
Comprehensive assessment of disulfidptosis-related long non-coding RNA index as biomarkers for predicting clinical outcomes and immune microenvironment in pancreatic cancer
Highlight box
Key findings
• This study identifies a disulfidptosis-related long non-coding RNA (lncRNA) index as a novel prognostic model for pancreatic adenocarcinoma (PAAD). The index robustly predicts patient survival and correlates with the tumor immune microenvironment, distinguishing between “hot” and “cold” immune tumors. This provides new insights into immune infiltration and personalized treatment strategies for PAAD.
What is known and what is new?
• Previously, disulfidptosis was recognized as a newly discovered form of cell death, but its potential in prognosis and tumor immunity was not fully explored.
• This study adds to the field by constructing a disulfidptosis-related lncRNA index that serves as a powerful tool to predict the prognosis of PAAD and assess immune infiltration status.
What is the implication, and what should change now?
• The developed index could guide the stratification of PAAD patients based on immune tumor status and help in the selection of personalized treatment strategies, especially in the context of immunotherapy. Further research and clinical trials are encouraged to validate the index’s potential as a biomarker for predicting immunotherapy responses and optimizing PAAD treatment decisions.
Introduction
Background
Pancreatic cancer (PC) is a malignant tumour with an extremely poor prognosis, of which pancreatic adenocarcinoma (PAAD) is the prevalent pathological category (about 90%) (1). According to the latest GLOBOCAN project, there were approximately 466,000 new deaths of PC worldwide in 2020, with a 5-year survival rate of only about 10% (2,3). PAAD is insidious in early stages, and most individuals have already developed metastases or are at an advanced stage by the time of diagnosis, depriving them of the opportunity for radical surgery (4). Over the last decade, immunotherapy has become a pillar of modern cancer treatment, complementing chemotherapy and targeted therapies. However, only a fraction of individuals can respond to immunotherapy and thus prolong survival. Hence, it is crucial to investigate specific biomarkers for PAAD prognosis and immunotherapy response prediction.
Rationale and knowledge gap
Programmed cell death (PCD) has been widely reported as an essential mechanism of cell death in anti-tumour therapy (5-7). Various anti-tumour therapies are designed to disrupt cancer cells through PCD (8,9). Therefore, understanding the relationship between PCD and cancer is of great importance to clinical oncology treatment. Recently, a team of researchers has identified and characterised a novel type of cell death, the disulfidptosis, which is distinct from all other known mechanisms of PCD (10). This study showed that in preclinical models, treatment with glucose inhibitors induced disulfidptosis in SLC7A11-high expressing cancer cells, effectively inhibiting tumour growth with no apparent toxicity to normal cells (10). Therefore, investigating the potential effects of disulfidptosis in different cancers may open the door to new cancer treatment strategies.
Long non-coding RNAs (lncRNAs) have been shown to modulate multiple biological behaviours through transcriptional, post-transcriptional and epigenetic mechanisms, including tumourigenesis, metastasis and invasion, and are considered to be potential targets for tumour therapy (11-13). Additionally, lncRNAs are widely involved in PCD processes including apoptosis, pyroptosis, ferroptosis and necroptosis, which in turn regulate the malignant biological behaviour of tumours (14-18). Furthermore, it has been demonstrated that lncRNAs are stably present in urine, plasma and serum and can be used as biomarkers of tumours (19,20). Nevertheless, disulfidptosis-related lncRNAs (DRLs) have yet to be identified and their potential application in tumours remains to be elucidated.
Objective
In the current research, we constructed a disulfidptosis-related lncRNA index (DRLncI) based on the Cox and LASSO algorithm, and systematically evaluated the prognostic predictive performance of the DRLncI. Additionally, we explored the predictive power of the DRLncI for tumour immune microenvironment (TIME) and drug sensitivity. Finally, consensus clustering analysis based on DRLncI identified different subtypes of TIME in PAAD and to determine the dominant population for treatment of immune checkpoint blockades (ICBs). The DRLncI-related lncRNAs identified in this study may provide the basis for the establishment of novel predictive biomarkers in PAAD. We present this article in accordance with the TRIPOD reporting checklist (available at https://tcr.amegroups.com/article/view/10.21037/tcr-24-1976/rc).
Methods
The study was conducted in accordance with the Declaration of Helsinki and its subsequent amendments.
Acquirement and preprocessing of data
We extracted RNA sequencing (RNA-seq) dataset, clinical phenotype data and nucleotide variation data matrices in The Cancer Genome Atlas (TCGA) cohort (https://portal.gdc.cancer.gov/repository). Ultimately, patients with PAAD containing both RNA-seq and survival information were included in our research. Subsequently, the RNA-seq data were processed utilizing Practical Extraction and Report Language (Perl) programming scripts, and the lncRNA and messenger RNA (mRNA) datasets for the TCGA-PAAD cohort were derived for subsequent studies, as were the gene mutation data matrices. A total of 10 disulfidptosis-related genes (DRGs) were identified from a previous study (21) (Table S1).
Co-expression algorithm to identify DRLs
The mRNA expression dataset of 10 DRGs was retrieved utilizing the ‘limma’ package. The lncRNAs associated with these 10 DRGs were identified using a co-expression algorithm and defined as DRLs. Pearson correlation threshold was set as Pearson coefficient >0.3, P<0.001. Sankey plots of DRGs and DRLs were plotted via the ‘igraph’ package. The ‘limma’ was also used to combine survival data with DRLs expression dataset for subsequent analysis.
Construction of a DRLncI
Firstly, we randomly divided the TCGA-PAAD individuals into training and validation cohorts (1:1), where the training cohort was selected for constructing the DRLncI and the validation set was selected for verifying the predictive power of the DRLncI. The Cox algorithm was employed to determine DRLs related with survival in the training cohort (P<0.05). The least absolute shrinkage and selection operator (LASSO) algorithm was used to avoid overfitting and multicollinearity, thereby filtering the optimal DRLs to participate in the construction of the DRLncI. According to the regression coefficients and expression of DRLncI, a risk formula for the DRLncI was constructed to derive a risk score for each individual:
The β in DRLncI denotes the risk coefficient for each lncRNA. Finally, all individuals were risk stratified according to the median risk score of the training set.
