
Identification and validation of hypoxia-associated genes positively correlated with HIF1A in pulmonary adenocarcinoma
Highlight box
Key findings
• This study identified five hypoxia-related genes (HRGs) in lung adenocarcinoma (LUAD) that are positively correlated with hypoxia-inducible factor 1 alpha (HIF1A): collagen type I alpha 1 chain (COL1A1), interleukin 11 (IL11), matrix metalloproteinase 14 (MMP14), notch receptor 3 (NOTCH3), and thymocyte differentiation antigen 1 (THY1). In vitro experiments showed that after chronic intermittent hypoxia (CIH) treatment, the mRNA expression of these HRGs and HIF1A increased in A549 cells. The developed model has a high predictive value for hypoxia-related LUAD.
What is known and what is new?
• It is known that hypoxia promotes the proliferation, migration, and chemoresistance of LUAD. HIF1A is a key factor in the hypoxic response.
• This study identified specific HRGs that are positively correlated with HIF1A, providing new therapeutic targets for LUAD. In vitro experiments confirmed the role of these HRGs in the hypoxia-driven progression of LUAD.
What is the implication, and what should change now?
• These HRGs may be key targets for the progression of LUAD under hypoxic conditions, providing insights into the diagnosis and treatment of patients with hypoxic diseases complicated by LUAD. Further research is needed to validate the clinical utility of these HRGs as biomarkers in a larger cohort. Developing targeted therapies against these HRGs may improve the management and prognosis of hypoxia-related LUAD patients.
Introduction
Lung cancer continues to be the most widespread form of cancer globally, comprising around 11.6% of all reported cancer cases and standing as a leading contributor to mortality associated with cancer (1). Among various types of lung cancers, the incidence of lung adenocarcinoma (LUAD) is the highest, accounting for approximately 50% of all reported lung cancer cases. Moreover, LUAD has a higher incidence in non-smokers, and its pathogenesis is complex. Over the past few decades, extensive efforts, including whole-genome sequencing, RNA sequencing, and proteomics, have been employed to study the molecular drivers of LUAD occurrence and progression. Despite a profound understanding of the risk, development, immune control, and treatment modalities of LUAD in humans, its mortality rate remains high (2). Therefore, an immediate imperative exists to devise predictive methodologies for LUAD, with the objective of discerning novel therapeutic targets and augmenting patient survival rates.
The majority of solid tumors, including LUAD, rely on a hypoxic microenvironment, and hypoxia has long been recognized as a hallmark of cancer (3). The hypoxic environment induces changes in the tumor microenvironment, influencing gene expression. Hypoxic signaling interacts with various cellular pathways to regulate cancer cell proliferation, migration, invasion, and chemoresistance, thereby impacting the outcomes of cancer treatment. Hypoxia-inducible factors (HIFs) represent core factors that respond to hypoxic stress and orchestrate downstream gene expression (4). The activation of HIFs stands out as a primary driver behind the heightened activity of cancer cells in a hypoxic milieu. HIFs dynamically reprogram gene transcription, protein synthesis, and cell cycle progression (5). As the pivotal subunit within HIFs, hypoxia-inducible factor 1 alpha (HIF1A) plays a crucial role in forming a vital link in our thorough exploration of the intricate relationship between hypoxia and tumors.
This study, utilizing transcriptome expression profiles from the Gene Expression Omnibus (GEO) database of LUAD patients and normal lung tissues, identified five core hypoxia-related genes (HRGs) that exhibit a positive correlation with HIF1A. An intermittent hypoxia (IH) cell model was established, and in vitro validation was performed using quantitative real-time polymerase chain reaction (qRT-PCR). Our research findings illuminate the role of HIF1A-associated HRGs in LUAD. This discovery holds the potential to offer novel insights and therapeutic targets for patients with clinical LUAD in conjunction with hypoxic conditions. We present this article in accordance with TRIPOD reporting checklist (available at https://tcr.amegroups.com/article/view/10.21037/tcr-2024-2265/rc).
Methods
This study was conducted in accordance with the Declaration of Helsinki and its subsequent amendments.
Data acquisition
The transcriptome data for this study were sourced from the GEO, specifically GSE116959 and GPL17077, the dataset comprises 57 LUAD samples and 11 normal lung tissue samples; GSE31210 (including 226 LUAD samples and 20 normal lung tissue samples) and GSE72094 (including 442 LUAD samples) serve as external validation datasets. Additionally, a set of 243 HRGs was retrieved from the gene set enrichment analysis (GSEA) database (https://www.gsea-msigdb.org/gsea/msigdb/human/geneset/WINTER_HYPOXIA_METAGENE.html?keywords=hypoxia).
Differentially expressed genes (DEGs) selection
The identification of DEGs in the GSE116959 dataset employed the “Limma” R package, applying filtering criteria of |logarithmic fold change| (|log FC|) >0.5 and P<0.05. Genes exhibiting log FC >0.5 and P<0.05 were classified as upregulated genes, whereas those with log FC <−0.5 and P<0.05 were classified as downregulated genes. Heatmaps and volcano plots were generated using the “Pheatmap” and “ggplot2” R packages, respectively.
Analysis of gene set variation analysis (GSVA) and weighted gene co-expression network analysis (WGCNA)
GSVA was performed using the “GSVA” R package (6) to obtain enrichment scores related to hypoxia in the GSE116959 dataset. Subsequently, based on the GSVA outcomes, WGCNA was applied to identify differentially regulated genes (DRGs) (7). The “WGCNA” R package, within the R programming language, was utilized to establish an unsupervised co-expression relationship among genes through Pearson correlation coefficients and to construct a topological overlap matrix (TOM). The determination of the soft-thresholding parameter (β) was guided by achieving a minimum power of 0.9 when fitting the scale-free topological model. The DynamicTreeCut algorithm was employed to partition gene modules, merging those with a similarity greater than or equal to 75% (cut Height =0.25). Gene significance (GS) and module membership (MM) values were calculated. Additionally, a hierarchical clustering dendrogram was generated based on the similarity of module eigengenes (ME). Pearson correlation analysis was conducted on co-expression modules in the hypoxia dataset. Genes within modules exhibiting a higher correlation with hypoxia were selected as DRGs identified through WGCNA, referred to as HRGs.
