/view/10.21037/tcr-2025-948/rc
Data Sharing Statement: Available at https://tcr.amegroups.com/article/view/10.21037/tcr-2025-948/dss
Peer Review File: Available at https://tcr.amegroups.com/article/view/10.21037/tcr-2025-948/prf
Funding: None.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tcr.amegroups.com/article/view/10.21037/tcr-2025-948/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This study was conducted in accordance with the Declaration of Helsinki and its subsequent amendments. This study was approved by the Ethics Committee of Henan Provincial People’s Hospital (No. 2023047). Written informed consent was obtained from all patients included in this study.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Bure IV, Nemtsova MV. Methylation and Noncoding RNAs in Gastric Cancer: Everything Is Connected. Int J Mol Sci 2021;22:5683. [Crossref] [PubMed]
- Greenberg MVC, Bourc'his D. The diverse roles of DNA methylation in mammalian development and disease. Nat Rev Mol Cell Biol 2019;20:590-607. [Crossref] [PubMed]
- Field AE, Robertson NA, Wang T, et al. DNA Methylation Clocks in Aging: Categories, Causes, and Consequences. Mol Cell 2018;71:882-95. [Crossref] [PubMed]
- Zheng X, Li W, Ren L, et al. The sphingosine kinase-1/sphingosine-1-phosphate axis in cancer: Potential target for anticancer therapy. Pharmacol Ther 2019;195:85-99. [Crossref] [PubMed]
- Sanwick AM, Chaple IF. Targeted radionuclide therapy for head and neck squamous cell carcinoma: a review. Front Oncol 2024;14:1445191. [Crossref] [PubMed]
- Acharya S, Yao J, Li P, et al. Sphingosine Kinase 1 Signaling Promotes Metastasis of Triple-Negative Breast Cancer. Cancer Res 2019;79:4211-26. [Crossref] [PubMed]
- Han Q, Zhou H, Xie W, et al. Association between the methylation of the STAT1 and SOCS3 in peripheral blood and gastric cancer. J Gastroenterol Hepatol 2020;35:1347-54. [Crossref] [PubMed]
- Bu YH, Wu H, Sun MH, et al. Role of sphingosine-1-phosphate and its signaling pathway in inflammation-related diseases. Chinese Pharmacological Bulletin 2019;35:1041-5.
- Hii LW, Chung FF, Mai CW, et al. Sphingosine Kinase 1 Regulates the Survival of Breast Cancer Stem Cells and Non-stem Breast Cancer Cells by Suppression of STAT1. Cells 2020;9:886. [Crossref] [PubMed]
- Jin L, Zhu J, Yao L, et al. Targeting SphK1/2 by SKI-178 inhibits prostate cancer cell growth. Cell Death Dis 2023;14:537. [Crossref] [PubMed]
- Bhadwal P, Randhawa V, Vaiphei K, et al. Clinical relevance of CERK and SPHK1 in breast cancer and their association with metastasis and drug resistance. Sci Rep 2022;12:18239. [Crossref] [PubMed]
- Yang Q, Wang XJ. Research progress of sphingosine kinase 1 inhibitors. J China Pharm Univ 2021;52:759-68.
- Yin S, Miao Z, Tan Y, et al. SPHK1-induced autophagy in peritoneal mesothelial cell enhances gastric cancer peritoneal dissemination. Cancer Med 2019;8:1731-43. [Crossref] [PubMed]
- Greenfield G, McMullin MF. Epigenetics in myeloproliferative neoplasms. Front Oncol 2023;13:1206965. [Crossref] [PubMed]
- Sun LX, Guo AB, Zhang YQ, et al. Research Progress on the Relationship between DNA Methylation and Gastric Cancer. Advances in Clinical Medicine 2021;11:965-73.
- Wang QJ, Yuan XM. Role of coiled-coil domain containing proteins in development of gastric cancer. World Chinese Journal of Digestology 2022;30:88-91.
- Zhou GQ, Han F, Shi ZL, et al. DNMT3A-mediated down-regulation of microRNA-105 promotes gastric cancer cell proliferation. Eur Rev Med Pharmacol Sci 2017;21:3377-83.
- Shao X, Liu L, Zhou Y, et al. High-fat diet promotes colitis-associated tumorigenesis by altering gut microbial butyrate metabolism. Int J Biol Sci 2023;19:5004-19. [Crossref] [PubMed]
- Xu W, Shen H. When RNA methylation meets DNA methylation. Nat Genet 2022;54:1261-2. [Crossref] [PubMed]
- Dong Q, Gong C, Jiang Q, et al. Identification of differentially expressed tumour-related genes regulated by UHRF1-driven DNA methylation. Sci Rep 2024;14:18371. [Crossref] [PubMed]
- Shida D, Fang X, Kordula T, et al. Cross-talk between LPA1 and epidermal growth factor receptors mediates up-regulation of sphingosine kinase 1 to promote gastric cancer cell motility and invasion. Cancer Res 2008;68:6569-77. [Crossref] [PubMed]
- Man X, Li Q, Wang B, et al. DNMT3A and DNMT3B in Breast Tumorigenesis and Potential Therapy. Front Cell Dev Biol 2022;10:916725. [Crossref] [PubMed]
- Richard Albert J, Au Yeung WK, Toriyama K, et al. Maternal DNMT3A-dependent de novo methylation of the paternal genome inhibits gene expression in the early embryo. Nat Commun 2020;11:5417. [Crossref] [PubMed]
- Zhang L, Wang S, Wu GR, et al. MBD2 facilitates tumor metastasis by mitigating DDB2 expression. Cell Death Dis 2023;14:303. [Crossref] [PubMed]
- Huang J, Soupir AC, Schlick BD, et al. Cancer Detection and Classification by CpG Island Hypermethylation Signatures in Plasma Cell-Free DNA. Cancers (Basel) 2021;13:5611. [Crossref] [PubMed]
(English Language Editor: J. Gray)