Systematic verification of the DRLncI
Firstly, we performed Kaplan-Meier (K-M) survival analyses on patients in the training and validation sets separately to compare the differences in survival between the high- and low-risk subgroups, and plotted survival curves, risk alignment and survivorship charts to visualise the differential outcomes. In addition, Cox analysis was conducted on the TCGA-PAAD cohort to identify whether the DRLncI predicts prognosis in individuals with PAAD independently of other clinical traits. Finally, receiver operating characteristic (ROC) curves were utilised to examine the predictive capability of the DRLncI in TCGA-PAAD cohort. The above process was implemented through R packages.
Assessment of the DRLncI in clinically stratified subgroups
To further validate the stability of DRLncI in individuals with distinct clinicopathological characteristics, we analysed survival differences between the two risk subgroups in terms of age, sex, tumour grade and TNM (Tumor, Node, Metastasis) stage, and visualised the outcomes using K-M curves. In addition, the ‘ComplexHeatmap’ was utilised to draw the status heat map for the risk groups and different clinicopathological parameters.
Enrichment analysis
We used the ‘limma’ package to extract differentially expressed genes (DEGs) in the high- and low-risk subgroups of the TCGA-PAAD cohort [|Log2 fold change| >1, false discovery rate (FDR) <0.05]. Subsequently, the “org.Hs. eg.db”, “clusterProfiler” and “DOSE” packages were applied to conduct Gene Ontology (GO) analysis of the DEGs. Additionally, the packages ‘limma’, ‘GSEABase (Gene Set Enrichment Analysis Base)’, ‘GSVA’ and ‘reshape2’ were utilised for the Gene Set Variance Analysis (GSVA) to obtain pathways enriched in the high- and low-risk subgroups (22). Finally, the association between lncRNA expression in DRLncI and major pathways was further analysed. The packages ‘Pheatmap’, ‘ggpubr’, ‘ggplot2’, ‘ComplexHeatmap’ and ‘circle’ were used to realize the visualization of enrichment analysis.
DRLncI-based tumor mutation burden (TMB) analysis
The ‘limma’ package was performed to investigate the TMB differences between the two risk subgroups in TCGA-PAAD cohort. In addition, we plotted the K-M curves of individuals in the different subgroup combinations, and compared the survival differences between different subgroup combinations. Furthermore, the 25 most frequently mutated genes were determined through the gene mutation data matrix, and the mutation waterfall maps of these 25 genes in the different risk subgroups were drawn through ‘maftools’ package.
Correlation between the DRLncI and TIME
The Estimation of STromal and Immune cells in MAlignant Tumor tissues using Expression data (ESTIMATE) is a method for estimating infiltrating immune cells in tumour tissue and imputing tumour purity (23). The ‘ESTIMATE’ and ‘limma’ packages were performed to evaluate the quantity of stromal and immune cells in the tumour tissue for each individual to obtain a corresponding score. The total of immune score and stromal score is ESTIMATE score, and it negatively correlated with tumour tissue purity. The three scores were further analysed between the two risk subgroups.
Tumor Immune Estimation Resource (TIMER) is a platform for the systematic investigate of immune infiltration in different types of cancer (24,25). We downloaded the tumour infiltrating immune cells file on TIMER. Further correlation analysis of risk scores and different immune cells was performed in the TCGA-PAAD cohort and visualised by the ‘ggtext’, ‘ggpubr’, ‘ggplot2’, ‘scales’ and ‘tidyverse’ packages for the Spearman correlation analysis.
Gene Set Enrichment Analysis (GSEA) allows for the categorisation of genomes that possess a common biological feature (26). We performed single-sample Gene Set Enrichment Analysis (ssGSEA) analysis on each patient in the TCGA-PAAD cohort utilizing the ‘GSEABase’ and ‘GSVA’ packages to quantify the degree of infiltration of different immune cells in each individual. The ‘ggpubr’, ‘reshape2’ and ‘pheatmap’ packages were used to draw the results of the ssGSEA analysis. Activation of immune checkpoints can come to suppress the immune function of T lymphocytes and induce tumour immune escape (27). We also analysed the variation in immune checkpoints expression across the different subgroups.
DRLncI-based drug sensitivity analysis
The ‘pRRophetic’ was developed to predict clinical response to many anti-cancer drugs from oncogene expression levels (28). We applied the ‘pRRophetic’ to predict the differences in IC50 (half maximal inhibitory concentration) of different antitumour drugs in distinct risk subgroups of the TCGA-PAAD cohort, to evaluate the significance of DRLncI in guiding clinical drug therapy for individuals with PAAD and to plot differential box plots for agents with differences in IC50 in the two subgroups.
Consensus clustering analysis
Consensus clustering algorithm offers quantitative and visually stable support for estimating the number of unsupervised classes in a dataset, and have been widely used in cancer genomics (29). We performed a consensus clustering analysis utilizing the package ‘ConsensusClusterPlus’ to classify all patients into clusters. The ‘ggplot2’ and ‘Rtsne’ were used for principal component analysis (PCA) of the clusters. The ‘ggplot2’ and ‘ggalluvial’ were performed to draw correlation sankey diagrams between different clusters and risk subgroups. Additionally, K-M curves were utilised to analyse the survival differences between the different clusters. Furthermore, the TIME profiles of the different clusters were further explored by ssGSEA, ESTIMATE, TIME 2.0 and immune checkpoints analysis. Finally, the drug sensitivity of different clusters was also analysed.
Statistical analysis
R Project (V.4.2.2) and corresponding software packages were used for statistical analyses. Strawberry-perl was conducted to process the dataset. Spearman correlation algorithm was conducted to analyse the correlation between DRLncI-lncRNAs and signalling pathways. The K-M method was utilised to compare survival differences between subgroups. P values <0.05 were regarded as statistically significant.
Results
Identification of DRLs
Figure 1 shows the flow chart for the current study. By co-expression analysis of the TCGA-PAAD cohort lncRNA expression matrix and the 10 DRGs mRNA expression matrix, we identified 146 DRLs (Figure 2A), with the highest number of lncRNAs associated with SLC7A11, followed by GYS1, with 40 and 34 related lncRNAs for both, respectively. In addition, there were 36 DRLs associated with more than one DRG.