GSEA
GSEA assesses microarray data at the level of gene sets, annotating genes within specific functional gene sets (8). We utilized the “GSEABase” package in the R language to analyze the expression matrix of samples and calculate the correlation between the dataset and hypoxia. Single-sample gene set enrichment analysis (ssGSEA) and single-gene gene set enrichment analysis (single-gene GSEA) were employed separately to compare the Mfuzz clustering modules between LUAD and normal groups and for functional annotation of individual HRGs.
Mfuzz analysis
We utilized the “Mfuzz” package to perform Fuzzy c-Means Clustering (FCM) on microarray data based on expression levels (9), calculate ssGSEA scores for different clustering modules with respect to HIF1A, assess the correlation of each module with HIF1A, and ultimately identify the cluster most closely associated with HIF1A.
Disease Ontology (DO), Gene Ontology (GO), and Kyoto Encyclopedia of Genes and Genomes (KEGG) enrichment analysis
DO (https://disease-ontology.org/), GO (https://www.geneontology.org/), and KEGG (https://www.genome.jp/kegg/) enrichment analysis is a crucial method in bioinformatics for exploring the characteristics and functional significance of genes (10-12). We utilized the “clusterProfiler” package in the R language to perform enrichment analysis on HRGs in terms of disease, biological processes (BP), cellular components (CC), molecular functions (MF), and signaling pathways. Visualization was conducted using the “ggplot2” R package. A significance threshold of P<0.05 was applied for enrichment analysis.
Protein-protein interaction network construction
We input HRGs and HIF1A into the Search Tool for the Retrieval of Interacting Genes/Proteins (STRING) database (http://string-db.org) and collected proteins with medium confidence >0.4 for interactions (13). We processed and created a Protein-Protein Interaction (PPI) network using Cytoscape. We subsequently filtered proteins directly associated with HIF1A.
Machine learning algorithm for core HRG selection
Support Vector Machine Recursive Feature Elimination (SVM-RFE) is an effective feature selection technique (14). We used the R packages “caret” and “e1071” for SVM-RFE, employing 40-fold cross-validation to select the minimum number of genes with the least error. Least absolute shrinkage and selection operator (LASSO) regression eliminates unimportant variables based on the regression coefficients of parameters (15). LASSO regression was executed using the “glmnet” package in the R language, with 10-fold cross-validation, and the final model retained all predictive variables for which the adjusted parameter (λ) was not equal to zero. Lastly, random forest (RF) analysis was employed for gene importance ranking, and we selected the top 10 important HRGs for core HRG selection.
Receiver operating characteristic (ROC) curve construction
ROC curve was used to evaluate the optimal diagnostic threshold for candidate genes (16), We used the “pROC” R package to calculate the area under the curve (AUC) and 95% Confidence Interval (CI) for each HRG. AUC >0.7 was considered to have a reliable diagnostic value (17).
Cell culture and chronic intermittent hypoxia (CIH) model
We obtained the LUAD A549 cells from Applied Biological Materials. The A549 cell line was cultured in Dulbecco’s Modified Eagle Medium (DMEM) containing 10% fetal bovine serum. A549 cells were seeded in 35 mm cell culture dishes, and after 24 hours of cultivation, cells were divided into two groups: (I) normoxia group cultured at 37 ℃ in a humidified incubator with 5% CO2; (II) hypoxia group subjected to IH in a high-end dynamic programming tri-gas cell culture chamber. The IH parameters were set to maintain the O2 concentration in the chamber at 5–21% cycling every 30 minutes, and the duration of CIH was 72 hours.
qRT-PCR in vitro validation
We conducted in vitro validation of core HRGs using qRT-PCR. Total RNA from A549 cells was extracted using Trizol reagent, and the RNA concentration was measured using a NanoDrop spectrophotometer (Thermo, USA). The HiScript III RT SuperMix for qPCR (+gDNA wiper) kit from Nanjing Novozymes was used for reverse transcription to cDNA. qRT-PCR was performed using the SYBR Green Kit reagent (Nanjing Novozymes), with glyceraldehyde-3-phosphate dehydrogenase (GAPDH) serving as the mRNA reference internal control. The primer sequences used are as follows (Table 1).
Table 1
| Gene | Forward | Reverse |
|---|---|---|
| HIF1A | GAAAGCGCAAGTCCTCAAAG | TGGGTAGGAGATGGAGATGC |
| GAPDH | TGACATCAAGAAGGTGGTGAAGCAG | GTGTCGCTGTTGAAGTCAGAGGAG |
| COL1A1 | AGGGCGACAGAGGCATAAAGG | GTTCACCAGGAGAGCCAGGAG |
| MMP14 | TCCATCAACACTGCCTACGAGAG | ACGCCTCATCAAACACCCAATG |
| IL11 | CGGGGACCACAACCTGGATTC | GCACACCTGGGAGCTGTAGAG |
| NOTCH3 | CATCTGGTTGCTGCTGACATCC | CTCATCCTCTTCAGTTGGCATTGG |
| THY1 | GCCTAGTGGACCAGAGCCTTC | CGGATAAGTAGAGGACCTTCATGTTG |
Statistical analysis
The experimental results were analyzed statistically three times using GraphPad Prism 9 software. Statistical methods included Student’s t-test and analysis of variance (ANOVA), and the results were expressed as mean ± standard deviation (SD). A significance level of P<0.05 was considered statistically significant.
Results
DEGs in LUAD and normal lung tissue
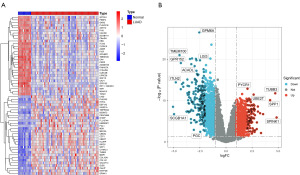
We used the “Limma” R package to identify DEGs in the dataset, obtaining a total of 20,434 DEGs. Among them, there are 4,749 DEGs with |log FC| >0.5 and probability value (P value) <0.05. The heatmap (Figure 1A) displays the top 30 upregulated and downregulated DEGs, while the volcano plot (Figure 1B) selects DEGs marked with P value <0.01 and |log FC| >2.
Correlation between the dataset and hypoxia
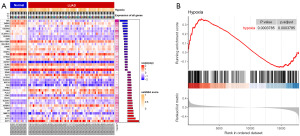
We obtained 243 HRGs from the GSEA database and analyzed the enrichment levels of hypoxia genes in each sample using ssGSEA. We found a high enrichment of HRGs in a significant number of samples (Figure 2A). GSEA analysis revealed the correlation between the LUAD dataset and the hypoxia gene set (Figure 2B). Validation was performed in the external dataset GSE31210 and yielded consistent conclusions (Figure S1A).
