
Cell intrinsic PD-1 checkpoint blockade releases the brake on human chimeric antigen receptor (CAR) T cells for solid tumors
The immune system plays an important role in controlling and eliminating cancer. Nevertheless, tumors often evade endogenous immune responses due to tolerogenic mechanisms which prevent the rejection of malignant cells that are recognized as ‘self’ or alternatively, not ‘dangerous.’ The complex network of biological pathways that maintain tolerance involves several mediators, including myeloid and lymphoid-derived regulatory cells, immunosuppressive cytokines and chemokines, as well as immune checkpoint molecules that down-modulate anti-tumor immunity. The programmed cell death protein 1 (PD-1)—PD-1 ligand 1 (PD-L1) receptor-ligand pair is a principal immune checkpoint axis operative in the tumor microenvironment that attenuates T cell receptor (TCR)-mediated activation, leading to inhibition of T cell expansion and cytokine production (1-3). Although the genetic re-direction of T cells with chimeric antigen receptors (CARs) is an attractive approach to break tolerance in the setting of cancer, there is a paucity of experimental evidence to suggest that CAR T cells are optimally functional within solid tumors and additionally, that blockade of immune checkpoints can potentiate their activity. Cherkassky et al. (4) address this long-standing question by engineering mesothelin-targeted CAR T cells to be intrinsically resistant to PD-1-induced inhibition. These findings should spur future investigations focused on more carefully defining the determinants of CAR T cell exhaustion and assessing the therapeutic potential of individual or combined checkpoint blockade in augmenting anti-tumor T cell responses.
In the case of most human cancers, the TCR repertoire is either lacking or inadequate for tumor control (5-7), which has paved the way for the concept of using CAR-redirected T cells for adoptive immunotherapy. Currently, we and others have demonstrated that CAR T cells can eradicate leukemia and induce long-term durable remissions in patients (8-10). In the setting of solid tumors, however, this approach has not achieved the same degree of clinical success, and this may be attributed to unique barriers imposed by solid tumors that are absent in hematological malignancies. Following the trafficking of CAR T cells to tumor tissues and infiltration through stromal elements, these engineered lymphocytes must overcome challenges present within a profoundly immunosuppressive landscape which, in addition to the aforementioned tolerogenic mechanisms, may include nutrient deprivation, oxidative stress, low pH and hypoxia [reviewed in (11)]. Furthermore, in this microenvironment where CAR T cells must function, there is evidence that TCR signaling becomes uncoupled independently of the effects of tumors on TCRζ expression (12,13). This led us and other groups to construct “bipartite receptors” comprised of TCRζ and intracellular signaling modules such as CD28, 4-1BB, OX40, ICOS or CD27 (14-19) to substantially improve the function and proliferation of adoptively transferred T cells. While the induction of costimulatory pathways can overcome some degree of solid tumor-mediated inhibitory signaling, the extent to which T cells expressing these “second generation” CARs could be inhibited upon in vivo antigen exposure within the solid tumor microenvironment is not well-characterized.
The studies by Cherkassky et al. (4) highlight the effects of specific types of co-stimulatory signaling on CAR T cell potency, which have been largely unappreciated due the use of immune sensitive model systems and the infusion of large numbers of T cells that do not accurately reflect effector to target cell ratios attained in patients with high tumor burdens. Similar to previous studies using different in vivo experimental systems, T cells expressing anti-mesothelin chimeric receptors bearing TCRζ signaling modules alone were not sufficient to drive sustained antitumor activity in an orthotopic model of malignant pleural mesothelioma (4,14). In contrast, the administration of high doses of CAR T cells incorporating CD28 or 4-1BB endodomains resulted in tumor clearance, regardless of the costimulatory pathway induced. Interestingly, upon transfer of low doses of T cells, only 4-1BBζ CAR T cells resulted in long-term tumor eradication, despite an intratumoral accumulation and persistence comparable to that of their CD28ζ counterparts. These findings were supported by the superior ability of 4-1BBζ CAR T cells to retain effector cytokine production and cytotoxic capacity following in vivo antigen exposure (4). In addition, when recall response was assessed (the so-called ability to function as “serial killers”), 4-1BBζ CAR T cells were more resistant to ongoing immune inhibition induced by repeated antigen stimulation in vitro or tumor re-challenge in vivo (4). These findings underscore that when selecting an optimal costimulatory signaling strategy, mere assessment of CAR T cell engraftment particularly as measured through peripheral blood sampling may not be a sufficient indication of potency in the setting of solid tumor indications. Pre-clinical studies that will ultimately inform clinical trial designs should thus move toward the evaluation of functional correlates of persistence in model systems that more accurately recapitulate tumor antigen load in patients.
