
Reduction in albumin binding function following liver resection in patients with and without cirrhosis
Introduction
Assessing liver function in patients undergoing liver resection is crucial. As albumin concentration is regarded as an important marker of liver function (1,2), serum albumin concentration rather than its binding function is usually determined. Albumin concentration, however, is not an accurate determinant of albumin binding activity, as circulating albumin may have lost its binding function. The three-dimensional structure of albumin is altered when liver function is impaired, reducing the ability of albumin to bind to its ligands. This may result in the failure to remove metabolic products, including bilirubin, and drugs from the body (3,4). Measuring both albumin concentration and binding function can better indicate actual liver function, thus guiding clinical practice.
The most commonly used indicators of albumin binding function are ischemia-modified albumin (IMA) and albumin fatty acid binding strength, which reflect the binding of albumin to metal ions and fatty acids, respectively (3,5,6). These activities were found to be lower in patients with liver cirrhosis, especially those with liver failure, than in normal subjects (7). Although albumin dialysis using a molecular adsorbent recirculating system removed albumin-bound metabolites from blood, it did not improve albumin binding function. Albumin binding function may be a more objective indicator of liver status, and IMA could be used to predict prognosis. We previously showed that the capacities of albumin to bind metal ions and fatty acids provided earlier and more sensitive indication than albumin concentration of liver damage in patients with nonalcoholic fatty liver disease, viral hepatitis and cirrhosis. (Ge PL, Yang HY, Lu JF, et al. Albumin binding function: The potential earliest indicator for liver function damage. Gastroent Res Pract. In Press.)
To our knowledge, however, there have been no reports on perioperative changes of albumin binding function in patients with and without cirrhosis undergoing liver resection. This observational study therefore assessed the effects of liver surgery on albumin binding function in patients with liver tumors due to cirrhosis and in non-cirrhotic patients with hepatic cavernous hemangiomas. Its findings suggest that albumin binding function may be a good indicator of liver function.
Methods
Patients
Patients who underwent liver surgery from January 2014 to March 2015 at the Department of Liver Surgery, Peking Union Medical College Hospital, were eligible. The study included 42 cirrhotic patients who underwent liver resection for hepatocellular carcinoma (HCC), and 17 non-cirrhotic patients who underwent resection for hepatic cavernous hemangioma. The control group included 50 healthy volunteers (Tables 1,2). All subjects provided written informed consent. The study was performed according to the principles of the Declaration of Helsinki and the study protocol was approved by the Ethics Committee of Peking Union Medical College Hospital.

Full table

Full table
Serum sample collection
Peripheral venous blood was drawn from all patients undergoing surgery on preoperative day 1 and on post-operative days (POD) 1, 3, and 7. Only one sample was obtained from the subjects in the control group. Blood samples were collected into additive-free vacuum tubes after at least 12 h of fasting, allowed to coagulate for 30–60 min, and centrifuged at 3000 rpm for 10 min. Serum samples were stored at −80 °C before analysis.
Routine serological tests
Routine tests included total bilirubin, alanine aminotransferase (ALT), creatinine, prothrombin time (PT), and international normalized ratio (INR). Child-Pugh score and model for end-stage liver disease (MELD) score were also determined of cirrhotic patients (1). Perioperative serum albumin concentrations were measured in samples from patients in the cirrhotic and non-cirrhotic groups.
Albumin cobalt binding capacity
Albumin cobalt binding capacity was measured essentially as described (6). Briefly, 100 µL of serum were mixed with 25 µL cobalt chloride (CoCl2, 1 mg/mL) (Sigma-Aldrich, USA) in each well of a 96-well plate and incubated at room temperature for 10 min. To each well was added 25 µL dithiothreitol (DTT, 1.5 mg/mL) (Sigma-Aldrich, USA). After incubation for 2 min, 150 µL saline were added to terminate the reaction, and absorbance was measured spectrophotometrically at 470 nm (Synergy H1; BioTek, USA). IMA was defined as the absorbance of the tested sample minus that of the control (no DTT added). High absorbance value indicated more free cobalt reacted with DTT, with less bound to albumin. To be more straightforward, results are presented as IMA transformed (IMAT), defined as 1-IMA, with a higher IMAT indicating a higher metal ion binding capacity. To assess metal ion binding function per unit of albumin, IMAT was also normalized to albumin concentration and reported as the IMAT/albumin ratio.