Selection of hypoxia-related DEGs
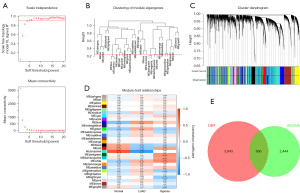
We constructed a scale-free network with a soft threshold parameter (β) of 4 (Figure 3A), merged modules with a similarity greater than 0.75, and plotted a dendrogram of co-expression network modules (Figures 3B,3C). We evaluated the correlation and p-values of modules with diseases and the hypoxia gene set (Figure 3D). Subsequently, we selected genes within the green, black, red, and white modules that showed relatively high and statistically significant correlations with diseases and the hypoxia gene set. The intersection of these genes with DEGs from the differential analysis with |log FC| >0.5 and P value <0.05 yielded a total of 906 HRGs (Figure 3E).
Selection of HIF1A positively correlated DEGs
We used the Mfuzz method to cluster genes with similar expression patterns based on the expression levels of HIF1A, obtaining a total of 100 clustering results (Figure 4A). We calculated ssGSEA scores for each cluster and compared the differences between the LUAD group and the normal lung tissue group (Figure 4B).

Next, we used the Spearman algorithm to examine the correlation between each clustering module and HIF1A. Finally, we selected genes within Cluster 9, 15, 44, 46, and 97 that showed a positive correlation with HIF1A as the objects for the next step of screening (Figure 5A,5B).
Identification of HIF1A positively correlated HRGs
We intersected the 906 HRGs identified with possible hypoxia correlation (|log FC| >0.5, P<0.05) with the 1043 DEGs related to HIF1A obtained from Mfuzz clustering, resulting in 62 HRGs potentially correlated with HIF1A (Figure 6A). DO enrichment analysis demonstrated a significant association of these genes with various cancers, with the highest enrichment observed in breast cancer (Figure 6B). GO enrichment analysis revealed that these HRGs have molecular functions related to catalyzing DNA replication and are mainly involved in biological processes related to cell DNA replication and division (Figure 6C). KEGG pathway analysis indicated the involvement of these HRGs in regulating pathways associated with the cell cycle, homologous recombination, p53, extracellular matrix (ECM)-receptor interaction, motor proteins, PI3K-Akt, and other cancer-related pathways (Figure 6D).
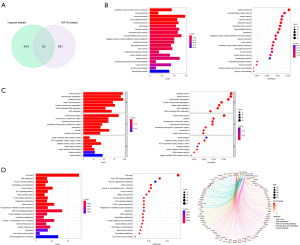
Selection of core HRGs
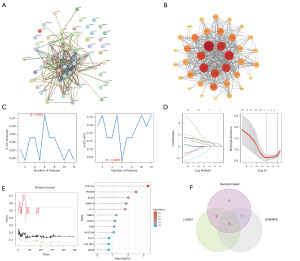
We inputted the 62 HRGs and HIF1A into the STRING database to create a protein-protein interaction network. After processing with Cytoscape, we identified 12 proteins directly associated with HIF1A (Figure 7A,7B). Further, using machine learning methods for core HRG selection, SVM-REF analysis indicated that 6 genes had the best accuracy for disease diagnosis (Figure 7C). LASSO regression model analysis identified a model of 7 genes that could accurately predict LUAD (Figure 7D), and random forest selected the top 10 genes for the next round of screening (Figure 7E). Combining the three approaches in a Venn diagram, we ultimately identified 5 core HRGs: collagen type I alpha 1 chain (COL1A1), interleukin 11 (IL11), matrix metallopeptidase 14 (MMP14), notch receptor 3 (NOTCH3), and thymocyte differentiation antigen 1 (THY1) (Figure 7F). We utilized external datasets to examine their expression in the samples. Except for the lack of difference in NOTCH3 expression levels between the two groups in GSE31210, the remaining four HRGs all exhibited high expression in LUAD tissues (Figure S1B).
Correlation analysis of core HRGs with HIF1A
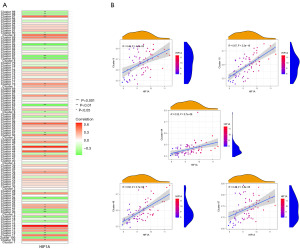
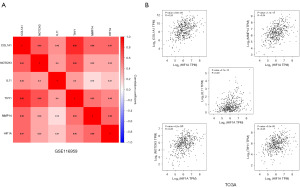
We conducted a correlation analysis between these five core HRGs and HIF1A and found that in the GSE116959 LUAD data cohort, all of them were positively correlated with HIF1A and had statistical significance (Figure 8A). The external datasets GSE31210 and GSE72094 also confirmed their positive correlation (Figure S1C). By using the Gene Expression Profiling Interactive Analysis (GEPIA2) online database and The Cancer Genome Atlas (TCGA) data cohort, we re-examined the correlation between the core HRGs and HIF1A. The results showed that these five HRGs were all positively correlated with HIF1A and had statistical significance (Figure 8B), further supporting the universality of our identification results in LUAD.
ROC curve analysis
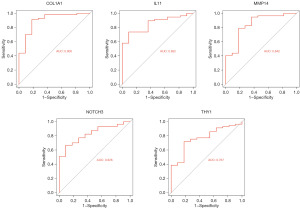
We conducted ROC analysis on these 5 core HRGs, and the results showed that the AUC values for these core genes were all >0.7. These results indicate that these genes have high accuracy and reliability for diagnosing LUAD (Figure 9).
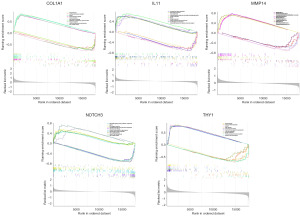
Potential functions of core HRGs in GSEA
We conducted single-gene GSEA analysis to explore the potential biological functions of COL1A1, IL11, MMP14, NOTCH3, and THY1. The high-expression groups of COL1A1, MMP14, and THY1 were associated with immune response, while the high-expression groups of IL11 and NOTCH3 were involved in the extracellular matrix pathway. The low-expression group of NOTCH3 participated in immune response, and the low-expression groups of COL1A1, IL11, MMP14, and THY1 were closely associated with cellular metabolism (Figure 10).