Despite the improved anti-tumor activities of second generation CAR T cells in which enhanced persistence is mainly attributed to the engagement of costimulatory pathways, these engineered lymphocytes also undergo an activation-induced upregulation of co-inhibitory receptors and pathways to naturally control the magnitude of immune responses. Accordingly, the balance between positive and negative signals is a critical determinant of the outcome of T cell-mediated immunity. However, the increased expression of inhibitory receptors following antigen encounter coupled with the overexpression of their cognate ligands by cancer cells greatly limits overall anti-tumor activity. Although blockade of negative regulatory checkpoint pathways has recently demonstrated promise in restoring defective T cell function (20-22), the clinical success of these approaches often relies on tumor mutation burden (23) and the localization of T cells within the microenvironment. Further, unabridged immune activation following checkpoint blockade is often accompanied by a number of toxicities and autoimmune sequelae (24). In patients with tumors of insufficient immunogenicity (i.e., “non-inflamed” tumors), treatment with CAR-redirected T cells may address these limitations. Nevertheless, adoptively-transferred T cells are susceptible to immune inhibition, and therefore, the removal of inhibitory checkpoint signals may potentiate their full anti-tumor effects.
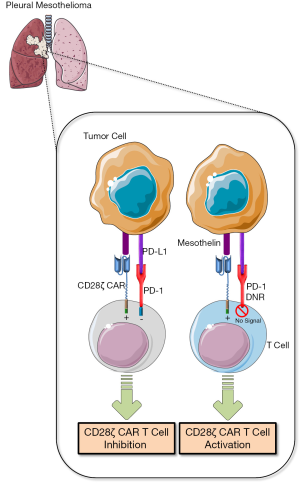
Cherkassky and colleagues (4) focused on repairing the defect observed in tumor-infiltrating CD28ζ-signaling CAR T cells that was largely characterized by PD-1 overexpression. Signaling through PD-1 results in the recruitment of phosphatases SHP-2 and to a lesser extent SHP-1 to the inhibitory receptor cytoplasmic domain that initiates dephosphorylation of antigen receptor proximal signaling molecules including ZAP70, PKCθ, and CD3ζ (1), leading presumably to attenuation of the CAR/CD28ζ signal. Co-expression of a PD-1 with a truncated intracellular signaling domain counteracted the inhibitory signaling conferred by endogenous PD-1 (4), which was likely attributed to competition for PD-L1 by the dominant negative receptor (Figure 1). This strategy resulted in enhanced in vitro CD28ζ CAR T cell activity and significantly improved in vivo anti-tumor efficacy with a single administration (4). The strength of this approach is elimination of the requirement for repeated anti-PD-1/PD-L1 antibody dosing, which the authors also demonstrate to be efficacious when administered concurrently with CD28ζ CAR T cells. Indeed, this genetic engineering strategy may offer an enhanced safety profile as well as a therapeutic benefit over PD-1/PD-L1-targeting antibodies due to pathway inhibition that is restricted to adoptively-transferred T cells. This permits tumor-targeted T cell reinvigoration within the microenvironment without dose-limiting toxicities that are inherent to broadly applied antibody-based checkpoint blockade (24). In future investigations focused on extending this approach into clinical development, consideration should be given to the incorporation of inducible suicide genes into CAR T cells that co-express a dominant negative PD-1 receptor. This “safety switch”-type engineering may ultimately prevent or limit the potential for adverse lymphoproliferative or autoimmunity.
How might these findings improve treatment strategies based on adoptive T cell transfer? Cherkassky et al. (4) illustrated the functional significance of a central mechanism underlying T cell exhaustion in an antigen-dependent model system that recapitulates certain features of inhibitory signaling in the tumor microenvironment. In addition, the authors demonstrate the importance of selecting an optimal costimulatory strategy that is capable of withstanding immune inhibition. It is intriguing to speculate that CAR T cells containing tripartite signaling domains may be more resistant to immune inhibitory effects imposed by this model, due to their “hardwired” costimulatory signals that are triggered upon encounter with tumor antigen. There are theoretical reasons to suggest that three signaling modules comprising a “third generation” chimeric receptor might have additive or synergistic effects. Although 4-1BB can function independently of CD28 (25), it is also possible that one of these co-stimulatory signals will be dominant. Nevertheless, deep immunophenotyping in addition to relevant functional analyses of pre- and post-infusion CAR T cells may provide clues about which T cells can proliferate and elicit superior anti-tumor activity following inhibition of the PD-1 inhibitory pathway. For example, it has been recently demonstrated that a certain PD-1+ CD8+ T cell population that proliferates following blockade of PD-1/PD-L1 signaling expresses several costimulatory molecules (e.g., ICOS, CD28) and is capable of self-renewal as well as effector differentiation (26). Thus, selection of such a population of T cells for CAR-based strategies that combine checkpoint blockade may optimize PD-1-directed immunotherapy. Finally, it is becoming increasingly evident that T cell exhaustion is regulated by co-expression of multiple inhibitory receptors including PD-1 (27). A systematic exploration of all inhibitory pathways that could limit CAR T cell potency in the setting of the solid tumor microenvironment may elucidate the therapeutic potential of individual or combined blockade of additional inhibitory receptors in restoring anti-tumor T cell function.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned and reviewed by the Section Editor Xian-Huo Wang (Department of Lymphoma, Sino-US Center for Lymphoma and Leukemia, Tianjin Medical University Cancer Institute and Hospital, National Clinical Research Center of Cancer, Key Laboratory of Cancer Prevention and Therapy, Tianjin, China).
