
The Wnt/β-catenin pathway is activated by miR-1246 in liver cancer stem cells
The Wnt/β-catenin signaling pathway drives stem cell proliferation and self-renewal (1). As the key transducer of the Wnt signal, the β-catenin transcriptional coactivator is tightly regulated (2). In the absence of Wnt, cytoplasmic β-catenin is found in a complex containing axis inhibition proteins 1 and 2 (AXIN1/2), adenomatous polyposis coli (APC), glycogen synthase kinase 3 beta (GSK3β), and casein kinase 1 epsilon (CK1ε). Here, it is phosphorylated on its amino terminal residues, ubiquitinated by beta-transducin repeat containing protein, and targeted for proteasomal degradation. When Wnt ligand is present, β-catenin levels are stabilized and it translocates into the nucleus to activate target gene expression.
Mutations in Wnt/β-catenin signaling components render the pathway aberrantly active in many cancers, including hepatocellular carcinoma (HCC) (3). HCC is associated with a high mortality rate because 80% of diagnoses occur after tumors have progressed to a late stage (4). In addition, HCC patients suffer from a 70% relapse rate within 5 years (5). This high HCC relapse rate can be attributed in part to a population of CD133+ liver cancer stem cells (CSCs) that have aberrant activation of stem-cell self-renewal pathways, including Wnt/β-catenin signaling (6). Around 20% of HCCs have mutations in the region of the CTNNB1 gene encoding its amino terminal phosphodegron (7), but these mutations are thought to arise late in carcinogenesis and also correlate with less aggressive disease (7,8). Despite this, β-catenin accumulation in the nucleus is found in up to 70% of HCCs, suggesting an alternative mechanism by which Wnt signaling is activated in liver CSCs (9).
A recent study by Chai et al. sought to identify how Wnt/β-catenin signaling is deregulated in liver CSCs (10). By sequencing small RNAs isolated from CD133+ and CD133- subsets of the PLC8024 HCC cell line, expression of the microRNA, miR-1246, was found to be elevated in the CD133+ subset. Because miR-1246 was predicted to target AXIN2 and GSK3β, the authors reasoned that downregulation of these genes by miR-1246 could drive HCC. After demonstrating that AXIN2 and GSK3β were directly targeted by miR-1246, they found that shRNA-mediated depletion of miR-1246 in HCC cells increased AXIN2 and GSK3β protein levels in the cytoplasm, reduced β-catenin levels in the nucleus, and decreased Wnt target gene expression.
Chai et al. then addressed the role of miR-1246 in controlling liver CSC-associated properties in vitro. First, they found that loss of miR-1246 inhibited HCC cell self-renewal, as assessed by hepatosphere formation after serial passage. Second, reducing miR-1246 levels sensitized HCC cells to apoptosis induced by both a general chemotherapeutic, cisplatin, and the primary targeted therapy in HCC, sorafenib. Finally, miR-1246 was shown to be required for the invasive, migratory, and angiogenic properties of HCC cells, reflecting its importance to the aggressive spread of HCC. Importantly, over-expressing miR-1246 in HCC cells exacerbated these phenotypes. Thus, miR-1246 is crucial for HCC cell tumorigenesis, drug resistance, and invasiveness in vitro.
The role of miR-1246 in driving tumorigenesis in vivo was assessed using a xenograft tumor mouse model. Strikingly, miR-1246 knockdown in Hep3B and Huh7 HCC cell lines abrogated tumor formation. The tumor-initiating potential of a more aggressive HCC cell line, BEL7402, was calculated in primary and secondary implants. As expected, depleting miR-1246 in BEL7402 cells dramatically reduced the rate of tumor formation. Additionally, the authors used orthotopic liver injections of luciferase-labeled control and knockdown HCC cells to demonstrate that reduction of miR-1246 levels resulted in significantly fewer lung metastases. This finding agrees with reports that aberrant activation of Wnt/β-catenin signaling correlates with metastasis in HCC patients (11). Chai et al. confirmed that miR-1246 is activating Wnt/β-catenin signaling in the orthotopic liver tumors by demonstrating increased levels of AXIN2 and GSK3β and decreased levels of β-catenin.