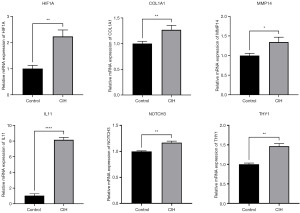
qRT-PCR validation of HIF1A-related HRGs
To validate the expression of the 5 core HRGs in the model, we performed qRT-PCR to detect gene expression in A549 cells under CIH and normoxic conditions. After CIH treatment, HIF1A was upregulated in A549 cells, and the expression of COL1A1, IL11, MMP14, NOTCH3, and THY1 also increased (Figure 11). This was consistent with our hypothesis.
Discussion
Cancer incidence and mortality rates are increasing globally, with lung cancer being the most common form. Approximately 80% are classified as non-small cell lung cancer (NSCLC), and 15% as small cell lung cancer (SCLC), with adenocarcinoma representing about 40% of all NSCLC types (18,19). Although adenocarcinoma has a slower growth rate compared to other types of lung cancer, it is more likely to be discovered before spreading (20). However, due to its larger base and heterogeneity leading to a relatively low 5-year survival rate after surgery (19), this heterogeneity is attributed to genomic instability, manifested as gene mutations (substitutions, insertions, deletions, rearrangements, etc.) (21). In recent years, sequencing technologies have provided a new perspective on understanding the development of lung cancer (22). Next-generation sequencing (NGS), single-cell RNA sequencing (scRNA-seq), and proteomic analysis technologies have helped reveal the mysteries of lung cancer heterogeneity, tumor microenvironment, and resistance mechanisms (23-25). This has benefitted numerous patients carrying carcinogenic drivers in targeted therapy (TT) and immunotherapy (26,27).
Hypoxia refers to a state with relatively lower oxygen levels compared with normal organs, tissues, or cells. It is categorized into acute hypoxia and chronic hypoxia, generally playing a pathological role in conditions such as cardiovascular diseases, pulmonary diseases, kidney diseases, metabolic diseases, etc. (28). However, for tumors, hypoxia is a crucial microenvironment that plays a significant role in tumor proliferation, migration, and chemoresistance (29). This is attributed to HIFs. The most important HIF, the heterodimeric HIF1A, consists of an oxygen-sensitive α subunit and a stable β subunit. Under aerobic conditions, prolyl hydroxylases (PHD) hydroxylate proline residues in the HIF-1A subunit, and von Hippel-Lindau protein (pVHL) is responsible for ubiquitinating HIF1A, leading to its degradation in the proteasome. When pVHL is defective or oxygen levels are low, HIF1A escapes ubiquitination, accumulates in the cell, binds with hypoxia-inducible factor-β (HIF-β), and transcriptionally activates genes containing hypoxia-responsive elements (HRE) (30). The activation of HIF1A further regulates a variety of pro-cancer or anti-cancer genes, thereby controlling tumor development (31-34). Therefore, targeting HIF1A serves as a bridge for us to deepen our understanding of the driver genes in lung cancer.
Our study started from the perspective of hypoxia, focusing on HIF1A as the entry point. We screened genes in LUAD samples that are positively correlated with HIF1A and associated with HRGs. Enrichment analysis explored the functions of these genes and the pathways they might affect. Machine learning further selected core HRGs, and ROC evaluation was used to assess their diagnostic value for LUAD. For in vitro validation, we chose the CIH model of obstructive sleep apnea syndrome (OSAS), a common hypoxic disease in clinical practice. OSAS is characterized by repetitive upper airway collapse during sleep, leading to CIH and fragmented sleep, accompanied by a series of pathological changes such as hypoxemia and hypercapnia. These changes increase the risk and mortality of various diseases (such as mental, cardiovascular, metabolic, or stress-related diseases) (35). For lung cancer, OSAS is closely related to the risk and development of lung cancer (36-38). Currently, the mechanisms by which OSAS promotes the occurrence and development of lung cancer are multifaceted. Firstly, the hypoxia and oxidative stress caused by OSAS alter the tumor immune microenvironment, inhibiting the immune system’s surveillance of tumors. Its main functions are to weaken the anti-tumor immune response, maintain cell proliferation, prevent cell apoptosis, maintain an immune-suppressive environment, and promote angiogenesis (39). Hypoxia has been shown to promote the migration and invasion of lung cancer cells by up-regulating transforming growth factor-beta (TGF-β) signaling to increase the activation of cancer-associated fibroblasts (CAFs) and the proportion of lung tumor-associated CAFs (37,40). Secondly, IH reshapes the HIF1A metabolic pathway, enriches lactate in the tumor immune microenvironment, and hinders T-cell proliferation, tumor invasion, and cytokine production. Additionally, a recent animal study has found that OSAS has an adverse impact on the aggressiveness and mortality of lung cancer. IH endows lung cancer with a stronger metastatic potential, manifested by increased tumor growth, tumor implantation, and up-regulation of genes related to lung cancer stem cells. The key factor in coping with oxidative stress conditions [the transcription factor BTB and CNC homolog 1 (Bach1)] is also up-regulated in lung cancer after IH exposure, and cancer stem cells are generated at a relatively high frequency (41). In terms of clinical research, Huang et al. found that among patients with stage III and IV lung cancer, those with severe OSAS had a higher cancer-related mortality rate than patients with mild-moderate OSAS. The apnea-hypopnea index (AHI) and the low oxygen saturation index (Tsat90%) were positively correlated with a higher overall mortality rate and shorter overall survival time and progression-free survival (PFS) time in patients with stage III and IV lung cancer. Their pathological staining provided direct evidence of a significant association between AHI and overexpression of HIF1A (42). A recent population-based cohort study and Mendelian randomization (MR) analysis have indicated that patients with OSAS are at a significantly increased risk of developing lung cancer. However, the results of the MR analysis do not support a causal relationship between OSAS and lung cancer (43). Further investigation is needed to uncover the underlying factors that may account for the observed association between OSAS and the risk of lung cancer. In order to identify these underlying factors, scholars have begun to explore the reasons that may lead to OSAS increasing the mortality rate of lung cancer patients. For example, Chen et al. used transcriptome sequencing to identify the key genes induced by CIH that promote the progression of lung cancer. The results showed that some of the differentially expressed genes between the IH group and the negative control (NC) group were upregulated in patients with LUAD, including apolipoprotein L1 (APOL1), electron transfer flavoprotein, beta subunit (ETFB), kallikrein-related peptidase 8 (KLK8), protein phosphatase 1 regulatory subunit 3G (PPP1R3G), prolactin (PRL), and spectrin alpha, erythrocytic 1 (SPTA1). High expression levels of these genes were significantly associated with poor overall survival (OS) in patients (44). In addition, a study published in the Journal of Cancer in 2024 identified 318 differentially expressed genes associated with hypoxia and mitochondrial score in LUAD. Ultimately, a prognostic model based on 16 genes was determined to have good predictive accuracy for the prognosis of LUAD (45). Their research coincides with our ideas, as we both hope to find interventional biological targets between OSAS and lung cancer and provide new diagnostic and therapeutic biomarkers for LUAD patients with OSAS in clinical practice.
