
A real-world retrospective cohort study: the clinical outcomes and characteristics of platinum-resistant recurrent ovarian cancer
Highlight box
Key findings
• The data of patients from six tertiary referral centers in China were examined to identify the clinical outcomes and characteristics of patients with platinum-resistant recurrent ovarian cancer (PRROC) diagnosed in real-world settings.
What is known, and what is new?
• Real-world research data can assist in optimizing treatment regimens for clinical patients; however, at present, real-world evidence regarding the characteristics, treatment modalities, and outcomes of PRROC remains limited.
• In this real-world cohort, PRROC patients, particularly those with a progression-free interval of >3–6 months, experienced substantial benefits from the re-administration of platinum agents.
What is the implication, and what should change now?
• Platinum rechallenge therapy represents a promising treatment approach for PRROC patients.
• We hope to precisely screen out drug-resistant patients who may benefit from the platinum rechallenge by using molecular markers.
Introduction
Ovarian cancer (OC) is the eighth most common cancer and the eighth most frequent cause of cancer-related death among women worldwide,and thus represents a serious global health concern (1). Despite an initially high response rate to platinum-based chemotherapy (~80%), most patients eventually experience relapse and die for platinum-resistant disease (2), which is commonly characterized by disease recurrence within 6 months following the completion of a platinum-based treatment regimen. Moreover, approximately 20% of patients exhibit no response to frontline platinum-based treatment, and are considered primary platinum refractory cases (i.e., they experience disease progression within 4 weeks of the last administration of platinum-based chemotherapy) (3). The development of resistance to platinum-based chemotherapy is a major hurdle in the treatment of OC and serves as a significant contributor to its unfavorable prognosis (4). Understanding the underlying mechanisms leading to platinum resistance and exploring avenues for its reversal or prevention are vital for improving the overall survival (OS) of OC patients.
Currently, no predictive biomarkers for assessing the likelihood of primary platinum-refractory or platinum-resistant disease in clinical practice have been established (3). Several genetic alterations are associated with acquired resistance to platinum-based chemotherapy, including the inactivation of tumor suppressor genes like RB1, RAD51B, and NF1; reversions of germline or somatic BRCA1/2 mutations; the overexpression of the drug efflux pump MDR1; and the amplification of CCNE1 (5). Ultimately, the mechanisms underpinning platinum resistance are heterogeneous and unclear (6). Notably, resistance to platinum-based therapy remains the leading cause of mortality in advanced OC patients, and there are limited treatment options and a conspicuous absence of standardized guidelines concerning treatment sequencing in the context of platinum resistance (7).
Currently, the standard treatment for platinum-resistant recurrent ovarian cancer (PRROC) involves the administration of sequential single-agent non-platinum chemotherapy or participation in a clinical research trial (6). However, none of the approved agents [including gemcitabine, topotecan, pegylated liposomal doxorubicin (PLD) or paclitaxel] have shown superiority in terms of OS. Response rates typically hover around 10–15%, while progression-free survival (PFS) ranges from 3–4 months, with OS typically falling short of a year (8). Clinically, platinum-resistant ovarian cancer patients have poor responses to alternative single-agent chemotherapy, thus, it is urgent to discover novel active drugs against this recurrent disease. The impact of targeted therapies and immunotherapies (9,10) on PFS and OS are currently under investigation, but the results are either unsatisfactory or very early, awaiting further clinical validation. Currently, clinical trials are the most promising way to establish a new treatment framework for such refractory diseases (11). A variety of strategies, including new chemotherapy drugs, molecular targeted therapies, immune checkpoint blockers and combination therapy regimens, have been evaluated in recent clinical trials or are being explored (12). However, patients treated in actual clinical settings often differ from the research participants of clinical trials who are selected based on strict inclusion and exclusion criteria (13,14). There are some obvious limitations to actual clinical settings, mostly inherent to the nature of non-interventional real-world studies. The patient population was heterogenous, and therapeutic decisions and patient evaluations were at the physician’s discretion and thus may not be homogenous between sites. Research data derived from real-world settings can enhance clinical trial findings and provide valuable insights into the efficacy of prevalent patient interventions in clinical practice (15). Currently, the availability of real-world evidence pertaining to the characteristics, treatment patterns, and outcomes of PRROC remains limited. This is partially due to the lack of specific information about cancer, such as the stage of disease, in large administrative claims databases.
In this study, we have collected clinical data from the National Union of Real-world Gynecological Oncology Research and Patient Management platform (NUWA), which was initiated by the National Clinical Research Center for Obstetrics and Gynecology in 2019. This platform integrates data from both inpatient and outpatient settings, genetic information, and post-treatment follow-up records. A multi-center real-world study was performed to explore the survival profile of PRROC patients and to help investigators understand the status, efficacy, and determinant factors influencing PRROC treatment, providing a basis for the further optimization of treatment options. We present this article in accordance with the STROBE reporting checklist (available at https://tcr.amegroups.com/article/view/10.21037/tcr-2025-641/rc).
Methods
Study population
The primary objective of this population-based, retrospective cohort study was to describe the practice patterns and outcomes observed in the routine care of patients with PRROC. The cohort included patients from six tertiary referral centers (Tongji Hospital affiliated with Huazhong University; The First Affiliated Hospital of Sun Yat-sen University; Shandong Tumor Hospital; The First Affiliated Hospital of Soochow University; The First Affiliated Hospital of Guangxi Medical University; Xuzhou Central Hospital) in China from January 2018 to December 2023. All study patients were followed up to December 1, 2023. To be eligible for inclusion in this study, the patients had to meet the following inclusion criteria: be aged 18 years or older; have a histologically confirmed diagnosis of OC (specifically including epithelial ovarian, fallopian tube, or peritoneal cancer); have platinum-resistant disease (defined as disease progression within 180 days post the last administration of the platinum-based treatment); and have complete clinical and follow-up data available. Individuals with fragmented clinicopathological data and platinum-refractory were excluded from the study (Figure 1). During January 2018 and December 2023, the total No. of platinum resistant recurrent ovarian cancer patients from six tertiary referral centers in China was 588. Excluding 52 patients who did not meet the inclusion criteria and 32 patients lost to follow-up, a total of 504 patients were included in the analysis, subdivided into platinum-based combination chemotherapy regimens (N=227) and single-agent chemotherapy regimens (N=277). The study was conducted in accordance with the Declaration of Helsinki and its subsequent amendments. This study received approval from two ethics committees: Tongji Hospital affiliated with Huazhong University of Science and Technology (No. 2019-S1102) and Xuzhou Central Hospital (No. XZSZXYY-LL-SOP-010-02). All participating hospitals/institutions were informed and agreed with this study. Individual consent for this retrospective analysis was waived.