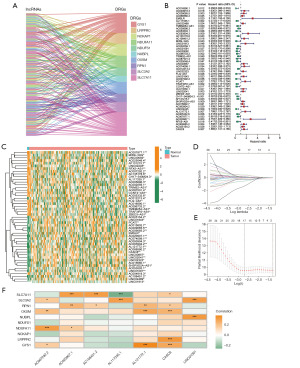
Establishment of DRLncI
All individuals were randomly classified into training and validation sets, and the clinical parameters of the patients in the different cohorts are shown in Table 1. The univariate Cox regression identified 49 DRLs associated with overall survival (OS), of which 23 were risk factors and the other 26 were protective factors (P<0.05) (Figure 2B). Additionally, all survival-related DRLs were abnormally expressed in tumour tissues (Figure 2C). To avoid overfitting, we further performed LASSO regression (Figure 2D,2E), which ultimately identified seven DRLs involved in DRLncI development (Figure 2F). Risk scores were obtained for each individual according to the expression of the DRLs and the coefficients in the DRLncI (Table 2). Risk score (DRLncI) = (−1.358343673 × AL117335.1) + (0.490680997 × AC108451.2) + (−1.331630996 × LINC01091) + (−3.943066884 × AC007292.2) + (0.749607907 × AL121772.1) + (0.729685317 × AC020907.1)+ (0.442813969 × CASC8).
Table 1
| Characteristics | Type | Training cohort | Validation cohort | P value |
|---|---|---|---|---|
| Age (years) | ≤65 | 47 (52.81%) | 47 (52.81%) | >0.99 |
| >65 | 42 (47.19%) | 42 (47.19%) | ||
| Gender | Female | 45 (50.56%) | 35 (39.33%) | 0.18 |
| Male | 44 (49.44%) | 54 (60.67%) | ||
| Grade | G1 | 16 (17.98%) | 15 (16.85%) | 0.41 |
| G2 | 49 (55.06%) | 46 (51.69%) | ||
| G3 | 21 (23.6%) | 27 (30.34%) | ||
| G4 | 2 (2.25%) | 0 (0%) | ||
| Unknown | 1 (1.12%) | 1 (1.12%) | ||
| Stage (TNM) | Stage I | 8 (8.99%) | 13 (14.61%) | 0.24 |
| Stage II | 74 (83.15%) | 73 (82.02%) | ||
| Stage III | 3 (3.37%) | 0 (0%) | ||
| Stage IV | 2 (2.25%) | 2 (2.25%) | ||
| Unknown | 2 (2.25%) | 1 (1.12%) | ||
| T stage | T1 | 3 (3.37%) | 4 (4.49%) | 0.35 |
| T2 | 11 (12.36%) | 13 (14.61%) | ||
| T3 | 70 (78.65%) | 72 (80.9%) | ||
| T4 | 3 (3.37%) | 0 (0%) | ||
| Unknown | 2 (2.25%) | 0 (0%) | ||
| N stage | N0 | 24 (26.97%) | 25 (28.09%) | >0.99 |
| N1 | 62 (69.66%) | 62 (69.66%) | ||
| Unknown | 3 (3.37%) | 2 (2.25%) | ||
| M stage | M0 | 39 (43.82%) | 41 (46.07%) | >0.99 |
| M1 | 2 (2.25%) | 2 (2.25%) | ||
| Unknown | 48 (53.93%) | 46 (51.69%) |
TNM, tumor node metastasis.
Table 2
| lncRNA | Coef | HR | P value |
|---|---|---|---|
| AL117335.1 | −1.358343673 | 0.2716 | 0.002 |
| AC108451.2 | 0.490680997 | 1.5947 | 0.02 |
| LINC01091 | −1.331630996 | 0.3910 | 0.03 |
| AC007292.2 | −3.943066884 | 0.1839 | 0.04 |
| AL121772.1 | 0.749607907 | 1.7995 | 0.008 |
| AC020907.1 | 0.729685317 | 2.7532 | 0.003 |
| CASC8 | 0.442813969 | 1.5651 | 0.007 |
Coef, coefficient; HR, hazard ratio; lncRNA, long non-coding RNA.
Verification of the DRLncI
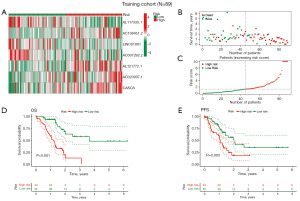
Heatmap of the training cohort showed differential expression of the seven DRLncI-related lncRNAs in the two risk groups (Figure 3A). Risk distribution and survivorship graphs indicated that the proportion of patients presenting with death increased as the risk score increased (Figure 3B,3C). Additionally, survival curves showed that the high-risk individuals had worse OS and progression-free survival (PFS) (Figure 3D,3E), suggesting the stability of the DRLncI.
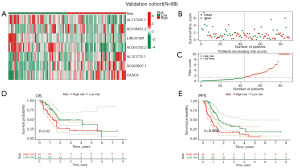
We then validated DRLncI in the validation set. Heatmap of the validation set showing differential expression of the seven DRLncI-related lncRNAs in the two risk groups (Figure 4A). Risk distribution and survivorship graphs indicated that the proportion of patients presenting with death increased as the risk score increased (Figure 4B,4C). Similarly, survival curves showed that the high-risk individuals had worse OS and PFS (Figure 4D,4E). Together, these results demonstrate the effectiveness and stability of the DRLncI.
Evaluation of the DRLncI
Uni- and multi-Cox analysis results revealed that the DRLncI were an independent risk variable for PAAD prognosis (Figure 5A,5B). Additionally, ROC curves showed that the area under the curve (AUC) values for the DRLncI at 1-, 3-, and 5-year were 0.744, 0.812 and 0.838 (Figure 5C). Furthermore, the AUC for the DRLncI at 1, 3, and 5 years were significantly higher than those for clinicopathological variables including sex, age, stage and grade (Figure 5D-5F). Finally, we also performed stratified validation of the index by clinical subtype and the outcomes indicated that survival was significantly better in the low-risk population across age, gender and tumour grade subgroups. Although there was no difference in survival between risk groups in stage III–IV group (P=0.31), a trend towards separate survival curves for the two groups could still be seen (Figure 5G-5N). Together, the above results suggest that the constructed DRLncI has high prognostic predictive efficacy. In addition, the heat map of clinical parameter status of patients in the TCGA-PAAD cohort showed that T stage differed across the groups (Figure 5O).