In our study, we identified 5 HRGs directly associated with HIF1A in LUAD: COL1A1, IL11, MMP14, NOTCH3, and THY1. COL1A1 is an important component of the ECM (46), serving as the framework for tumor tissue, maintaining the physical and biochemical characteristics of cancer cells, and guiding cell proliferation, migration, and differentiation (47). Existing studies have demonstrated the crucial role of COL1A1 in the progression and prognosis of lung cancer, gastric cancer, breast cancer, and malignant pleural mesothelioma (48-51). The positive correlation between HIF1A and COL1A1 under hypoxia promotes ECM production and enhances the migration, invasion, and chemoresistance of pancreatic cancer cells (52). However, HIF1A exhibits a negative correlation with COL1A1 transcription in chondrocyte engineering by binding to the Sp3 transcription factor (53). This may highlight the heterogeneity of tumors. IL11 is a member of the interleukin-6 (IL6) family of cytokines discovered in 1990 (54), IL11 along with other IL6 family members, is a significant contributor to inflammation-driven cancer factors (55). An early study suggested that IL11 might not be an accomplice in solid tumor progression (56), However, increasing evidence from later studies suggests that the classical signaling transduction or reverse signaling transduction of IL11 is a driver in the development of various cancers (57-62). Another study has indicated that hypoxia regulates IL11 through HIF1A-targeted transcription factor v-maf musculoaponeurotic fibrosarcoma oncogene homolog F (MAFF) in breast cancer (63). Hypoxia can also regulate IL11 through non-HIF1A pathways, such as miR-495 and miR-5688 targeting IL11 directly to regulate the proliferation, migration, and invasion of LUAD (64). MMP14 is a member of the zinc-dependent enzyme family (MMP) capable of degrading ECM components. MMP14 is commonly expressed in invasive cancer cells, endothelial cells, fibroblasts, and immune system cells (65). The function of MMP14 depends on its cytoplasmic tail (CT), which promotes MMP14 movement and signal transduction within cells. Under hypoxic conditions, MMP14 transcription is induced in an HIF1A-dependent manner. Even in normoxic conditions, MMP14 within some tumor cells can activate HIF1A by binding with its CT to asparagine hydroxylase (FIH-1). MMP14 seems to be a key player in maintaining hypoxic metabolism (66). The NOTCH signaling pathway is a highly conserved pathway that regulates tissue development and homeostasis. It has a dual role, promoting both tissue development and cancer development, as well as causing cell death and tumor suppression (67,68). Reports on NOTCH3 in breast cancer development have both promoted and inhibited cancer progression (69,70). This may be due to differences in cell background details (71). For LUAD, activating neutrophils upregulates NOTCH3 expression to promote self-invasion and migration (72), while NOTCH3 negatively regulates iron death in LUAD through reactive oxygen species (ROS) (73). Recent research indicates that hypoxia-driven NOTCH3 to promote tumor development depends on HIF1A (74). This is consistent with our research results. THY1 (CD90) is a glycosylphosphatidylinositol (GPI)-anchored protein attached to the extracellular membrane, first discovered in mouse T lymphocytes. Subsequent studies have found its expression in various cancers, with extensive research in liver cancer and hematological tumors (75,76). Similar to NOTCH3, THY1 has contradictory roles, serving as a characteristic of cancer stem cells, promoting cancer cell migration and metastasis, and acting as an inhibitory molecule in ovarian adenocarcinoma and nasopharyngeal carcinoma (77), Life sciences are complex and puzzling. Hypoxia can regulate the secretion of CD90 by mesenchymal stem cells (78,79), but there is limited research on whether hypoxia can regulate CD90 through HIF1A to promote lung cancer development, requiring further exploration.
However, our study has many limitations. Firstly, the samples included in our study can only represent a small part of the population. Due to the large individual differences among patients, such as age, gender, smoking history, and genetic background, these factors may all affect the heterogeneity of LUAD and the hypoxic state. In the data we obtained, there was no record of whether the patients suffered from OSAS and the severity of hypoxia. At present, there is still a lack of a large number of adenocarcinoma patient samples with OSAS for research. Secondly, the tumor tissue itself has great heterogeneity. Tumor cells in different regions differ in gene expression, protein expression, and metabolic characteristics. For example, the degree of hypoxia and the types of cells may be different in the central and peripheral areas of the tumor (80). Additionally, tumors progress in a stepwise manner, with significant differences in the genome and immunological profile at different stages (81), which is attributed to the fact that aggressive LUAD exhibits high levels of tumor mutation burden (TMB) and genomic instability (82), therefore, the collected samples cannot fully represent all the characteristics of the tumor. There are also certain limitations in technology. The evidence on which our study relies, obtained solely from the transcriptome, seems so insignificant and feeble in supporting the relationship between OSAS and LUAD. Hence, the combination of multi-omics technologies (single-cell sequencing, proteomics, metabolomics, and spatial omics) will provide a more comprehensive perspective for humanity to unravel the mysteries of various tumors, including LUAD (83). Additionally, the cell models we constructed can only simulate some of the biological characteristics of tumors under hypoxic conditions. There are many hypoxic states of diseases in clinical practice that may affect the development of LUAD, such as asthma, chronic obstructive pulmonary disease (COPD), interstitial lung disease, etc. These diseases are not represented by our model. Moreover, the human body environment is extremely complex. In vitro cell models lack the complexity and dynamic changes of the tumor microenvironment, and animal models cannot fully simulate the heterogeneity and hypoxic state of human LUAD. It is necessary to establish a variety of models to continuously explore the hypoxic characteristics of LUAD.