Albumin fatty acid binding strength
To measure albumin fatty acid binding strength, 100 µL serum and 20 µL spin label 3.0 mmol/L 16-doxyl stearic acid (Sigma-Aldrich, USA) were added to a 0.5-mL Eppendorf tube. Each mixture was stirred with a glass rod for 3 min at 4 °C. The tubes were sealed and incubated in a 37 °C water bath for 20 min. A 10-µL aliquot of each sample was transferred to an electron paramagnetic resonance instrument (Bruker EMX A200, Bruker BioSpin GmbH, Germany), operated with a center field 3427.4 G, sweep width 100 G, microwave frequency 9.6 GHz, microwave power 6.5 MW, and modulation frequency 100 KHz with modulation amplitude 2.5 G.
Data were analyzed with Origin 8 image processing software (OriginLab, USA), and a spectral diagram of the spin label and albumin complex was constructed (Figure 1). The ratio of the number of high-affinity to low-affinity fatty acid binding sites (H/L) could be calculated from the spectrum. H/L reflected not only the binding strength of albumin to fatty acid, but also its conformational alteration.
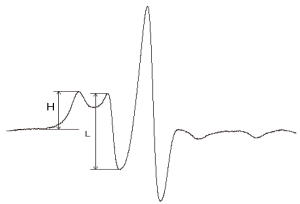
Statistical analysis
Continuous variables were expressed as mean ± standard deviation. Statistical analyses were performed with SPSS 13.0 statistical software (SPSS Inc., USA). The data conformed to normal distribution and homogeneity of variance. The difference between preoperative tumor sizes was analyzed by independent sample t-tests, and the differences in other preoperative parameters among the three groups were analyzed by one-way analysis of variance.
The differences between the cirrhotic and non-cirrhotic patient groups at each preoperative and postoperative time point were analyzed by two factor repeated measurements analysis of variance, whereas within-group differences among time points were analyzed by paired t tests. Multivariate analysis of variance was used to test differences at the same time point between the two operated groups. Two-way tests were used for all analyses. A P value <0.05 was considered statistically significant.
Results
Tumor size was significantly smaller in the cirrhotic than in the non-cirrhotic group (5.94±3.46 vs. 10.88±5.64 cm, P<0.001). Patients in both groups underwent laparotomy for resection of one or more liver segments or hemihepatectomy, whereas some patients in the non-cirrhotic group underwent hepatic hemangioma stripping (Table 3).
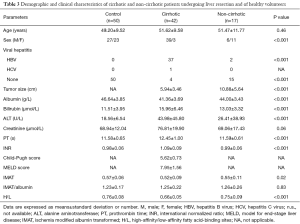
Full table
Following surgery, all patients in the cirrhotic and non-cirrhotic groups received prophylactic antibiotics for 1–3 days and nutritional support. Patients in the cirrhotic group who underwent resection of several liver segments or hemihepatectomy received infusions of 20 g/day commercial albumin (200 g/L, Hualan Bio, China) for 3–5 days. Albumin infusions were not administered to patients in the cirrhotic group who underwent resection of a single liver segment or to patients in the non-cirrhotic group. All patients recovered well and were discharged from the hospital 7–10 days after surgery. No patients died within 30 days postoperation.
Albumin concentration
Preoperative albumin concentration was significantly higher in healthy volunteers than in cirrhotic patients (P<0.001). Albumin concentrations sharply decreased in both the cirrhotic and non-cirrhotic groups after surgery (P<0.001), gradually increasing along with improvements in liver function. Albumin concentration rose faster and higher in the cirrhotic than in the non-cirrhotic group, and was higher in the former on POD 7 (P=0.03) (Figure 2, Table 4).
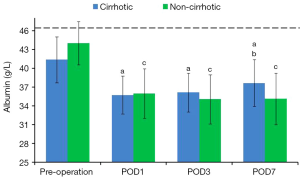
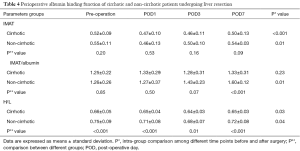
Full table
Albumin cobalt binding capacity
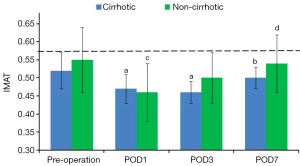
IMAT
IMAT in both operated groups was significantly lower on POD 1 than preoperatively (P=0.01). Minimum IMAT was observed on POD 1 in the non-cirrhotic group and on POD 3 in the cirrhotic group. On POD 7, IMAT had returned to preoperative concentration in both the non-cirrhotic (P=0.65) and cirrhotic (P=0.28) groups, but there were no significant between group differences at any time point (P=0.07) (Figure 3, Table 4).