After elucidating the role for miR-1246 in promoting HCC in both cell culture and mouse xenograft models, the authors also demonstrated its importance in patients, which added substantially to the clinical relevance of this study. Compared to adjacent normal liver tissue, levels of miR-1246 in HCC tumors are dramatically increased. Increased levels of miR-1246 negatively correlated with both overall survival and disease-free survival. Notably, the authors detected miR-1246 in the serum of HCC patients and found that it acts as a highly sensitive biomarker, clearly distinguishing control individuals from HCC patients. This is a significant finding given the existing troubles detecting HCC at a stage where therapeutic intervention may be more effective. Additionally, the authors show that miR-1246 overexpression and β-catenin mutation in HCC are mutually exclusive events, suggesting that miR-1246 may be driving oncogenic Wnt/β-catenin signaling in tumors that do not harbor stabilizing β-catenin mutations. This mutual exclusivity is supported by findings that altering miR-1246 levels in HCC cell lines containing mutant β-catenin abrogates the tumorigenic, drug-resistant, and invasive phenotypes described previously.
This cumulative data raises the question of how miR-1246 levels are elevated in HCC. By analyzing transcription factor binding motifs in the miR-1246 promoter region, Chai et al. identified four putative octamer-binding transcription factor 4 (OCT4) binding sites. Subsequently, OCT4 binding to three of these sites was confirmed and a positive correlation between OCT4 and miR-1246 expression in HCC tumors was reported. Additionally, OCT4 is expressed more abundantly in CD133+ CSCs cells relative to non-CSCs, and its depletion reduced both miR-1246 and β-catenin expression. While OCT4 is a stem cell transcription factor that is associated with self-renewal transcriptional programs and has been identified as an oncogene in HCC (12,13), it is unknown how it’s expression is elevated in HCC liver CSCs. Interestingly, OCT4 has previously been shown to directly activate expression of Wnt target gene, CCND1 (13). Perhaps OCT4 activates a subset of Wnt target genes both directly and indirectly through upregulation of miR-1246.
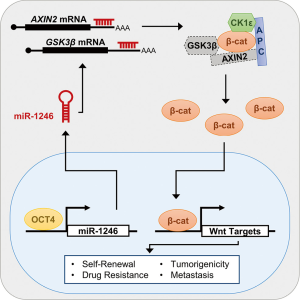
Cumulatively, the results presented in Chai et al. reveal a novel mechanism for β-catenin accumulation in CD133+ liver CSCs. Overexpression of the transcription factor OCT4 in HCC cells leads to elevated levels of miR-1246, which targets AXIN2 and GSK3β mRNA. The subsequent reduction in levels of these regulatory proteins allows accumulation of nuclear β-catenin and aberrant activation of genes controlling self-renewal, drug-resistance, tumorigenicity, and metastasis (Figure 1). Importantly, miR-1246 may also serve as a highly sensitive serum biomarker for early detection of HCC. While early detection is ideal, this study may also contribute to the development of novel therapeutics. The current treatment for late-stage HCC, sorafenib, only prolongs survival three months (14). Given that Chai et al. demonstrate increased sensitivity to sorafenib in miR-1246 depleted HCC cells, combining sorafenib and a miR-1246 targeted therapy could improve outcomes. Interestingly, a combination therapy clinical trial of sorafenib and a Wnt pathway inhibitor is currently underway (NCT02069145). The Wnt inhibitor in this trial, OMP-54F28, is a frizzled family receptor 8 decoy receptor that acts as a Wnt ligand sponge, preventing pathway activation by exogenous Wnt ligand.
In summary, Chai et al. have elucidated an important mechanism driving HCC. This study raises many new questions that should be addressed in future research. First, whether miR-1246 is therapeutically targetable is unknown. Several miRNA-targeted therapies have had success, yet difficulties remain in preventing off-target effects and assuring effective delivery (15). Targeting upstream of miR-1246, for example OCT4, may prove to be effective although the results presented in the current study do not preclude the involvement of another transcriptional regulator of miR-1246 expression. Indeed, p53 has previously been shown to induce miR-1246 expression in HCC cell lines (16). Second, it will be interesting to determine what other targets of miR-1246 are implicated in HCC development. Cell adhesion molecule 1 (CADM1) and nuclear factor 1 B (NF1B) have also been confirmed as miR-1246 targets in HCC (16,17). Finally, Wnt/β-catenin signaling is deregulated in numerous cancer subtypes as well as a myriad of diseases (18). Therefore, while important for HCC diagnosis and treatment, the novel mechanism of Wnt/β-catenin pathway activation by miR-1246 uncovered in this study may have a more global impact on human health and disease.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned and reviewed by the Section Editor Bo Zhai, MD, PhD (Department of Hepatobiliary Surgery, The Fourth Hospital of Harbin Medical University, Harbin, China).