Data collection
All clinical data were sourced from the NUWA initiative launched by the National Clinical Research Center for Obstetrics and Gynecology in 2019. The integrated data included data from both inpatient and outpatient settings, genetic information, and post-treatment follow-up records. The data were extracted from electronic medical records using a big data platform, and then underwent meticulous processing, standardization, and construction in accordance with pre-defined data nodes. To guarantee the quality of the data, cross-verification was conducted by two independent investigators.
We examined the PFS and OS of the PRROC patients. Efficacy data were stratified based on the platinum sensitivity status of the patients. Platinum-resistant disease was defined as recurrence within 6 months after the final administration of platinum-based chemotherapy. Among the population treated with platinum-containing regimens, we conducted a survival stratification analysis based on the stratification and classification of the reference, progression-free interval (PFI) with a boundary of 3 months [3< PFI ≤6 vs. 0< PFI ≤3 months] (8). PFS was defined as the time from the initiation of the drug treatment to the first sign of disease progression or mortality, whichever occurred first. OS was defined as the time interval between the date of PRROC diagnosis and the date of death from any cause or the date of the last follow-up.
Statistical analysis
The data cut-off date for all analyses was December 1, 2023. Statistical analyses were carried out using SPSS 22.0 statistical software (SPSS Inc., USA). Demographic data are presented as descriptive statistics and summarized as the median or frequency. The Chi-square test or Mann-Whitney U test was used to assess the differences in the patient characteristics between groups. The survival analysis was conducted using the Kaplan-Meier approach and the log-rank test. Both univariate and multivariate analyses were conducted using the Cox proportional hazard regression model. A P value <0.05 was considered statistically significant.
Results
Patient characteristics
In total, 504 PRROC patients met the eligibility criteria for this cohort, of whom 236 (46.83%) were classified as primary platinum resistant, and 268 (53.17%) were classified as acquired platinum resistant. The baseline characteristics of the patients are outlined in Table 1. The vast majority of patients (90.07%) had advanced stage disease. All patients received tumor cell reduction, while 145 (28.77%) received neoadjuvant chemotherapy, 80.36% underwent surgery through conventional laparotomy, and 19.64% underwent minimally invasive surgery. Satisfactory tumor cell reduction was achieved in 279 patients (55.36%). Among the histological types, 414 (82.14%) patients had high-grade serous carcinoma (HGSC), 45 (8.93%) clear cell carcinoma (CCC), 17 (3.37%) endometrioid carcinoma (EC), 15 (2.98%) mucinous carcinoma (MC), and 13 (2.58%) low-grade serous carcinoma (LGSC). The median number of prior treatment regimens was three, and 23.61% of the patients had received extensive pretreatment involving three or more regimens. Specifically, before PRROC diagnosis, 30 patients (5.95%) had been treated with poly (adenosine diphosphate-ribose) polymerase inhibitor (PARPi), 88 (17.46%) had received antiangiogenic therapy, and 24 (4.76%) had participated in clinical trials. All cases were diagnosed as PRROC until December 1, 2023, and the average follow-up time was 16.50 months.
Table 1
| Characteristics | Values |
|---|---|
| Stage | |
| I | 23 (4.56) |
| II | 27 (5.36) |
| III | 331 (65.67) |
| IV | 123 (24.40) |
| Histology | |
| HGSC | 414 (82.14) |
| CCC | 45 (8.93) |
| EC | 17 (3.37) |
| MC | 15 (2.98) |
| LGSC | 13 (2.58) |
| Platinum status | |
| Primary platinum resistant | 236 (46.83) |
| Acquired platinum resistant | 268 (53.17) |
| Debulking | |
| Optimal | 279 (55.36) |
| Suboptimal | 130 (25.79) |
| Unknown | 95 (18.85) |
| Surgical procedures | |
| Epigastric | 405 (80.36) |
| Minimally invasive | 99 (19.64) |
| Neoadjuvant | |
| Yes | 145 (28.77) |
| No | 359 (71.23) |
| Treatment received before PRROC diagnosis | |
| Surgery | 504 (100.00) |
| Neoadjuvant | 145 (28.77) |
| Antiangiogenic therapy | 88 (17.46) |
| PARPi | 30 (5.95) |
| No. of previous lines of anticancer therapy | |
| 1–2 | 385 (76.39) |
| ≥3 | 119 (23.61) |
| Clinical trial participation | 24 (4.76) |
| Follow-up time, months | 16.50±10.46 |
Data are presented as mean ± standard deviation or n (%). HGSC, high-grade serous carcinoma; CCC, clear cell carcinoma; EC, endometrioid carcinoma; MC, mucinous carcinoma; LGSC, low-grade serous carcinoma; PRROC, platinum-resistant recurrent ovarian cancer; PARPi, poly (adenosine diphosphate-ribose) polymerase inhibitor.