Conclusions
We conducted a bioinformatics analysis on the GEO dataset and identified five HRGs, namely COL1A1, IL11, MMP14, NOTCH3, and THY1, which are positively correlated with HIF1A in LUAD. These genes serve as valuable biomarkers for LUAD. We will continue to explore the underlying mechanisms by which hypoxia promotes the progression of LUAD through HIF1A-mediated regulation of COL1A1, IL11, MMP14, NOTCH3, and THY1. It is hoped that in the near future, these findings will provide novel diagnostic and therapeutic approaches for clinical LUAD patients with hypoxia-related diseases.
Acknowledgments
None.
Footnote
Reporting Checklist: The authors have completed the TRIPOD reporting checklist. Available at https://tcr.amegroups.com/article/view/10.21037/tcr-2024-2265/rc
Data Sharing Statement: Available at https://tcr.amegroups.com/article/view/10.21037/tcr-2024-2265/dss
Peer Review File: Available at https://tcr.amegroups.com/article/view/10.21037/tcr-2024-2265/prf
Funding: This study was supported by
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tcr.amegroups.com/article/view/10.21037/tcr-2024-2265/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This study was conducted in accordance with the Declaration of Helsinki and its subsequent amendments.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- The Lancet. Lung cancer: some progress, but still a lot more to do. Lancet 2019;394:1880. [Crossref]
- Bade BC, Dela Cruz CS. Lung Cancer 2020: Epidemiology, Etiology, and Prevention. Clin Chest Med 2020;41:1-24. [Crossref] [PubMed]
- Ye Y, Hu Q, Chen H, et al. Characterization of Hypoxia-associated Molecular Features to Aid Hypoxia-Targeted Therapy. Nat Metab 2019;1:431-44. [Crossref] [PubMed]
- Luo Z, Tian M, Yang G, et al. Hypoxia signaling in human health and diseases: implications and prospects for therapeutics. Signal Transduct Target Ther 2022;7:218. [Crossref] [PubMed]
- Gordan JD, Thompson CB, Simon MC. HIF and c-Myc: sibling rivals for control of cancer cell metabolism and proliferation. Cancer Cell 2007;12:108-13. [Crossref] [PubMed]
- Hänzelmann S, Castelo R, Guinney J. GSVA: gene set variation analysis for microarray and RNA-seq data. BMC Bioinformatics 2013;14:7. [Crossref] [PubMed]
- Pei G, Chen L, Zhang W. WGCNA Application to Proteomic and Metabolomic Data Analysis. Methods Enzymol 2017;585:135-58. [Crossref] [PubMed]
- Subramanian A, Tamayo P, Mootha VK, et al. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc Natl Acad Sci U S A 2005;102:15545-50. [Crossref] [PubMed]
- Futschik ME, Carlisle B. Noise-robust soft clustering of gene expression time-course data. J Bioinform Comput Biol 2005;3:965-88. [Crossref] [PubMed]
- Schriml LM, Mitraka E, Munro J, et al. Human Disease Ontology 2018 update: classification, content and workflow expansion. Nucleic Acids Res 2019;47:D955-62. [Crossref] [PubMed]
- Ashburner M, Ball CA, Blake JA, et al. Gene ontology: tool for the unification of biology. The Gene Ontology Consortium. Nat Genet 2000;25:25-9. [Crossref] [PubMed]
- Kanehisa M, Furumichi M, Sato Y, et al. KEGG for taxonomy-based analysis of pathways and genomes. Nucleic Acids Res 2023;51:D587-92. [Crossref] [PubMed]
- Szklarczyk D, Morris JH, Cook H, et al. The STRING database in 2017: quality-controlled protein-protein association networks, made broadly accessible. Nucleic Acids Res 2017;45:D362-8. [Crossref] [PubMed]
- Lin X, Yang F, Zhou L, et al. A support vector machine-recursive feature elimination feature selection method based on artificial contrast variables and mutual information. J Chromatogr B Analyt Technol Biomed Life Sci 2012;910:149-55. [Crossref] [PubMed]
- Tibshirani R. The lasso method for variable selection in the Cox model. Stat Med 1997;16:385-95. [Crossref] [PubMed]
- Martínez Pérez JA, Pérez Martin PS. ROC curve. Semergen. 2023;49:101821. [Crossref] [PubMed]
- Robin X, Turck N, Hainard A, et al. pROC: an open-source package for R and S+ to analyze and compare ROC curves. BMC Bioinformatics 2011;12:77. [Crossref] [PubMed]
- Herbst RS, Morgensztern D, Boshoff C. The biology and management of non-small cell lung cancer. Nature 2018;553:446-54. [Crossref] [PubMed]
- Chen Z, Fillmore CM, Hammerman PS, et al. Non-small-cell lung cancers: a heterogeneous set of diseases. Nat Rev Cancer 2014;14:535-46. [Crossref] [PubMed]
- Zappa C, Mousa SA. Non-small cell lung cancer: current treatment and future advances. Transl Lung Cancer Res 2016;5:288-300. [Crossref] [PubMed]
- Alexandrov LB, Nik-Zainal S, Wedge DC, et al. Signatures of mutational processes in human cancer. Nature 2013;500:415-21. [Crossref] [PubMed]
- Stratton MR, Campbell PJ, Futreal PA. The cancer genome. Nature 2009;458:719-24. [Crossref] [PubMed]
- Larson NB, Oberg AL, Adjei AA, et al. A Clinician’s Guide to Bioinformatics for Next-Generation Sequencing. J Thorac Oncol 2023;18:143-57. [Crossref] [PubMed]
- Fan XX, Wu Q. Decoding Lung Cancer at Single-Cell Level. Front Immunol 2022;13:883758. [Crossref] [PubMed]
- Qiu T, Zhi X, Ren S. Recent advance of next-generation sequencing in patients with lung cancer. Expert Rev Mol Diagn 2023;23:959-70. [Crossref] [PubMed]