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2016.12.57). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Barker N. The canonical Wnt/beta-catenin signalling pathway. Methods Mol Biol. 2008;468:5-15. [Crossref] [PubMed]
- MacDonald BT, Tamai K, He X. Wnt/beta-catenin signaling: components, mechanisms, and diseases. Dev Cell 2009;17:9-26. [Crossref] [PubMed]
- Giles RH, van Es JH, Clevers H. Caught up in a Wnt storm: Wnt signaling in cancer. Biochim Biophys Acta 2003;1653:1-24. [PubMed]
- Pez F, Lopez A, Kim M, et al. Wnt signaling and hepatocarcinogenesis: molecular targets for the development of innovative anticancer drugs. J Hepatol 2013;59:1107-17. [Crossref] [PubMed]
- Forner A, Llovet JM, Bruix J. Hepatocellular carcinoma. Lancet 2012;379:1245-55. [Crossref] [PubMed]
- Ma S, Chan KW, Hu L, et al. Identification and characterization of tumorigenic liver cancer stem/progenitor cells. Gastroenterology 2007;132:2542-56. [Crossref] [PubMed]
- Park JY, Park WS, Nam SW, et al. Mutations of beta-catenin and AXIN I genes are a late event in human hepatocellular carcinogenesis. Liver Int 2005;25:70-6. [Crossref] [PubMed]
- Lachenmayer A, Alsinet C, Savic R, et al. Wnt-pathway activation in two molecular classes of hepatocellular carcinoma and experimental modulation by sorafenib. Clin Cancer Res 2012;18:4997-5007. [Crossref] [PubMed]
- Suzuki T, Yano H, Nakashima Y, et al. Beta-catenin expression in hepatocellular carcinoma: a possible participation of beta-catenin in the dedifferentiation process. J Gastroenterol Hepatol 2002;17:994-1000. [Crossref] [PubMed]
- Chai S, Ng KY, Tong M, et al. Octamer 4/microRNA-1246 signaling axis drives Wnt/β-catenin activation in liver cancer stem cells. Hepatology 2016;64:2062-76. [Crossref] [PubMed]
- Lai TY, Su CC, Kuo WW, et al. β-catenin plays a key role in metastasis of human hepatocellular carcinoma. Oncol Rep 2011;26:415-22. [PubMed]
- Yuan F, Zhou W, Zou C, et al. Expression of Oct4 in HCC and modulation to wnt/β-catenin and TGF-β signal pathways. Mol Cell Biochem 2010;343:155-62. [Crossref] [PubMed]
- Cao L, Li C, Shen S, et al. OCT4 increases BIRC5 and CCND1 expression and promotes cancer progression in hepatocellular carcinoma. BMC Cancer 2013;13:82. [Crossref] [PubMed]
- Llovet JM, Di Bisceglie AM, Bruix J, et al. Design and endpoints of clinical trials in hepatocellular carcinoma. J Natl Cancer Inst 2008;100:698-711. [Crossref] [PubMed]
- Li Z, Rana TM. Therapeutic targeting of microRNAs: current status and future challenges. Nat Rev Drug Discov 2014;13:622-38. [Crossref] [PubMed]
- Zhang Q, Cao LY, Cheng SJ, et al. p53-induced microRNA-1246 inhibits the cell growth of human hepatocellular carcinoma cells by targeting NFIB. Oncol Rep 2015;33:1335-41. [PubMed]
- Sun Z, Meng C, Wang S, et al. MicroRNA-1246 enhances migration and invasion through CADM1 in hepatocellular carcinoma. BMC Cancer 2014;14:616. [Crossref] [PubMed]
- Clevers H, Nusse R. Wnt/β-catenin signaling and disease. Cell 2012;149:1192-205. [Crossref] [PubMed]