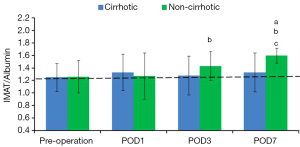
IMAT/albumin
Preoperative IMAT/albumin in the cirrhotic and non-cirrhotic groups was similar to that in healthy volunteers (P=0.83). IMAT/albumin in the cirrhotic group slightly fluctuated after surgery (P=0.23). In the non-cirrhotic group, IMAT/albumin on POD 1 was similar to that preoperatively (P=0.89). IMAT/albumin in this group increased subsequently, peaking on POD 7 (P<0.001), at which time it was significantly higher than in the cirrhotic group (P<0.001). (Figure 4, Table 4).
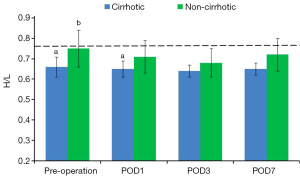
Albumin fatty acid binding strength
Preoperative H/L ratio was significantly lower in the cirrhotic group than in the non-cirrhotic group and in healthy volunteers, but did not differ significantly in the latter two groups. H/L ratios in both the cirrhotic and non-cirrhotic groups declined for 3 days after hepatectomy, being significantly lower on day 3 than preoperatively in the cirrhotic (P=0.01) and non-cirrhotic (P=0.03) groups. Subsequently, these ratios began to rise, reaching preoperative concentrations in the cirrhotic (P=0.17) and non-cirrhotic (P=0.24) groups. H/L ratios were higher in the non-cirrhotic than in the cirrhotic group at all time points (P<0.001 preoperatively and on PODs 1 and 7; P=0.01 on POD 3) (Figure 5, Table 4).
Discussion
Albumin concentration has been used more frequently than albumin binding activity to assess liver function in patients with liver disease (8). Albumin activity may better reflect changes in liver function, decreasing when liver function is impaired, albumin conformation changes, or metabolic burden increases (9). Our previous study found that albumin binding activity was significantly lower in patients with viral hepatitis than in normal individuals, although albumin concentrations in the two groups did not differ. Furthermore, albumin concentrations and binding activities in patients with various degrees of cirrhosis differed from each other. (Ge PL, Yang HY, Lu JF, et al. Albumin binding function: The potential earliest indicator for liver function damage. Gastroent Res Pract. In Press.) Albumin metal ion binding capacity was found to decrease in patients with both chronic hepatitis and cirrhosis (10). As these results were less affected by albumin infusion, albumin binding capacity was regarded as more objective.
To assess whether albumin binding activity correlated with albumin concentration in patients undergoing liver surgery and whether these parameters differed in patients with and without cirrhosis, we compared cirrhotic patients with HCC and non-cirrhotic patients with hepatic cavernous hemangioma. To our knowledge, this study is the first to compare albumin binding activity in patients with and without cirrhosis.
IMA, originally an indicator of myocardial ischemia, can be measured by testing the binding capacity of the N-terminal metal ion binding sites of albumin to cobalt ions (6). Studies have found that IMA is higher in patients with type 2 diabetes mellitus, stroke, mesenteric ischemia, and Alzheimer’s disease than in healthy individuals (11-14). IMA was altered earlier than other indicators of oxidative stress (15), perhaps because the impact of oxygen free radicals on albumin structure can be detected earlier than their impact on other parameters (16). IMA is regarded as an indicator of liver damage (7,9). Our study found that albumin metal ion binding capacity could be used as a parameter to assess the recovery state of patients following hepatectomy.
Our results showed that preoperative metal ion binding capacity and albumin concentration were both lower in patients with cirrhosis than in healthy volunteers (P=0.02). Although preoperative albumin concentration was also lower in patients without cirrhosis than in healthy volunteers, their albumin binding activity was similar (P=0.79). These findings indicate that cirrhosis not only reduces albumin concentration, but lowers albumin binding capacity. This activity was also slightly lower in non-cirrhotic patients with huge hepatic cavernous hemangiomas (tumor size 10.88±5.64 cm). In non-cirrhotic patients, binding activity was lowest on POD 1 (P=0.01), rising to preoperative concentrations on POD 3 (P=0.19), although no albumin was infused. In cirrhotic patients, however, albumin binding capacity was lower on POD 1 (P=0.01). Despite infusion of exogenous albumin, binding activity in this group remained low until POD 3. By POD 7, albumin binding activity returned to preoperative concentrations in both the non-cirrhotic (P=0.65) and cirrhotic (P=0.28) groups, whereas the albumin concentration in both groups remained significantly lower than preoperatively (both P<0.001). Although surgery greatly affected albumin concentration and binding function, these effects differed from each other, as well as differing in cirrhotic and non-cirrhotic patients. Specifically, surgical trauma had a more profound and prolonged impact on albumin binding function in patients with liver cirrhosis.