Real-world treatment patterns and outcomes in PRROC
In this cohort of 504 PRROC patients, 277 (54.96%) received single-agent non-platinum chemotherapy. Of these, 184 (66.43%) also received bevacizumab (Bev). 227 (45.04%) received a platinum-containing regimen, and 94 (41.41%) received additional Bev. Meanwhile, among the 504 patients, the treatment regimens of 36 (7.14%) patients contained PARPi monotherapy. The median PFS and OS in the single-agent non-platinum chemotherapy group were 4.0 and 13.0 months, respectively. The median PFS and OS in the platinum-based combination chemotherapy group were 5.6 and 15.9 months, respectively. The median PFS and OS in the PARPi monotherapy group were 4.5 and 13.1 months, respectively.
Compared to the total single-agent non-platinum chemotherapy group, the total platinum-based combination chemotherapy group had significantly longer OS [15.9 vs. 13.0 months, hazard ratio (HR): 0.766, 95% confidence interval (CI): 0.638–0.919, P=0.005] and PFS [5.6 vs. 4.0 months, HR: 0.431, 95% CI: 0.356–0.521, P<0.001] (Figure 2A,2B). The PARPi monotherapy group tended to have a statistically non-significant, minimally improved OS (13.1 vs. 13.0 months, HR: 1.101, 95% CI: 0.767–1.580, P=0.56) and PFS (4.5 vs. 4.0 months, HR: 0.788, 95% CI: 0.573–1.085, P=0.15) compared with the single-agent non-platinum chemotherapy group (Figure 2C,2D).
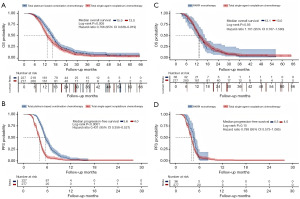
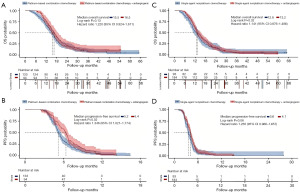
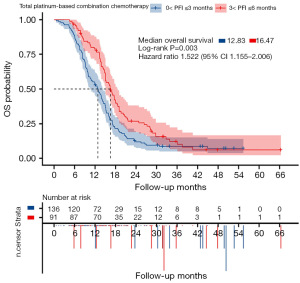
The OS non-significantly (16.5 vs. 15.5 months, HR: 1.220, 95% CI: 0.924–1.611, P=0.13) improved and median PFS was significantly (6.4 vs. 5.2 months, HR: 1.346, 95% CI: 1.021–1.774, P=0.02) when Bev was added to the platinum-based combination chemotherapy (Figure 3A,3B). Improvements in efficiencies were observed after adding Bev to platinum-based combination chemotherapy and single-agent non-platinum chemotherapy. Compared with patients undergoing single-agent chemotherapy alone, those who received additional Bev showed a trend towards prolonged OS (13.2 vs. 12.8 months, HR: 1.141, 95% CI: 0.876–1.486, P=0.32) and median PFS (4.1 vs. 3.6 months, HR: 1.265, 95% CI: 0.966–1.657, P=0.06) (Figure 3C,3D). In the subgroup of patients who received platinum-based combination chemotherapy, those with a PFI of >3–6 months re-treated with platinum-based chemotherapy had longer OS than those with a PFI of 0–3 months (16.47 vs. 12.83 months, HR: 1.522, 95% CI: 1.155–2.006, P=0.003) (Figure 4).
Prognostic factors of OS and PFS
Univariate and multivariate analyses of OS were performed for the prognostic factors listed in Tables 2,3. Our results showed that the PFI, histological type, and clinical trial participation were independent prognostic factors for OS. The patients receiving platinum-based combination chemotherapy were further classified into two subgroups based on whether they had a PFI of >3–6 or >0–3 months (Table 2), and the OS for each group was calculated and compared. The median OS of the groups with a PFI of >3–6 and >0–3 months were 16.47 and 12.83 months, respectively, and the difference was statistically significant (95% CI: 1.155–2.006, P=0.003) (Figure 4).
Table 2
| Variables | HR | 95% CI | P |
|---|---|---|---|
| Age at diagnosis (<50 vs. ≥50 years) | 1.080 | 0.848–1.376 | 0.53 |
| Stage (I vs. II vs. III vs. IV) | 0.960 | 0.833–1.106 | 0.57 |
| Histology (HGSC vs. CCC vs. EC vs. MC vs. LGSC) | 1.186 | 1.071–1.313 | 0.001 |
| Platinum status (primary vs. acquired) | 0.765 | 0.635–0.921 | 0.005 |
| Hydroperitoneum (yes vs. no vs. unknown) | 1.017 | 0.904–1.144 | 0.77 |
| Neoadjuvant (yes vs. no) | 0.939 | 0.766–1.152 | 0.54 |
| Surgical procedures (transabdominal vs. minimally invasive) | 1.161 | 0.921–1.462 | 0.20 |
| Debulking (optimal vs. suboptimal vs. unknown) | 0.959 | 0.852–1.080 | 0.49 |
| No. of previous lines of anticancer therapy (1–2 vs. ≥3) | 0.970 | 0.756–1.245 | 0.81 |
| 0 < PFI ≤3 months vs. 3 < PFI ≤6 months | 0.415 | 0.340–0.508 | <0.001 |
| PARPi received before PRROC diagnosis (yes vs. no) | 0.772 | 0.519–1.149 | 0.20 |
| Bev received before PRROC diagnosis (yes vs. no) | 0.883 | 0.692–1.127 | 0.31 |
| Clinical trial participation (yes vs. no) | 5.703 | 2.356–13.801 | <0.001 |
OS, overall survival; HR, hazard ratio; CI, confidence interval; HGSC, high-grade serous carcinoma; CCC, clear cell carcinoma; EC, endometrioid carcinoma; MC, mucinous carcinoma; LGSC, low-grade serous carcinoma; PFI, progression-free interval; PRROC, platinum-resistant recurrent ovarian cancer; PARPi, poly (adenosine diphosphate-ribose) polymerase inhibitor.