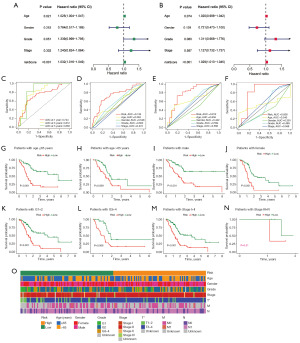
GSVA and GO analysis
Differential analyses identified 1,524 DEGs across the different risk groups. Subsequently, GO analysis revealed that DEGs were mainly enriched in immune-related biological processes such as T cell activation, positive regulation of leukocyte and lymphocyte activation and adaptive immune response. Regarding cellular component, the sites of enrichment included plasma membrane signaling receptors, T cell receptor and immunoglobulin complex. Regarding molecular function, DEGs were mainly enriched in signaling receptor activator activity, receptor ligand activity, G protein-coupled receptor binding and immunoglobulin receptor binding (Figure 6A). Additionally, Spearman correlation analysis revealed extensive correlations between the seven DRLs in the DRLncI and tumour-related signaling pathways (Figure 6B). Furthermore, the heat map of enrichment status showed that the Kyoto Encyclopedia of Genes and Genomes (KEGG) enriched in the high-risk subgroup included the pentose phosphate pathway, basic excision repair, glycolytic gluconeogenesis, and steroid biosynthesis. In contrast, in the low-risk cohort, enriched pathways included Toll like receptor, T cell, B cell, mTOR and JAK-STAT signaling pathways (Figure 6C).
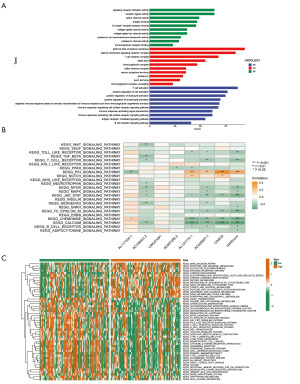
DRLncI-based TMB analysis
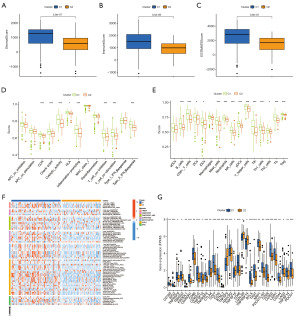
We used simple nucleotide variant data from the TCGA-PAAD cohort to calculate TMB values. Box plots show higher TMB levels in the high-risk group (P<0.001) (Figure 7A). K-M curves of TMB and risk score revealed that samples with lower risk scores and lower TMB had better clinical outcomes than other samples (P<0.001) (Figure 7B), suggesting that the DRLncI combined with TMB levels could better predict the prognosis in PAAD. Among them, TP53 (54%) had the highest mutation rate in the low-risk subgroup, while KRAS (79%) had the highest mutation rate in the high-risk subgroup (Figure 7C,7D).
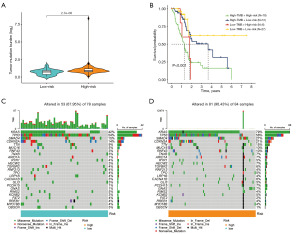
Association of DRLncI with immune landscape
We further investigated the relevance of DRLncI to the TIME of PAAD. ESTIMATE algorithm revealed that the ESTIMATE, immune and stromal scores were lower in the high-risk subgroup (P<0.001) (Figure 8A-8C). Additionally, the TIMER algorithm showed that the degree of infiltration of most immune cells was negatively associated with the DRLncI score (Figure 8D). Furthermore, the findings of the ssGSEA also corroborated with the results of the ESTIMATE and TIMER platform analysis, suggesting that most immune cell scores and immune function scores were higher in the low-risk population (Figure 8E,8F). Further analysis showed that most of the immune checkpoints, including CD274, CTLA-4, PDCD1 and LAG3, present higher expression in the low-risk populations (Figure 8G). Together, the above results demonstrate that risk stratification based on the DRLncI can well differentiate the TIME characteristics of individuals with PAAD.
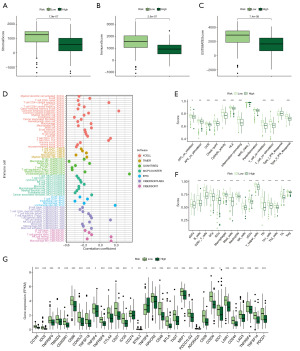
The predictive role of the DRLncI on the sensitivity of chemical compounds
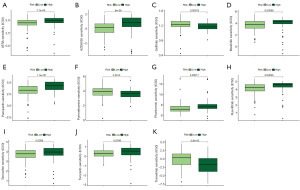
To explore the significance of DRLncI in guiding the clinical management of individuals with advanced PAAD, we performed an IC50 analysis of different drugs using the ‘pRRophtic’ algorithm. Box plots show that the IC50 of gefitinib, pyrimethamine and trametinib in the low-risk subgroup is higher than that in the high-risk one. However, for all-trans retinoic acid (ATRA), AZD8055, masitinib, pazopanib, phenformin, ruxolitinib, tamoxifen and tivozanib, the IC50 was higher in the high-risk population (Figure 9A-9K). The above results suggest differences in the sensitivity of different risk subgroups to different drugs.
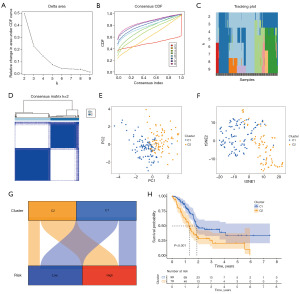
DRLncI-based molecular subtyping
The consensus clustering algorithm can be used to identify tumour features with different molecular subtypes. All individuals were divided into cluster 1 (n=99) and cluster 2 (n=79) (Figure 10A-10D). PCA and t-distributed stochastic neighbor embedding (tSNE) show the distribution of the clusters (Figure 10E,10F). Furthermore, the most part of individuals in high-risk population belonged to cluster 2, while the majority of low-risk individuals belonged to cluster 1 (Figure 10G). K-M survival analysis showed that survival was better for individuals in cluster 1 (P<0.001) (Figure 10H).