The mechanism underlying the return of albumin binding function, but not albumin concentration, to preoperative concentrations by POD 7 remains unclear, but may be due to albumin compensation, which helps the liver resist surgical stress. We therefore calculated the ratio of albumin binding to metal ion to unit albumin concentration (IMAT/albumin). Although preoperative albumin concentration and binding function were both lower in patients with cirrhosis than in healthy volunteers, IMAT/albumin did not differ (P=0.83), suggesting that albumin binding function in cirrhotic patients began compensating for liver injury before hepatectomy. Compensatory ability, however, did not increase after liver resection (P=0.06), suggesting that the compensatory ability of binding function per unit of albumin is limited in cirrhotic patients. Furthermore, the metal ion binding capacity of commercially infused albumin (200 g/L) was 1.34 times that of native serum albumin of healthy volunteers with the same volume, and 4.29 times for albumin concentration. Thus, the binding capacity of commercial albumin was 31.24% that of the same concentration of native albumin, in agreement with previous findings (17). Thus, albumin infusion contributed more to increasing concentration than binding function. In contrast, preoperative IMAT/albumin was similar in non-cirrhotic patients with hepatic cavernous hemangioma and in healthy volunteers (P=0.83), indicating that these lesions had no discernible effect on albumin binding function, and therefore did not induce a compensatory increase. Nevertheless, hepatectomy could damage albumin binding function of patients with non-cirrhosis. Per unit albumin binding function in these patients gradually recovered after surgery, peaking on POD 7 (P<0.001). These findings indicate the compensatory ability of albumin binding function was better in non-cirrhotic than in cirrhotic patients, accounting for the differences in recovery between albumin concentration and albumin binding function.
Normally functioning binding sites are the structural basis for the transport and metabolic roles of albumin. However, functioning of these binding sites may be affected by many factors, including albumin structure, toxins, and drugs in the blood. The three-dimensional structure of albumin may be damaged in patients with cirrhosis, hepatitis, and liver ischemia. The binding to ligands changed, resulting in changes in the ratio of high- to low-affinity binding sites to fatty acid (18). To explore the reason for these differences, we assessed albumin structures in the three groups of subjects.
H/L reflects the strength of albumin binding to fatty acids. This ratio was similar in non-cirrhotic patients preoperatively and in healthy volunteers (P=0.96). In contrast, the preoperative binding function of cirrhotic patients was significantly lower, which indicated that instead of the size of tumor, cirrhosis was to blame. Following surgery, both cirrhotic and non-cirrhotic patients showed reductions in binding capacity, being minimal in both groups on POD 3 and slowly returning to preoperative concentrations on POD 7. Liver surgery may have damaged the three-dimensional structure of albumin, with these conformational changes reducing albumin fatty acid binding capacity. The postoperative recovery of damaged albumin structure and newly generated albumin gradually enhanced fatty acid binding capacity to preoperative level. Although postoperative infusion of commercial albumin into cirrhotic patients increased their serum albumin concentrations, making it higher than in non-cirrhotic patients, the fatty acid binding capacity of albumin remained lower in cirrhotic than in non-cirrhotic patients at all time points.
Albumin binding function reflects synthetic capacity of liver. On the other side, clinically relevant postoperative prognosis is mainly affected by heterogenous perioperative treatment and overall liver function, so correlation analysis between them isn’t done.
One limitation of the study is the small sample size. The heterogeneity of data and complex effect of liver surgery to albumin concentration and binding function limit us to get a solid conclusion.
Conclusions
To our knowledge, this study is the first to show that albumin binding function decreases following liver resection in patients with and without cirrhosis, especially the former. Binding capacity is a better indicator of liver damage than albumin concentration, with the results less affected by albumin infusion. Albumin binding capacity may be an important indicator of liver function.
Acknowledgments
The authors thank Ya-Nan Wang for the valuable advice on test technique, Zi-Xing Wang for the help on statistical analysis, and Edanz Group China for the English language revision.