Table 3
| Variables | B | SE | Wald | HR | 95% CI | P |
|---|---|---|---|---|---|---|
| Histology (HGSC vs. CCC vs. EC vs. MC vs. LGSC) | 0.128 | 0.052 | 6.146 | 1.137 | 1.027–1.258 | 0.01 |
| Platinum status (primary vs. acquired) | –0.114 | 0.096 | 1.418 | 0.892 | 0.739–1.077 | 0.23 |
| 0 < PFI ≤3 months vs. 3 < PFI ≤6 months | –0.768 | 0.105 | 53.784 | 0.464 | 0.378–0.570 | <0.001 |
| Clinical trial participation (yes vs. no) | 1.389 | 0.455 | 9.308 | 4.012 | 1.644–9.769 | 0.002 |
OS, overall survival; HR, hazard ratio; CI, confidence interval; HGSC, high-grade serous carcinoma; CCC, clear cell carcinoma; EC, endometrioid carcinoma; MC, mucinous carcinoma; LGSC, low-grade serous carcinoma; PFI, progression-free interval; SE, standard error.
Discussion
Finding effective therapies for PRROC is an ongoing challenge. The applicability of clinical trials in PRROC settings to real-world patient cohorts may be limited, as the enrollment criteria of such trials often target individuals with moderate pretreatment history and potentially fewer concurrent conditions (16). A growing number of researchers have recognized the importance of real-world evidence in decisions related to drug development, regulatory approval, and prescribing practices. However, real-world data on the treatment modalities of PRROC patients remain limited (17). In this study, we describe practice patterns and outcomes in the routine care of patients with PRROC. In our cohort of 504 PRROC patients, 46.83% had primary PRROC, and an additional 53.17% developed acquired platinum resistance during the follow-up period.
Following the emergence of platinum resistance, patients typically undergo treatment with a single-agent chemotherapy, such as paclitaxel, topotecan, PLD, or gemcitabine. Our results showed that the overall response rates to these drugs are poor. Further, no cytotoxic agent demonstrated superior efficacy compared to others in the context of our study. These outcomes align with previous research; for example, a phase III randomized trial comparing PLD against topotecan found no significant differences in response rate, PFS, and OS in patients with platinum-resistant, recurrent ovarian cancer (P=0.11, P=0.73, P=0.45) (18).
In recent years, targeted therapeutics like Bev and olaparib have been approved by the Food and Drug Administration (FDA) for the treatment of recurrent OC. A growing body of evidence indicates that combination therapy with Bev can significantly improve the PFS of patients with PRROC (19,20).
In our cohort, patients receiving a combination of single-agent chemotherapy with Bev (n=184) showed a trend towards prolonged median PFS (4.1 vs. 3.6 months, 95% CI: 0.966–1.657, P=0.06) and OS (13.2 vs. 12.8 months, 95% CI: 0.876–1.486, P=0.32) compared to patients receiving single-agent chemotherapy alone (n=93) (Figure 3C,3D). These findings are consistent with the results of the AURELIA trial that reported (based on a total of 361 patients) that the addition of Bev extended PFS by 3 months, but also reported no statistically significant difference between the regimens in terms of OS (19). The median PFS and OS of the patients in this study treated with Bev and chemotherapy in routine practice were shorter than those reported in the AURELIA trial, which indicates that there is a substantial effectiveness gap between trial and real-world outcomes. Based on the AURELIA trial, the FDA sanctioned the use of Bev in combination with paclitaxel, PLD, or topotecan for the treatment of patients with PRROC (21). Moreover, due to its endorsement as a frontline induction and maintenance therapy for platinum-sensitive recurrent OC (3), most patients will already have received Bev before they develop PRROC (22). Current evidence indicates that previous Bev treatment is unlikely to influence subsequent treatment outcomes; thus, Bev re-treatment remains an option for patients with PRROC (23,24).
A number of PARPi have been evaluated as monotherapy treatments in recurrent epithelial OC. Olaparib was the first PARPi to obtain approval in gBRCA epithelial OC patients who have undergone at least three lines of therapy. Among our cohort of patients, 36 received PARPi monotherapy. Our data show a trend toward longer PFS for patients receiving PARPi monotherapy, without reaching the level of statistical significance. OS was at least non-inferior (Figure 2C,2D).
The BRCA status remained undisclosed for the majority of the participants in our study, as the genetic testing data of these individuals had not been uploaded to the data platform. In a separate study, the therapeutic efficacy of olaparib as monotherapy was evaluated in BRCA mutation carriers who had previously received at least three lines of chemotherapy. The results revealed that platinum-resistant patients had a response rate of 30% and a response duration of 8 months (25). However, the results of recent post-hoc analyses (with clear limitations) reported that patients with recurrent OC who received PARPi monotherapy showed worse OS compared to those who received chemotherapy (26,27). Specifically, the SOLO3 (26) trial compared PARPi monotherapy and chemotherapy in patients with recurrent OC, and reported a median OS of 29.9 and 39.4 months, respectively, in patients with three prior lines of therapy, while the ARIEL4 (27) trial reported a median OS of 19.4 and 25.4 months, respectively, in patients with two prior lines of therapy. With an increasing number of patients receiving PARPi as maintenance therapy, there is a growing population of recurrent patients who have been previously exposed to (and potentially developed resistance to) PARPi. But data on the post-progression outcomes of patients following PARPi maintenance therapy are still limited. The association between PARP inhibition and sensitivity to treatments is not yet well established, and further prospective research is needed to examine this phenomenon (28). For patients who experience recurrence after PARPi maintenance therapy, the optimal management strategy remains unclear (29).