We further explored the TIME differences between the two clusters. First, the ESTIMATE analysis showed that the stromal, immune and ESTIMATE scores were significantly higher in cluster 1 (P<0.001) (Figure 11A-11C). Additionally, the ssGSEA difference box plot showed that cytokine-cytokine receptor, T-cell co-inhibition/co-stimulation, human leukocyte antigen, immune checkpoint, cytolytic activity, inflammation promotion and type II interferon (IFN) response were stronger in the cluster 1 (Figure 11D). In terms of immune cells, cluster 1 had significantly higher levels of infiltration of CD8+ T, mast cells, B cells, dendritic cells, neutrophils, tumour infiltrating lymphocytes, natural killer (NK) cells, helper T cells, follicular helper T lymphocytes, Th1/2 cells and Tregs (Figure 11E). The immune cell infiltration heat map similarly suggested a high infiltration status of immune cells in cluster 1 (Figure 11F). Furthermore, differential box plots of immune checkpoints showed that most immune checkpoints, including PDCD1, CTLA-4, TIGIT and LAG3, were highly expressed in cluster 1 (Figure 11G). Finally, IC50 of some chemotherapeutics and targeted drugs varies across clusters (Figure S1).
Discussion
Cell death is a physiological process that maintains biological development and the homeostasis of the internal environment (30,31). Targeting cell death-related pathways to kill cancer cells has long been a major direction in cancer therapy (32,33). A previous study (34) has shown that in the absence of glucose, nicotinamide adenine dinucleotide phosphate (NADPH) is rapidly depleted in SLC7A11 overexpressing cells and disulfides such as cystine accumulate abnormally, triggering disulfide stress and rapid cell death. Just recently, the team published a follow-up study revealing the mechanism of this disulfide stress-induced cell death and named this novel type of cell death as disulfidptosis (10). It was found that glucose deprivation-induced death of SLC7A11 overexpressing cancer cells does not belong to any of the known types of cell death. This novel cell death type could neither be inhibited by drugs typically used to inhibit cell death nor prevented by knocking out key genes for ferroptosis or apoptosis. Instead, thiol oxidants can significantly enhance this cell death (10). Targeting disulfidptosis opens up new possibilities for cancer therapy. Exploring the mechanism of action of disulfidptosis death in different tumours could provide additional targets for cancer therapy.
There is growing evidence that lncRNAs play a complex regulatory role in tumours by acting as regulators of oncogenes or cancer suppressor genes (35,36). Meanwhile, it has been shown that lncRNAs are engaged in various forms of cell death (37,38). Additionally, the role of lncRNAs as diagnostic and prognostic biomarkers of tumours is increasingly recognised (39-42). However, DRLs have not been established in cancer. It is important to identify DRLs and investigate their potential value in the diagnosis and treatment of tumours.
In the current research, we identified the DRLs in PAAD and constructed DRLncI to predict the clinical outcomes and TIME of individuals with PAAD. The results show that DRLncI has good predictive specificity and sensitivity and can independently predict prognosis in individuals with PAAD. Among the seven DRLs constructing the DRLncI, CASC8 has previously been shown to be associated with pancreatic carcinogenesis and tumour-associated fibroblasts, and is a poor prognostic factor for PAAD (43,44). However, the remaining six DRLs have not been reported in PAAD (AL117335.1, AC108451.2, LINC01091, AC007292.2, AL121772.1 and AC020907.1), with AL121772.1 was demonstrated to have considerable affinity for sphingomyelin and to be localized in membrane lesions (45). LINC01091 can promote gastric cancer development by activating the ELF4/CDX2 axis through miR-128-3p downregulation (46). Considering the potential roles of these DRLs, their regulatory mechanisms deserve further in-depth elucidation.
PAAD is known for its unique and challenging tumor microenvironment (TME), which significantly contributes to the aggressive nature of the disease and its resistance to treatment (47). The TME in PAAD is characterized by a dense stroma, high interstitial pressure, and an immunosuppressive environment that limits the efficacy of both chemotherapy and immunotherapy (48). The progress of treatment research in PAAD has been slow but promising in recent years. While traditional chemotherapy remains a cornerstone of treatment, its effectiveness is limited due to the rapid development of resistance and the inability to effectively target the TME (49). Targeted therapies that focus on specific molecular pathways, such as KRAS mutations, have shown limited success, but recent advancements in understanding the molecular mechanisms of PAAD are opening up new therapeutic avenues (49,50). Immunotherapy, particularly immune checkpoint inhibitors, has shown promise in certain cancers, but their efficacy in PAAD has been limited due to the immunosuppressive TME (51). However, ongoing research into combination therapies, such as the use of immune checkpoint inhibitors alongside chemotherapy or targeted therapies, may offer new hope for improving treatment outcomes.
Despite the significant advances in immunotherapy represented by ICBs in solid tumours, their use in PAAD has not achieved significant clinical efficacy. The tumour microenvironment (TME) in PAAD has a low infiltration rate of cytotoxic T cells for highly suppressed TIME (52). This may partly explain the poor immunotherapeutic outcome in individuals with PAAD and so-called immune ‘cold tumours’ (53,54). Also, there is a lack of clinically validated predictive biomarkers to determine the TIME of PAAD. Although microsatellite instability-high (MSI-H) can be served as a predictive biomarker for the response of ICBs treatment, the MSI-H population represents less than 2% of PAAD (55,56). The systematic analysis of the ESTIMATE, ssGSEA and TIMER platforms in this study suggests that the constructed DRLncI and DRLncI-based consensus clusters are able to accurately determine patients with different TIME characteristics. Patients in the low-risk subgroup and cluster 1 not only had a better prognosis, but also had a higher immune cell infiltration status. In addition, most immune checkpoints, including PDCD1, CTLA-4 and LAG3, were expressed higher in the low-risk subgroup and cluster 1. Together, these results indicated that the low-risk group and cohort 1 were more consistent with an ‘immuno-hot tumour’ profile and may have responded better to ICBs treatment (57).