- Lee JM, McNamee CJ, Toloza E, et al. Neoadjuvant Targeted Therapy in Resectable NSCLC: Current and Future Perspectives. J Thorac Oncol 2023;18:1458-77. [Crossref] [PubMed]
- Borgeaud M, Friedlaender A, Addeo A. Bringing immune-checkpoint inhibitors earlier in the management of non-small cell lung cancer. Transl Lung Cancer Res 2023;12:2353-8. [Crossref] [PubMed]
- Chen PS, Chiu WT, Hsu PL, et al. Pathophysiological implications of hypoxia in human diseases. J Biomed Sci 2020;27:63. [Crossref] [PubMed]
- Schito L, Semenza GL. Hypoxia-Inducible Factors: Master Regulators of Cancer Progression. Trends Cancer 2016;2:758-70. [Crossref] [PubMed]
- Kaelin WG Jr, Ratcliffe PJ. Oxygen sensing by metazoans: the central role of the HIF hydroxylase pathway. Mol Cell 2008;30:393-402. [Crossref] [PubMed]
- Cheng JC, Klausen C, Leung PC. Hypoxia-inducible factor 1 alpha mediates epidermal growth factor-induced down-regulation of E-cadherin expression and cell invasion in human ovarian cancer cells. Cancer Lett 2013;329:197-206. [Crossref] [PubMed]
- Joseph JV, Conroy S, Pavlov K, et al. Hypoxia enhances migration and invasion in glioblastoma by promoting a mesenchymal shift mediated by the HIF1α-ZEB1 axis. Cancer Lett 2015;359:107-16. [Crossref] [PubMed]
- Yang MH, Wu MZ, Chiou SH, et al. Direct regulation of TWIST by HIF-1alpha promotes metastasis. Nat Cell Biol 2008;10:295-305. [Crossref] [PubMed]
- Xu YR, Wang AL, Li YQ. Hypoxia-inducible factor 1-alpha is a driving mechanism linking chronic obstructive pulmonary disease to lung cancer. Front Oncol 2022;12:984525. [Crossref] [PubMed]
- Garbarino S, Lanteri P, Durando P, et al. Co-Morbidity, Mortality, Quality of Life and the Healthcare/Welfare/Social Costs of Disordered Sleep: A Rapid Review. Int J Environ Res Public Health 2016;13:831. [Crossref] [PubMed]
- Seijo LM, Pérez-Warnisher MT, Giraldo-Cadavid LF, et al. Obstructive sleep apnea and nocturnal hypoxemia are associated with an increased risk of lung cancer. Sleep Med 2019;63:41-5. [Crossref] [PubMed]
- Cui Z, Ruan Z, Li M, et al. Obstructive sleep apnea promotes the progression of lung cancer by modulating cancer cell invasion and cancer-associated fibroblast activation via TGFβ signaling. Redox Rep 2023;28:2279813. [Crossref] [PubMed]
- Cui Z, Ruan Z, Li M, et al. Intermittent hypoxia inhibits anti-tumor immune response via regulating PD-L1 expression in lung cancer cells and tumor-associated macrophages. Int Immunopharmacol 2023;122:110652. [Crossref] [PubMed]
- Riera-Domingo C, Audigé A, Granja S, et al. Immunity, Hypoxia, and Metabolism-the Ménage à Trois of Cancer: Implications for Immunotherapy. Physiol Rev 2020;100:1-102. [Crossref] [PubMed]
- Ma L, Shan W, Ding X, et al. Intermittent hypoxia induces tumor immune escape in murine S180 solid tumors via the upregulation of TGF-β(1) in mice. Sleep Breath 2021;25:719-26. [Crossref] [PubMed]
- Hao S, Zhu X, Liu Z, et al. Chronic intermittent hypoxia promoted lung cancer stem cell-like properties via enhancing Bach1 expression. Respir Res 2021;22:58. [Crossref] [PubMed]
- Huang HY, Lin SW, Chuang LP, et al. Severe OSA associated with higher risk of mortality in stage III and IV lung cancer. J Clin Sleep Med 2020;16:1091-8. [Crossref] [PubMed]
- Yao J, Duan R, Li Q, et al. Association between obstructive sleep apnea and risk of lung cancer: findings from a collection of cohort studies and Mendelian randomization analysis. Front Oncol 2024;14:1346809. [Crossref] [PubMed]
- Chen LD, Lin L, Chen JZ, et al. Identification of key genes in chronic intermittent hypoxia-induced lung cancer progression based on transcriptome sequencing. BMC Cancer 2024;24:41. [Crossref] [PubMed]
- Zhao W, Huang H, Zhao Z, et al. Identification of Hypoxia and Mitochondrial-related Gene Signature and Prediction of Prognostic Model in Lung Adenocarcinoma. J Cancer 2024;15:4513-26. [Crossref] [PubMed]
- Gelse K, Pöschl E, Aigner T. Collagens--structure, function, and biosynthesis. Adv Drug Deliv Rev 2003;55:1531-46. [Crossref] [PubMed]
- Pickup MW, Mouw JK, Weaver VM. The extracellular matrix modulates the hallmarks of cancer. EMBO Rep 2014;15:1243-53. [Crossref] [PubMed]
- Ma B, Li F, Ma B. Down-regulation of COL1A1 inhibits tumor-associated fibroblast activation and mediates matrix remodeling in the tumor microenvironment of breast cancer. Open Life Sci 2023;18:20220776. [Crossref] [PubMed]
- Li J, Ding Y, Li A. Identification of COL1A1 and COL1A2 as candidate prognostic factors in gastric cancer. World J Surg Oncol 2016;14:297. [Crossref] [PubMed]
- Li B, Pu YQ, Li ZL, et al. Expression and clinical significance of COL1A1 and COL1A2 genes in malignant pleural mesothelioma tissues. Zhonghua Lao Dong Wei Sheng Zhi Ye Bing Za Zhi 2022;40:487-94. [PubMed]
- Zhang H, Li X, Jia M, et al. Roles of H19/miR-29a-3p/COL1A1 axis in COE-induced lung cancer. Environ Pollut 2022;313:120194. [Crossref] [PubMed]
- Zhao Z, Liu L, Chen H, et al. Thymoquinone affects the gemcitabine sensitivity of pancreatic cancer by regulating collagen via hypoxia inducible factor-1α. Front Pharmacol 2023;14:1138265. [Crossref] [PubMed]
- Duval E, Bouyoucef M, Leclercq S, et al. Hypoxia inducible factor 1 alpha down-regulates type i collagen through Sp3 transcription factor in human chondrocytes. IUBMB Life 2016;68:756-63. [Crossref] [PubMed]