In the current study, 227 (45.04%) patients received platinum rechallenge therapy, and had a median PFS of 5.6 months, and an OS of 15.9 months (Figure 2A,2B). These results showed that patients who received the platinum rechallenge therapy had better survival outcomes than those who received non-platinum monotherapies (15.9 vs. 13.0 months, respectively) (Figure 2A). The idea of re-treating resistant cancer patients with platinum is not novel. Havrilesky et al. reported that three out of eight patients with PRROC responded to treatment with low-dose carboplatin and paclitaxel on a weekly basis (consisting of one cycle of carboplatin at an area under curve (AUC) of 2, and paclitaxel at 80 mg/m2 on days 1, 8, and 15 for a 28-day cycle) (30). Hansen et al. reported that PRROC patients who received carboplatin in combination with Bev (i.e., one cycle of carboplatin at an AUC of 5, and Bev at 10 mg/kg on day 1 for a 21-day cycle) (31) had a response rate of 57%, and PFS and OS of 5.0 and 11.2 months, respectively. Tatsuki et al. conducted a multicenter retrospective study to evaluate the efficacy and safety of platinum rechallenge in patients with PRROC, and found that platinum rechallenge therapy for patients with PRROC did not result in previously unreported adverse events, and any such events were manageable. They also reported higher response rates and disease control rates and prolonged OS (32). Thus, platinum rechallenge therapy for PRROC may be a viable treatment option.
Our research outcomes support the consensus guidelines promulgated by the European Society for Medical Oncology (ESMO) and European Society of Gynaecological Oncology (ESGO) in 2019 (3), which state that for some patients without contraindications to platinum rechallenge, platinum rechallenge therapy (monotherapy or combination therapy) should be considered. Although not statistically significant, our results indicated that platinum-based combination chemotherapy plus Bev improved OS compared with platinum-based combination chemotherapy alone, which suggests that Bev may exert a synergistic beneficial effect (Figure 3A). Previous investigations have reported marked enhancements in therapeutic efficacy in both platinum-sensitive and platinum-resistant settings when Bev was incorporated into gemcitabine-carboplatin combination therapy and single-agent therapy, respectively (19,33). The OCEANS trial examined the efficacy and safety of the platinum-based combination chemotherapy plus Bev regimen in platinum-sensitive relapsed OC patients, and our research further supports that the combination of platinum-based combination chemotherapy and Bev may yield relevant improvements for PRROC patients (Figure 3A,3B).
Univariate and multivariate analyses of OS were conducted to examine the background factors listed in Tables 2,3. Histological type was an independent prognostic factor for OS. A large number of molecular studies have shown that different histological types of OC effectively represent different diseases, and the etiology, mechanism, disease progression, and clinical treatment strategies for different histological types differ greatly; thus, histological type is an important prognostic factor of OC (34). The common types of epithelial ovarian cancer (EOC) are SOC, EC, MC, and CCC. The most frequent subtype, SOC can be further divided into HGSC and LGSC. In general, CCC, MC, and LGSC tend to be relatively resistant to platinum-based chemotherapy (35). CCC exhibits a relatively unfavorable prognosis compared to other histological subtypes of EOC (36-39). A study reviewed data from patients with FIGO Stage I–IV EOC who participated in 12 prospective randomized GOG protocols and found that CCC exhibited superior PFS compared to SOC in early stage cases. However, in advanced-stage patients, CCC was significantly associated with reduced OS (40). In our study, HGSC accounted for the majority of cases (82.14%), followed by CCC (8.93%), EC (3.37%), MC (2.98%), and LGSC (2.58%). Patients with HGSC also presented with more advanced tumor stages. In clinical research on PRROC, the inclusion of patients with diverse subtypes of OC is likely to introduce biases, making results difficult to interpret. At the very least, patients with different histological types of OC should be stratified. Ideally, they should be studied independently, as the different histological subtypes are representative of distinct diseases. For the less frequent subtypes this would, however, require a larger cohort size.
Our results also showed that PFI was an independent prognostic factor for OS (Table 3). Patients receiving platinum-based combination chemotherapy were classified into two groups based on whether they had a PFI of >3–6 or >0–3 months, and their respective OS durations were calculated and compared. The median OS for the >3–6 months PFI group was 16.47 months, while that for the >0–3 months PFI group was 12.83 months. The difference was statistically significant (HR =1.518, 95% CI: 1.155–2.006, P=0.003) (Figure 4). A longer PFI has been associated with an increased response to platinum-based therapy, but PFI has been shown to decrease with each line of therapy (3).
PFI, which is defined as the duration between the last administration of platinum-based therapy and the date of recurrence, is recognized as the most widely employed and accepted clinical surrogate marker for predicting chemotherapy response, evaluating prognosis, and facilitating patient cohort selection and stratification (41). With the progress in relapsed disease detection and the advent of novel targeted therapies, some authors argue that these traditional definitions are becoming a less reliable indication for treatment options, signaling a potential shift toward more personalized treatment strategies for relapsed disease (42). Our findings further challenge the validity of using a 6-month PFI as an arbitrary threshold for subsequent treatment decisions. Patients in our study who were considered “platinum resistant” still exhibited clinical benefits from platinum-based chemotherapy, especially within the PFI range of >3–6 months. In clinical practice, the identification of biomarkers indicative of platinum sensitivity is essential for determining the eligibility of potential responders for platinum-based re-treatment (43).
The further categorization of the time following previous therapy as treatment-free intervals (TFIs) has been suggested. TFIs include the TFI associated with the most recent platinum-based therapy (TFIp), the TFI related to the last non-platinum-based therapy (TFInp), and the TFI corresponding to the final biological agent administered (TFIb) (12). Predictive biomarkers for response to targeted therapies are expected to be more important than TFIp in terms of predicting benefits, particularly in scenarios in which chemotherapy is not administered. The incorporation of biomarkers in future clinical trials will help to establish their relevance in OC and their relationship with efficacy endpoints. This approach will facilitate the identification of parameters to enhance patient response beyond the 6-month PFI cutoff (16).