Somatic mutations in driver genes are a major driver and molecular genetic feature in a large proportion of individuals with PAAD (56), with KRAS mutations being the most common, which affect tumour cell recognition by T lymphocytes and promote immune escape of tumour cells (58,59). Additionally, mutations in TP53 can allow tumour cells to evade immune cell killing by suppressing T cell recruitment, and its mutation frequency in PAAD is second only to KRAS (60). In our findings, KRAS and TP53 were more frequently mutated in the high-risk patients, which also explains the poor clinical outcomes in the high-risk subgroup from a genetic point of view. Currently, there are limited reports on the relationship between TMB and prognosis in PAAD. Previous studies have indicated that PAAD falls into the category of cancer types with low TMB (61), while TMB-ultra-low has been demonstrated to be a protective feature for PC (62). Our results indicate that TMB is higher in high-risk individuals. Furthermore, the DRLncI combined with TMB levels allows for a more accurate risk stratification of individuals.
There is no standard clinical treatment protocol for individuals with advanced PAAD treated beyond second line. How to develop an individualised and precise treatment plan to improve prognosis is the primary goal of advanced PAAD treatment. Trametinib is an inhibitor that targets MEK, a downstream effector of KRAS. A randomised controlled phase 2 clinical trial has shown that the combination of trametinib plus anti-programmed cell death protein-1 (PD-1) and stereotactic body radiotherapy may be a new option for patients with local recurrence of PC (63). This study revealed that high-risk individuals were more sensitive to trametinib. ruxolitinib is a STAT3 inhibitor, and its combination with MEK inhibitors may reduce PAAD interstitial inflammation to overcome immunotherapy resistance (64). In addition, tivozanib, an oral vascular endothelial growth factor receptor (VEGFR) tyrosine kinase inhibitor, in combination with ICB may improve the response to immunotherapy in immunologically cold tumors, including PC (65). The present study showed that low-risk patients were more sensitive to both ruxolitinib and tivozanib. Together, these results suggest the potential role of the DRLncI in the individualized comprehensive treatment.
While this study provides valuable insights into the role of DRLncI in PAAD, several limitations should be noted. First, the analysis in this study is based on public RNA-sequencing data from the TCGA-PAAD cohort, and no experimental validation through cell models or animal studies has been performed. Therefore, while the DRLncI is promising as a prognostic tool, its exact biological functions and mechanisms in PAAD need further validation in laboratory settings. Second, although we used multi-omics data to explore potential mechanisms, the heterogeneity of these data and the lack of independent cohort validation may introduce bias. Further studies with larger and more diverse cohorts are needed to validate the robustness and generalizability of the DRLncI. Finally, while we explored the predictive value of DRLncI for TIME and drug efficacy, the clinical application of DRLncI in personalized treatment requires further investigation through prospective clinical trials. Despite these limitations, this study lays the foundation for future research into the potential therapeutic strategies based on DRLs in PAAD.
Conclusions
The DRLncI and the DRLncI-based consensus clusters developed in this work can effectively predict clinical outcomes in PAAD patients and help to differentiate patients with different TIME profiles, thus determining the potential beneficiary population of immunotherapy for ICBs. Furthermore, DRLncI can provide the basis for personalised treatment options for some clinical agents.
Acknowledgments
None.
Footnote
Reporting Checklist: The authors have completed the TRIPOD reporting checklist. Available at https://tcr.amegroups.com/article/view/10.21037/tcr-24-1976/rc
Peer Review File: Available at https://tcr.amegroups.com/article/view/10.21037/tcr-24-1976/prf
Funding: This work was supported by
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tcr.amegroups.com/article/view/10.21037/tcr-24-1976/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki and its subsequent amendments.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Gao AY, Diaz Espinosa AM, Gianì F, et al. Pim-1 kinase is a positive feedback regulator of the senescent lung fibroblast inflammatory secretome. Am J Physiol Lung Cell Mol Physiol 2022;323:L685-97. [Crossref] [PubMed]
- Sung H, Ferlay J, Siegel RL, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin 2021;71:209-49. [Crossref] [PubMed]
- Siegel RL, Miller KD, Fuchs HE, et al. Cancer statistics, 2022. CA Cancer J Clin 2022;72:7-33. [Crossref] [PubMed]
- Hessmann E, Buchholz SM, Demir IE, et al. Microenvironmental Determinants of Pancreatic Cancer. Physiol Rev 2020;100:1707-51. [Crossref] [PubMed]
- Yu H, Yan J, Li Z, et al. Recent trends in emerging strategies for ferroptosis-based cancer therapy. Nanoscale Adv 2023;5:1271-90. [Crossref] [PubMed]
- Li M, Kim J, Rha H, et al. Photon-Controlled Pyroptosis Activation (PhotoPyro): An Emerging Trigger for Antitumor Immune Response. J Am Chem Soc 2023;145:6007-23. [Crossref] [PubMed]
- Tong X, Tang R, Xiao M, et al. Targeting cell death pathways for cancer therapy: recent developments in necroptosis, pyroptosis, ferroptosis, and cuproptosis research. J Hematol Oncol 2022;15:174. [Crossref] [PubMed]