Funding: This work was supported by National Natural Science Foundation of China (81201566) and National Key Technology Research and Development Program of China (2012BA I06B01).
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2016.12.13). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was performed according to the principles of the Declaration of Helsinki (as revised in 2013) and the study protocol was approved by the Ethics Committee of Peking Union Medical College Hospital and written informed consent was obtained from all patients.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Ge PL, Du SD, Mao YL. Advances in preoperative assessment of liver function. Hepatobiliary Pancreat Dis Int 2014;13:361-70. [Crossref] [PubMed]
- Cheung TT, Lo CM. Laparoscopic liver resection for hepatocellular carcinoma in patients with cirrhosis. Hepatobiliary Surg Nutr 2015;4:406-10. [PubMed]
- Garcia-Martinez R, Caraceni P, Bernardi M, et al. Albumin: pathophysiologic basis of its role in the treatment of cirrhosis and its complications. Hepatology 2013;58:1836-46. [Crossref] [PubMed]
- Ishizawa T, Saiura A, Kokudo N. Clinical application of indocyanine green-fluorescence imaging during hepatectomy. Hepatobiliary Surg Nutr 2016;5:322-8. [Crossref] [PubMed]
- Kazmierczak SC, Gurachevsky A, Matthes G, et al. Electron spin resonance spectroscopy of serum albumin: a novel new test for cancer diagnosis and monitoring. Clin Chem 2006;52:2129-34. [Crossref] [PubMed]
- Bar-Or D, Lau E, Winkler JV. A novel assay for cobalt-albumin binding and its potential as a marker for myocardial ischemia-a preliminary report. J Emerg Med 2000;19:311-5. [Crossref] [PubMed]
- Jalan R, Schnurr K, Mookerjee RP, et al. Alterations in the functional capacity of albumin in patients with decompensated cirrhosis is associated with increased mortality. Hepatology 2009;50:555-64. [Crossref] [PubMed]
- Liu Y, Xue X. Systematic review of peri-operative nutritional support for patients undergoing hepatobiliary surgery. Hepatobiliary Surg Nutr 2015;4:304-12. [PubMed]
- Klammt S, Mitzner SR, Stange J, et al. Improvement of impaired albumin binding capacity in acute-on-chronic liver failure by albumin dialysis. Liver Transpl 2008;14:1333-9. [Crossref] [PubMed]
- Chen CY, Tsai WL, Lin PJ, et al. The value of serum ischemia-modified albumin for assessing liver function in patients with chronic liver disease. Clin Chem Lab Med 2011;49:1817-21. [Crossref] [PubMed]
- Kaefer M, Piva SJ, De Carvalho JA, et al. Association between ischemia modified albumin, inflammation and hyperglycemia in type 2 diabetes mellitus. Clin Biochem 2010;43:450-4. [Crossref] [PubMed]
- Nayak AR, Kashyap RS, Kabra D, et al. Prognostic significance of ischemia-modified albumin in acute ischemic stroke patients: A preliminary study. Ann Neurosci 2011;18:5-7. [Crossref] [PubMed]
- Kadioğlu H, Ömür D, Bozkurt S, et al. Ischemia modified albumin can predict necrosis at incarcerated hernias. Dis Markers 2013;35:807-10. [Crossref] [PubMed]
- Altunoglu E, Guntas G, Erdenen F, et al. Ischemia-modified albumin and advanced oxidation protein products as potential biomarkers of protein oxidation in Alzheimer's disease. Geriatr Gerontol Int 2015;15:872-80. [Crossref] [PubMed]
- Sinha MK, Roy D, Gaze DC, et al. Role of "Ischemia modified albumin", a new biochemical marker of myocardial ischaemia, in the early diagnosis of acute coronary syndromes. Emerg Med J 2004;21:29-34. [Crossref] [PubMed]
- Sinha MK, Gaze DC, Tippins JR, et al. Ischemia modified albumin is a sensitive marker of myocardial ischemia after percutaneous coronary intervention. Circulation 2003;107:2403-5. [Crossref] [PubMed]
- Matthes G, Seibt G, Muravsky V, et al. Albumin transport analysis of different collected and processed plasma products by electron spin resonance spectroscopy. Transfus Apher Sci 2002;27:129-35. [Crossref] [PubMed]
- Junk MJ, Spiess HW, Hinderberger D. The distribution of fatty acids reveals the functional structure of human serum albumin. Angew Chem Int Ed Engl 2010;49:8755-9. [Crossref] [PubMed]