- Paul SR, Bennett F, Calvetti JA, et al. Molecular cloning of a cDNA encoding interleukin 11, a stromal cell-derived lymphopoietic and hematopoietic cytokine. Proc Natl Acad Sci U S A 1990;87:7512-6. [Crossref] [PubMed]
- Unver N, McAllister F. IL-6 family cytokines: Key inflammatory mediators as biomarkers and potential therapeutic targets. Cytokine Growth Factor Rev 2018;41:10-7. [Crossref] [PubMed]
- Soda H, Raymond E, Sharma S, et al. Recombinant human interleukin-11 is unlikely to stimulate the growth of the most common solid tumors. Anticancer Drugs 1999;10:97-101. [Crossref] [PubMed]
- To SQ, Dmello RS, Richards AK, et al. STAT3 Signaling in Breast Cancer: Multicellular Actions and Therapeutic Potential. Cancers (Basel) 2022;14:429. [Crossref] [PubMed]
- Lokau J, Kespohl B, Kirschke S, et al. The role of proteolysis in interleukin-11 signaling. Biochim Biophys Acta Mol Cell Res 2022;1869:119135. [Crossref] [PubMed]
- Metcalfe RD, Putoczki TL, Griffin MDW. Structural Understanding of Interleukin 6 Family Cytokine Signaling and Targeted Therapies: Focus on Interleukin 11. Front Immunol 2020;11:1424. [Crossref] [PubMed]
- Lokau J, Schoeder V, Garbers C. The role of interleukin-11 in osteosarcoma. Der Pathologe 2020;41:163-7. [Crossref] [PubMed]
- Leung JH, Ng B, Lim WW. Interleukin-11: A Potential Biomarker and Molecular Therapeutic Target in Non-Small Cell Lung Cancer. Cells 2022;11:2257. [Crossref] [PubMed]
- Putoczki TL, Ernst M. IL-11 signaling as a therapeutic target for cancer. Immunotherapy 2015;7:441-53. [Crossref] [PubMed]
- Moon EJ, Mello SS, Li CG, et al. The HIF target MAFF promotes tumor invasion and metastasis through IL11 and STAT3 signaling. Nat Commun 2021;12:4308. [Crossref] [PubMed]
- Zhao M, Chang J, Liu R, et al. miR-495 and miR-5688 are down-regulated in non-small cell lung cancer under hypoxia to maintain interleukin-11 expression. Cancer Commun (Lond) 2020;40:435-52. [Crossref] [PubMed]
- Itoh Y. Membrane-type matrix metalloproteinases: Their functions and regulations. Matrix Biol 2015;44-46:207-23. [Crossref] [PubMed]
- Strouhalova K, Tolde O, Rosel D, et al. Cytoplasmic Tail of MT1-MMP: A Hub of MT1-MMP Regulation and Function. Int J Mol Sci 2023;24:5068. [Crossref] [PubMed]
- Bray SJ. Notch signalling in context. Nat Rev Mol Cell Biol 2016;17:722-35. [Crossref] [PubMed]
- Nowell CS, Radtke F. Notch as a tumour suppressor. Nat Rev Cancer 2017;17:145-59. [Crossref] [PubMed]
- Leontovich AA, Jalalirad M, Salisbury JL, et al. NOTCH3 expression is linked to breast cancer seeding and distant metastasis. Breast Cancer Res 2018;20:105. [Crossref] [PubMed]
- Lin HY, Liang YK, Dou XW, et al. Notch3 inhibits epithelial-mesenchymal transition in breast cancer via a novel mechanism, upregulation of GATA-3 expression. Oncogenesis 2018;7:59. [Crossref] [PubMed]
- Hosseini-Alghaderi S, Baron M. Notch3 in Development, Health and Disease. Biomolecules 2020;10:485. [Crossref] [PubMed]
- Peng W, Sheng Y, Xiao H, et al. Lung Adenocarcinoma Cells Promote Self-Migration and Self-Invasion by Activating Neutrophils to Upregulate Notch3 Expression of Cancer Cells. Front Mol Biosci 2021;8:762729. [Crossref] [PubMed]
- Li Z, Xiao J, Liu M, et al. Notch3 regulates ferroptosis via ROS-induced lipid peroxidation in NSCLC cells. FEBS Open Bio 2022;12:1197-205. [Crossref] [PubMed]
- Nakamura H, Sekine H, Kato H, et al. Hypoxia-inducible factor-1α and poly [ADP ribose] polymerase 1 cooperatively regulate Notch3 expression under hypoxia via a noncanonical mechanism. J Biol Chem 2022;298:102137. [Crossref] [PubMed]
- Balçık-Erçin P, Aysan A, Salık N, et al. SIX1 Downregulation Suppresses Self-renewal Capacity and THY1 Expression in Hepatocellular Carcinoma and SIX1 Dominate the Survival in Liver Cancer. Turk J Gastroenterol 2023;34:881-9. [Crossref] [PubMed]
- ElMenshawy N, El-Chennawi F, Darwish A, et al. CD44, CD90 and CD96 expression in immune thrombocytopenia purpura (ITP) patients. J Immunoassay Immunochem 2023;44:326-37. [Crossref] [PubMed]
- Sauzay C, Voutetakis K, Chatziioannou A, et al. CD90/Thy-1, a Cancer-Associated Cell Surface Signaling Molecule. Front Cell Dev Biol 2019;7:66. [Crossref] [PubMed]
- Arfianti A. Hipoxia modulates the secretion of growth factors of human umbilical cord-derived mesenchymal stem cells. Biomedicine (Taipei) 2023;13:49-56. [Crossref] [PubMed]
- Wang P, Zhu P, Yu C, et al. The Proliferation and Stemness of Peripheral Blood-Derived Mesenchymal Stromal Cells Were Enhanced by Hypoxia. Front Endocrinol (Lausanne) 2022;13:873662. [Crossref] [PubMed]
- Katsumata S, Aokage K, Miyoshi T, et al. Differences of tumor microenvironment between stage I lepidic-positive and lepidic-negative lung adenocarcinomas. J Thorac Cardiovasc Surg 2018;156:1679-1688.e2. [Crossref] [PubMed]
- Chen H, Carrot-Zhang J, Zhao Y, et al. Genomic and immune profiling of pre-invasive lung adenocarcinoma. Nat Commun 2019;10:5472. [Crossref] [PubMed]
- Comprehensive molecular profiling of lung adenocarcinoma. Nature 2014;511:543-50. [Crossref] [PubMed]
- Eisenstein M. Why tumour geography matters - and how to map it. Nature 2024;635:1031-3. [Crossref] [PubMed]