- Meng Q, Ding B, Ma P, et al. Interrelation between Programmed Cell Death and Immunogenic Cell Death: Take Antitumor Nanodrug as an Example. Small Methods 2023;7:e2201406. [Crossref] [PubMed]
- Luo Y, Tang W, Xiang S, et al. Non-coding RNAs in breast cancer: Implications for programmed cell death. Cancer Lett 2022;550:215929. [Crossref] [PubMed]
- Liu X, Nie L, Zhang Y, et al. Actin cytoskeleton vulnerability to disulfide stress mediates disulfidptosis. Nat Cell Biol 2023;25:404-14. [Crossref] [PubMed]
- Nandwani A, Rathore S, Datta M. LncRNAs in cancer: Regulatory and therapeutic implications. Cancer Lett 2021;501:162-71. [Crossref] [PubMed]
- Gong CY, Tang R, Liu KX, et al. Long non-coding RNA TP73-AS1 in cancers. Clin Chim Acta 2020;503:151-6. [Crossref] [PubMed]
- Shuai Y, Ma Z, Liu W, et al. TEAD4 modulated LncRNA MNX1-AS1 contributes to gastric cancer progression partly through suppressing BTG2 and activating BCL2. Mol Cancer 2020;19:6. [Crossref] [PubMed]
- Zhao J, Yang S, Lv C, et al. Cancer-associated fibroblasts suppressed ferroptosis in glioblastoma via upregulating lncRNA DLEU1. Am J Physiol Cell Physiol 2023;324:C1039-52. [Crossref] [PubMed]
- Qu X, Liu B, Wang L, et al. Loss of cancer-associated fibroblast-derived exosomal DACT3-AS1 promotes malignant transformation and ferroptosis-mediated oxaliplatin resistance in gastric cancer. Drug Resist Updat 2023;68:100936. [Crossref] [PubMed]
- Wu Z, Zhang F, Wang Y, et al. Identification and Validation of the lncRNA MYOSLID as a Regulating Factor of Necroptosis and Immune Cell Infiltration in Colorectal Cancer following Necroptosis-Related LncRNA Model Establishment. Cancers (Basel) 2022;14:4364. [Crossref] [PubMed]
- Iwai M, Kajino T, Nakatochi M, et al. Long non-coding RNA TILR constitutively represses TP53 and apoptosis in lung cancer. Oncogene 2023;42:364-73. [Crossref] [PubMed]
- Cheng Z, Han J, Jiang F, et al. Prognostic pyroptosis-related lncRNA signature predicts the efficacy of immunotherapy in hepatocellular carcinoma. Biochem Biophys Rep 2022;32:101389. [Crossref] [PubMed]
- Gencel-Augusto J, Wu W, Bivona TG. Long Non-Coding RNAs as Emerging Targets in Lung Cancer. Cancers (Basel) 2023;15:3135. [Crossref] [PubMed]
- Lou UK, Wong CH, Chen Y. A simple and rapid colorimetric detection of serum lncRNA biomarkers for diagnosis of pancreatic cancer. RSC Adv 2020;10:8087-92. [Crossref] [PubMed]
- Shao D, Shi L, Ji H. Disulfidptosis: Disulfide Stress Mediates a Novel Cell Death Pathway via Actin Cytoskeletal Vulnerability. Mol Cells 2023;46:414-6. [Crossref] [PubMed]
- Zhao P, Zhen H, Zhao H, et al. Identification of hub genes and potential molecular mechanisms related to radiotherapy sensitivity in rectal cancer based on multiple datasets. J Transl Med 2023;21:176. [Crossref] [PubMed]
- Liu Q, Fang Y, Wang J. ESTIMATE algorithm is not appropriate for inferring tumor purity and stromal and immune cell admixture in hematopoietic or stromal tumors. Cancer Immunol Immunother 2020;69:1153-4. [Crossref] [PubMed]
- Li T, Fan J, Wang B, et al. TIMER: A Web Server for Comprehensive Analysis of Tumor-Infiltrating Immune Cells. Cancer Res 2017;77:e108-10. [Crossref] [PubMed]
- Li T, Fu J, Zeng Z, et al. TIMER2.0 for analysis of tumor-infiltrating immune cells. Nucleic Acids Res 2020;48:W509-14. [Crossref] [PubMed]
- Mubeen S, Tom Kodamullil A, Hofmann-Apitius M, et al. On the influence of several factors on pathway enrichment analysis. Brief Bioinform 2022;23:bbac143. [Crossref] [PubMed]
- Yi M, Niu M, Xu L, et al. Regulation of PD-L1 expression in the tumor microenvironment. J Hematol Oncol 2021;14:10. [Crossref] [PubMed]
- Wang Y, Qu X, Li L, et al. Integrative Analysis Reveals a Nine TP53 Pathway-Related lncRNA Prognostic Signature in Endometrial Cancer. Biomed Res Int 2022;2022:5432806. [Crossref] [PubMed]
- Yan J, Zhou X, Yang H. TGF-β signaling-related signature for predicting prognosis and therapeutic response in lower-grade glioma. Transl Cancer Res 2024;13:4985-5002. [Crossref] [PubMed]
- Frank D, Vince JE. Pyroptosis versus necroptosis: similarities, differences, and crosstalk. Cell Death Differ 2019;26:99-114. [Crossref] [PubMed]
- Bedoui S, Herold MJ, Strasser A. Emerging connectivity of programmed cell death pathways and its physiological implications. Nat Rev Mol Cell Biol 2020;21:678-95. [Crossref] [PubMed]
- Hsu SK, Li CY, Lin IL, et al. Inflammation-related pyroptosis, a novel programmed cell death pathway, and its crosstalk with immune therapy in cancer treatment. Theranostics 2021;11:8813-35. [Crossref] [PubMed]
- Xu T, Ding W, Ji X, et al. Molecular mechanisms of ferroptosis and its role in cancer therapy. J Cell Mol Med 2019;23:4900-12. [Crossref] [PubMed]
- Liu X, Olszewski K, Zhang Y, et al. Cystine transporter regulation of pentose phosphate pathway dependency and disulfide stress exposes a targetable metabolic vulnerability in cancer. Nat Cell Biol 2020;22:476-86. [Crossref] [PubMed]
- Xu J, Wang X, Zhu C, et al. A review of current evidence about lncRNA MEG3: A tumor suppressor in multiple cancers. Front Cell Dev Biol 2022;10:997633. [Crossref] [PubMed]
- Yang Z, Xu F, Teschendorff AE, et al. Insights into the role of long non-coding RNAs in DNA methylation mediated transcriptional regulation. Front Mol Biosci 2022;9:1067406. [Crossref] [PubMed]