A study has indicated that individuals participating in clinical trials are significantly younger, possess higher income levels, and demonstrate a lower prevalence of comorbidities compared to the general cancer population (44). However, for patients with PRROC, enrollment in early phase clinical trials of novel drugs or combination therapies represents a promising option. In our study, patients who participated in clinical trials benefited greatly and had a comparatively good prognosis (Table 3). Despite the difficulties in identifying new treatment options for PRROC, substantial optimism remains that novel targeted treatments or immunotherapies may yield substantial clinical benefits. Recently, mirvetuximab soravtansine (MIRV) has been approved for adult patients with Folate receptor (FR) α-positive, PROC, primary peritoneal and tubular cancer by the Food and Drug Administration (FDA), for people who have undergone one to three prior treatment courses (45). A report has demonstrated that MIRV can bind to FRα can trigger antibody-dependent cellular cytotoxicity and phagocytosis, boosting the immune system’s capacity to identify and eliminate cancer cells, provides a comprehensive approach to treating ovarian cancer (46). Striking correlations between intratumoral T cells and survival (47,48) also imply that immune responses are important in ovarian cancer. Still, available treatment modalities have so far failed to overcome tumor-associated immunosuppression (49). With regard to targeted cytotoxic therapies, the p53 protein-reactivating drug, eprenetapopt (APR-246), has shown the ability to resensitize PRROC cells to platinum treatment in culture (50,51). Furthermore, early phase clinical study of small-molecule tyrosine kinase inhibitors or its combination with single-agent nonplatinum chemotherapy for PRROC treatment are underway (52). There are no data to support immunotherapy alone or in combination with chemotherapy for patients with a platinum-free interval of less than 6 months. The NRGGY003 trial reported that the combination of programmed cell death protein 1 (PD-1) and cytotoxic T-lymphocyte associated protein 4 (CTLA-4) inhibitors may lead to clinical improvement. More evidence is needed to evaluate other combination strategies involving PARP inhibitors or investigational drugs (such as antibody-drug conjugates) (53). The identification of additional biomarkers and the development of optimized diagnostic assays for biomarker assessment should be the key focus of future research in PRROC.
This study had several limitations, including its retrospective and observational design, as well as the potential for selection bias. Additionally, variations in chemotherapy regimens, dosages, and treatment strategies were influenced by individual practitioners and institutional practices. Moreover, the study was not powered to separately assess different subtypes of PRROC. In addition, we did not assess quality of life (QOL). QOL should be taken into account when formulating therapeutic strategies for PRROC due to the diminished physical vitality and bone marrow reserves observed in patients following multiple treatment regimens (51). Therefore, our findings should be interpreted cautiously.
Conclusions
In the real-world setting, the sequential administration of a single non-platinum chemotherapy agent remains the predominant treatment protocol for patients with PRROC. Compared to the single-agent non-platinum chemotherapy group, the platinum-based combination chemotherapy group had significantly longer PFS and OS, particularly those with a PFI of >3–6 months. Platinum rechallenges therapy currently represents the most promising treatment approach for PRROC, as the majority of patients with platinum-resistance do not benefit from conventional standard therapies. In the current landscape of intensive clinical research, early trials are often considered a key option for treating patients with drug-resistant diseases. The heterogeneity of cancer genomes and their significant adaptability suggest that overcoming treatment resistance necessitates a diverse array of strategies. In this context, extending our understanding of the factors driving OC will be essential in advancing new therapeutic approaches.
Acknowledgments
The abstract of this article has been accepted for Poster presentation (display) at the ESMO Asia Congress 2024 (6–8 December), Singapore.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://tcr.amegroups.com/article/view/10.21037/tcr-2025-641/rc
Data Sharing Statement: Available at https://tcr.amegroups.com/article/view/10.21037/tcr-2025-641/dss
Peer Review File: Available at https://tcr.amegroups.com/article/view/10.21037/tcr-2025-641/prf
Funding: This work was supported by funding from
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tcr.amegroups.com/article/view/10.21037/tcr-2025-641/coif). Colton Ladbury serves as an unpaid editorial board member of Translational Cancer Research from August 2024 to July 2026. Jörg Wischhusen is an inventor on several patents related to cancer immunotherapy, and a co-founder of the biotech companies CatalYm GmbH and Toleris Biotherapeutics GmbH, outside the submitted work. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki and its subsequent amendments. The study was approved by the Ethics Committee of Tongji Hospital affiliated with Huazhong University of Science and Technology (No. 2019-S1102) and Xuzhou Central Hospital (No. XZSZXYY-LL-SOP-010-02). All participating hospitals/institutions were informed and agreed with this study. Individual consent for this retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Sung H, Ferlay J, Siegel RL, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin 2021;71:209-49. [Crossref] [PubMed]
- Lheureux S, Braunstein M, Oza AM. Epithelial ovarian cancer: Evolution of management in the era of precision medicine. CA Cancer J Clin 2019;69:280-304. [Crossref] [PubMed]
- Colombo N, Sessa C, du Bois A, et al. ESMO-ESGO consensus conference recommendations on ovarian cancer: pathology and molecular biology, early and advanced stages, borderline tumours and recurrent disease†. Ann Oncol 2019;30:672-705. [Crossref] [PubMed]
- Van Nyen T, Planque M, van Wagensveld L, et al. Serine metabolism remodeling after platinum-based chemotherapy identifies vulnerabilities in a subgroup of resistant ovarian cancers. Nat Commun 2022;13:4578. [Crossref] [PubMed]
- Patch AM, Christie EL, Etemadmoghadam D, et al. Whole-genome characterization of chemoresistant ovarian cancer. Nature 2015;521:489-94. [Crossref] [PubMed]
- Richardson DL, Eskander RN, O'Malley DM. Advances in Ovarian Cancer Care and Unmet Treatment Needs for Patients With Platinum Resistance: A Narrative Review. JAMA Oncol 2023;9:851-9. [Crossref] [PubMed]
- Yang L, Xie HJ, Li YY, et al. Molecular mechanisms of platinum-based chemotherapy resistance in ovarian cancer Oncol Rep 2022;47:82. (Review). [Crossref] [PubMed]
- Gaillard S, Oaknin A, Ray-Coquard I, et al. Lurbinectedin versus pegylated liposomal doxorubicin or topotecan in patients with platinum-resistant ovarian cancer: A multicenter, randomized, controlled, open-label phase 3 study (CORAIL). Gynecol Oncol 2021;163:237-45. [Crossref] [PubMed]
- How JA, Dang M, Lee S, et al. Pembrolizumab plus chemotherapy in frontline treatment of advanced ovarian cancer: Clinical and translational results from a phase 2 trial. Med 2025;6:100494. [Crossref] [PubMed]
- Rosario SR, Long MD, Chilakapati S, et al. Integrative multi-omics analysis uncovers tumor-immune-gut axis influencing immunotherapy outcomes in ovarian cancer. Nat Commun 2024;15:10609. [Crossref] [PubMed]
- Oronsky B, Ray CM, Spira AI, et al. A brief review of the management of platinum-resistant-platinum-refractory ovarian cancer. Med Oncol 2017;34:103. [Crossref] [PubMed]
- Pujade-Lauraine E, Banerjee S, Pignata S. Management of Platinum-Resistant, Relapsed Epithelial Ovarian Cancer and New Drug Perspectives. J Clin Oncol 2019;37:2437-48. [Crossref] [PubMed]
- Zhang N, Zheng H, Gao Y, et al. Real-world study of bevacizumab treatment in patients with ovarian cancer: a Chinese single-institution study of 155 patients. BMC Womens Health 2023;23:178. [Crossref] [PubMed]
- Wang DF, Shi XW, Zhang C, et al. Real-world applications of PARPi maintenance therapy for recurrent ovarian cancer: A single-center study in China. Gynecol Oncol 2023;170:25-31. [Crossref] [PubMed]
- Blonde L, Khunti K, Harris SB, et al. Interpretation and Impact of Real-World Clinical Data for the Practicing Clinician. Adv Ther 2018;35:1763-74. [Crossref] [PubMed]
- Eskander RN, Moore KN, Monk BJ, et al. Overcoming the challenges of drug development in platinum-resistant ovarian cancer. Front Oncol 2023;13:1258228. [Crossref] [PubMed]
- Hall JP, Chang J, Moon R, et al. Real-world treatment patterns in patients with advanced (stage III-IV) ovarian cancer in the USA and Europe. Future Oncol 2020;16:1013-30. [Crossref] [PubMed]
- Gordon AN, Fleagle JT, Guthrie D, et al. Recurrent epithelial ovarian carcinoma: a randomized phase III study of pegylated liposomal doxorubicin versus topotecan. J Clin Oncol 2001;19:3312-22. [Crossref] [PubMed]
- Pujade-Lauraine E, Hilpert F, Weber B, et al. Bevacizumab combined with chemotherapy for platinum-resistant recurrent ovarian cancer: The AURELIA open-label randomized phase III trial. J Clin Oncol 2014;32:1302-8. [Crossref] [PubMed]
- Seol A, Kim SI, Yoon HY, et al. Impact of Bevacizumab on Clinical Outcomes in Patients With Platinum-resistant Relapsed Ovarian Cancer. In Vivo 2024;38:1338-50. [Crossref] [PubMed]
- Saif MW U.S.. Food and Drug Administration approves paclitaxel protein-bound particles (Abraxane®) in combination with gemcitabine as first-line treatment of patients with metastatic pancreatic cancer. JOP 2013;14:686-8. [Crossref] [PubMed]
- Moore K, Mirza M, Gourley C, et al. Evolution of the Ovarian Cancer Treatment Paradigm, Including Maintenance Treatment, in the US and Europe: A Real-World Chart Review Analysis (2017-2020) (028). Gynecol Oncol 2022;166:S20-1.
- Haunschild CE, Tewari KS. Bevacizumab use in the frontline, maintenance and recurrent settings for ovarian cancer. Future Oncol 2020;16:225-46. [Crossref] [PubMed]
- Pignata S, Lorusso D, Joly F, et al. Carboplatin-based doublet plus bevacizumab beyond progression versus carboplatin-based doublet alone in patients with platinum-sensitive ovarian cancer: a randomised, phase 3 trial. Lancet Oncol 2021;22:267-76. [Crossref] [PubMed]
- Domchek SM, Aghajanian C, Shapira-Frommer R, et al. Efficacy and safety of olaparib monotherapy in germline BRCA1/2 mutation carriers with advanced ovarian cancer and three or more lines of prior therapy. Gynecol Oncol 2016;140:199-203. [Crossref] [PubMed]
- Lee JY, Lee YY, Park JY, et al. Major clinical research advances in gynecologic cancer in 2022: highlight on late-line PARP inhibitor withdrawal in ovarian cancer, the impact of ARIEL-4, and SOLO-3. J Gynecol Oncol 2023;34:e51. [Crossref] [PubMed]