- Shi W, Sethi G. Long noncoding RNAs induced control of ferroptosis: Implications in cancer progression and treatment. J Cell Physiol 2023;238:880-95. [Crossref] [PubMed]
- Yang H, Zhang Y, Du Z, et al. Hair follicle mesenchymal stem cell exosomal lncRNA H19 inhibited NLRP3 pyroptosis to promote diabetic mouse skin wound healing. Aging (Albany NY) 2023;15:791-809. [Crossref] [PubMed]
- Wang W, Ye Y, Zhang X, et al. Construction of a Necroptosis-Associated Long Non-Coding RNA Signature to Predict Prognosis and Immune Response in Hepatocellular Carcinoma. Front Mol Biosci 2022;9:937979. [Crossref] [PubMed]
- Jiang Y, Ye Y, Huang Y, et al. Identification and validation of a novel anoikis-related long non-coding RNA signature for pancreatic adenocarcinoma to predict the prognosis and immune response. J Cancer Res Clin Oncol 2023;149:15069-83. [Crossref] [PubMed]
- Zhao Q, Ye Y, Zhang Q, et al. PANoptosis-related long non-coding RNA signature to predict the prognosis and immune landscapes of pancreatic adenocarcinoma. Biochem Biophys Rep 2024;37:101600. [Crossref] [PubMed]
- Bao L, Ye Y, Zhang X, et al. Identification and verification of a PANoptosis-related long noncoding ribonucleic acid signature for predicting the clinical outcomes and immune landscape in lung adenocarcinoma. Heliyon 2024;10:e29869. [Crossref] [PubMed]
- López de Maturana E, Rodríguez JA, Alonso L, et al. A multilayered post-GWAS assessment on genetic susceptibility to pancreatic cancer. Genome Med 2021;13:15. [Crossref] [PubMed]
- Ye Y, Zhao Q, Wu Y, et al. Construction of a cancer-associated fibroblasts-related long non-coding RNA signature to predict prognosis and immune landscape in pancreatic adenocarcinoma. Front Genet 2022;13:989719. [Crossref] [PubMed]
- Wu E, Guo X, Teng X, et al. Discovery of Plasma Membrane-Associated RNAs through APEX-seq. Cell Biochem Biophys 2021;79:905-17. [Crossref] [PubMed]
- Wang Q, Zhang C, Cao S, et al. Tumor-derived exosomes orchestrate the microRNA-128-3p/ELF4/CDX2 axis to facilitate the growth and metastasis of gastric cancer via delivery of LINC01091. Cell Biol Toxicol 2023;39:519-36. [Crossref] [PubMed]
- Hartupee C, Nagalo BM, Chabu CY, et al. Pancreatic cancer tumor microenvironment is a major therapeutic barrier and target. Front Immunol 2024;15:1287459. [Crossref] [PubMed]
- Ho WJ, Jaffee EM, Zheng L. The tumour microenvironment in pancreatic cancer - clinical challenges and opportunities. Nat Rev Clin Oncol 2020;17:527-40. [Crossref] [PubMed]
- Wood LD, Canto MI, Jaffee EM, et al. Pancreatic Cancer: Pathogenesis, Screening, Diagnosis, and Treatment. Gastroenterology 2022;163:386-402.e1. [Crossref] [PubMed]
- Hu ZI, O'Reilly EM. Therapeutic developments in pancreatic cancer. Nat Rev Gastroenterol Hepatol 2024;21:7-24. [Crossref] [PubMed]
- Mukherji R, Debnath D, Hartley ML, et al. The Role of Immunotherapy in Pancreatic Cancer. Curr Oncol 2022;29:6864-92. [Crossref] [PubMed]
- Elyada E, Bolisetty M, Laise P, et al. Cross-Species Single-Cell Analysis of Pancreatic Ductal Adenocarcinoma Reveals Antigen-Presenting Cancer-Associated Fibroblasts. Cancer Discov 2019;9:1102-23. [Crossref] [PubMed]
- Galon J, Bruni D. Approaches to treat immune hot, altered and cold tumours with combination immunotherapies. Nat Rev Drug Discov 2019;18:197-218. [Crossref] [PubMed]
- Bonaventura P, Shekarian T, Alcazer V, et al. Cold Tumors: A Therapeutic Challenge for Immunotherapy. Front Immunol 2019;10:168. [Crossref] [PubMed]
- von den Driesch J, Flöttmann J, Prall F, et al. HROP68: A rare case of medullary pancreatic cancer-characterization and chemosensitivity of the first patient-derived cell line. Front Oncol 2022;12:1082927. [Crossref] [PubMed]
- Cao L, Huang C, Cui Zhou D, et al. Proteogenomic characterization of pancreatic ductal adenocarcinoma. Cell 2021;184:5031-5052.e26. [Crossref] [PubMed]
- Liu YT, Sun ZJ. Turning cold tumors into hot tumors by improving T-cell infiltration. Theranostics 2021;11:5365-86. [Crossref] [PubMed]
- Cullis J, Das S, Bar-Sagi D. Kras and Tumor Immunity: Friend or Foe? Cold Spring Harb Perspect Med 2018;8:a031849. [Crossref] [PubMed]
- Waters AM, Der CJ. KRAS: The Critical Driver and Therapeutic Target for Pancreatic Cancer. Cold Spring Harb Perspect Med 2018;8:a031435. [Crossref] [PubMed]
- Blagih J, Buck MD, Vousden KH. p53, cancer and the immune response. J Cell Sci 2020;133:jcs237453. [Crossref] [PubMed]
- Chalmers ZR, Connelly CF, Fabrizio D, et al. Analysis of 100,000 human cancer genomes reveals the landscape of tumor mutational burden. Genome Med 2017;9:34. [Crossref] [PubMed]
- Imamura T, Ashida R, Ohshima K, et al. Characterization of pancreatic cancer with ultra-low tumor mutational burden. Sci Rep 2023;13:4359. [Crossref] [PubMed]
- Zhu X, Cao Y, Liu W, et al. Stereotactic body radiotherapy plus pembrolizumab and trametinib versus stereotactic body radiotherapy plus gemcitabine for locally recurrent pancreatic cancer after surgical resection: an open-label, randomised, controlled, phase 2 trial. Lancet Oncol 2022;23:e105-15. [Crossref] [PubMed]
- Datta J, Dai X, Bianchi A, et al. Combined MEK and STAT3 Inhibition Uncovers Stromal Plasticity by Enriching for Cancer-Associated Fibroblasts With Mesenchymal Stem Cell-Like Features to Overcome Immunotherapy Resistance in Pancreatic Cancer. Gastroenterology 2022;163:1593-612. [Crossref] [PubMed]
- Ramnaraign BH, Lee JH, Ali A, et al. Atezolizumab plus tivozanib for immunologically cold tumor types: the IMMCO-1 trial. Future Oncol 2022; Epub ahead of print. [Crossref]