- Kristeleit R, Lisyanskaya A, Fedenko A, et al. Rucaparib versus standard-of-care chemotherapy in patients with relapsed ovarian cancer and a deleterious BRCA1 or BRCA2 mutation (ARIEL4): an international, open-label, randomised, phase 3 trial. Lancet Oncol 2022;23:465-78. [Crossref] [PubMed]
- El Bairi K, Madariaga A, Trapani D, et al. New horizons for platinum-resistant ovarian cancer: insights from the 2023 American Society of Clinical Oncology (ASCO) and European Society for Medical Oncology (ESMO) Annual Meetings. Int J Gynecol Cancer 2024;34:760-72. [Crossref] [PubMed]
- Park J, Kim SI, Jeong SY, et al. Second-line olaparib maintenance therapy is associated with poor response to subsequent chemotherapy in BRCA1/2-mutated epithelial ovarian cancer: A multicentre retrospective study. Gynecol Oncol 2022;165:97-104. [Crossref] [PubMed]
- Havrilesky LJ, Alvarez AA, Sayer RA, et al. Weekly low-dose carboplatin and paclitaxel in the treatment of recurrent ovarian and peritoneal cancer. Gynecol Oncol 2003;88:51-7. [Crossref] [PubMed]
- Hansen MKG, Smerdel MP, Waldstrøm M, et al. Carboplatin re-treatment in platinum-resistant epithelial ovarian cancer patients. Cancer Chemother Pharmacol 2020;86:751-9. [Crossref] [PubMed]
- Tatsuki S, Shoji T, Abe M, et al. Efficacy and Safety of Platinum Rechallenge in Patients With Platinum-resistant Ovarian, Fallopian Tube or Primary Peritoneal Cancer: A Multicenter Retrospective Study. Anticancer Res 2022;42:4603-10. [Crossref] [PubMed]
- Demirkiran A, Eryilmaz MK, Karaagac M, et al. Low-dose (7.5 mg/kg) bevacizumab may be a viable option in recurrent ovarian cancer: A retrospective study. J Cancer Res Ther 2023;19:595-600. [Crossref] [PubMed]
- Köbel M, Kang EY. The Evolution of Ovarian Carcinoma Subclassification. Cancers (Basel) 2022;14:416. [Crossref] [PubMed]
- Reid BM, Permuth JB, Sellers TA. Epidemiology of ovarian cancer: a review. Cancer Biol Med 2017;14:9-32. [Crossref] [PubMed]
- Chan JK, Kapp DS. Clear Cell Ovarian Cancer: Optimum Management and Prognosis Remain Hazy. Gynecol Oncol 2017;147:237-9. [Crossref] [PubMed]
- Wu Y, Lyu X, Zhang H, et al. Clinicopathological factors of ovarian clear cell carcinoma: A single institutional analysis of 247 cases in China. Biomol Biomed 2025;25:436-44. [Crossref] [PubMed]
- Chao A, Huang CY, Yu W, et al. Molecular profiling reveals novel therapeutic targets and clonal evolution in ovarian clear cell carcinoma. BMC Cancer 2024;24:1403. [Crossref] [PubMed]
- Mabuchi S, Sugiyama T, Kimura T. Clear cell carcinoma of the ovary: molecular insights and future therapeutic perspectives. J Gynecol Oncol 2016;27:e31. [Crossref] [PubMed]
- Oliver KE, Brady WE, Birrer M, et al. An evaluation of progression free survival and overall survival of ovarian cancer patients with clear cell carcinoma versus serous carcinoma treated with platinum therapy: An NRG Oncology/Gynecologic Oncology Group experience. Gynecol Oncol 2017;147:243-9. [Crossref] [PubMed]
- Joly F, Hilpert F, Okamoto A, et al. Fifth Ovarian Cancer Consensus Conference of the Gynecologic Cancer InterGroup: Recommendations on incorporating patient-reported outcomes in clinical trials in epithelial ovarian cancer. Eur J Cancer 2017;78:133-8. [Crossref] [PubMed]
- Lindemann K, Gao B, Mapagu C, et al. Response rates to second-line platinum-based therapy in ovarian cancer patients challenge the clinical definition of platinum resistance. Gynecol Oncol 2018;150:239-46. [Crossref] [PubMed]
- Unger JM, Hershman DL, Fleury ME, et al. Association of Patient Comorbid Conditions With Cancer Clinical Trial Participation. JAMA Oncol 2019;5:326-33. [Crossref] [PubMed]
- Ludmir EB, Mainwaring W, Lin TA, et al. Factors Associated With Age Disparities Among Cancer Clinical Trial Participants. JAMA Oncol 2019;5:1769-73. [Crossref] [PubMed]
- Dilawari A, Shah M, Ison G, et al. FDA Approval Summary: Mirvetuximab Soravtansine-Gynx for FRα-Positive, Platinum-Resistant Ovarian Cancer. Clin Cancer Res 2023;29:3835-40. [Crossref] [PubMed]
- Shaukat A, Shakeel L, Khan A, et al. Addressing the challenge of platinum-resistant ovarian cancer: the role of mirvetuximab soravtansine. Ann Med Surg (Lond) 2025;87:1-7. [Crossref] [PubMed]
- Zhang L, Conejo-Garcia JR, Katsaros D, et al. Intratumoral T cells, recurrence, and survival in epithelial ovarian cancer. N Engl J Med 2003;348:203-13. [Crossref] [PubMed]
- Curiel TJ, Coukos G, Zou L, et al. Specific recruitment of regulatory T cells in ovarian carcinoma fosters immune privilege and predicts reduced survival. Nat Med 2004;10:942-9. [Crossref] [PubMed]
- Mollaoglu G, Tepper A, Falcomatà C, et al. Ovarian cancer-derived IL-4 promotes immunotherapy resistance. Cell 2024;187:7492-7510.e22. [Crossref] [PubMed]
- Mohell N, Alfredsson J, Fransson Å, et al. APR-246 overcomes resistance to cisplatin and doxorubicin in ovarian cancer cells. Cell Death Dis 2015;6:e1794. [Crossref] [PubMed]
- Wilson MK, Pujade-Lauraine E, Aoki D, et al. Fifth Ovarian Cancer Consensus Conference of the Gynecologic Cancer InterGroup: recurrent disease. Ann Oncol 2017;28:727-32. [Crossref] [PubMed]
- Hou Z, Lan C, Huang X, et al. Efficacy, safety and pharmacokinetics of apatinib plus etoposide versus apatinib alone for platinum-resistant recurrent ovarian cancer: protocol of a multicenter, open-label, randomized phase 2 trial. Transl Cancer Res 2023;12:2959-67. [Crossref] [PubMed]
- Bogani G, Moore KN, Ray-Coquard I, et al. Incorporating immune checkpoint inhibitors in epithelial ovarian cancer. Gynecol Oncol 2025;193:30-40. [Crossref] [PubMed]