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2016.12.06). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Sheppard KA, Fitz LJ, Lee JM, et al. PD-1 inhibits T-cell receptor induced phosphorylation of the ZAP70/CD3zeta signalosome and downstream signaling to PKCtheta. FEBS Lett 2004;574:37-41. [Crossref] [PubMed]
- Herbst RS, Soria JC, Kowanetz M, et al. Predictive correlates of response to the anti-PD-L1 antibody MPDL3280A in cancer patients. Nature 2014;515:563-7. [Crossref] [PubMed]
- Tumeh PC, Harview CL, Yearley JH, et al. PD-1 blockade induces responses by inhibiting adaptive immune resistance. Nature 2014;515:568-71. [Crossref] [PubMed]
- Cherkassky L, Morello A, Villena-Vargas J, et al. Human CAR T cells with cell-intrinsic PD-1 checkpoint blockade resist tumor-mediated inhibition. J Clin Invest 2016;126:3130-44. [Crossref] [PubMed]
- Morgan DJ, Kreuwel HT, Fleck S, et al. Activation of low avidity CTL specific for a self epitope results in tumor rejection but not autoimmunity. J Immunol 1998;160:643-51. [PubMed]
- Liu GY, Fairchild PJ, Smith RM, et al. Low avidity recognition of self-antigen by T cells permits escape from central tolerance. Immunity 1995;3:407-15. [Crossref] [PubMed]
- Lyman MA, Nugent CT, Marquardt KL, et al. The fate of low affinity tumor-specific CD8+ T cells in tumor-bearing mice. J Immunol 2005;174:2563-72. [Crossref] [PubMed]
- Porter DL, Hwang WT, Frey NV, et al. Chimeric antigen receptor T cells persist and induce sustained remissions in relapsed refractory chronic lymphocytic leukemia. Sci Transl Med 2015;7:303ra139 [Crossref] [PubMed]
- Maude SL, Frey N, Shaw PA, et al. Chimeric antigen receptor T cells for sustained remissions in leukemia. N Engl J Med 2014;371:1507-17. [Crossref] [PubMed]
- Lee DW, Kochenderfer JN, Stetler-Stevenson M, et al. T cells expressing CD19 chimeric antigen receptors for acute lymphoblastic leukaemia in children and young adults: a phase 1 dose-escalation trial. Lancet 2015;385:517-28. [Crossref] [PubMed]
- Newick K, Moon E, Albelda SM. Chimeric antigen receptor T-cell therapy for solid tumors. Mol Ther Oncolytics 2016;3:16006. [Crossref] [PubMed]
- Staveley-O'Carroll K, Sotomayor E, Montgomery J, et al. Induction of antigen-specific T cell anergy: An early event in the course of tumor progression. Proc Natl Acad Sci U S A 1998;95:1178-83. [Crossref] [PubMed]
- Clark JM, Annenkov AE, Panesar M, et al. T cell receptor zeta reconstitution fails to restore responses of T cells rendered hyporesponsive by tumor necrosis factor alpha. Proc Natl Acad Sci U S A 2004;101:1696-701. [Crossref] [PubMed]
- Carpenito C, Milone MC, Hassan R, et al. Control of large, established tumor xenografts with genetically retargeted human T cells containing CD28 and CD137 domains. Proc Natl Acad Sci U S A 2009;106:3360-5. [Crossref] [PubMed]
- Guedan S, Chen X, Madar A, et al. ICOS-based chimeric antigen receptors program bipolar TH17/TH1 cells. Blood 2014;124:1070-80. [Crossref] [PubMed]
- Song DG, Ye Q, Poussin M, et al. CD27 costimulation augments the survival and antitumor activity of redirected human T cells in vivo. Blood 2012;119:696-706. [Crossref] [PubMed]
- Hombach AA, Heiders J, Foppe M, et al. OX40 costimulation by a chimeric antigen receptor abrogates CD28 and IL-2 induced IL-10 secretion by redirected CD4(+) T cells. Oncoimmunology 2012;1:458-66. [Crossref] [PubMed]
- Finney HM, Akbar AN, Lawson AD. Activation of resting human primary T cells with chimeric receptors: costimulation from CD28, inducible costimulator, CD134, and CD137 in series with signals from the TCR zeta chain. J Immunol 2004;172:104-13. [Crossref] [PubMed]
- Imai C, Mihara K, Andreansky M, et al. Chimeric receptors with 4-1BB signaling capacity provoke potent cytotoxicity against acute lymphoblastic leukemia. Leukemia 2004;18:676-84. [Crossref] [PubMed]
- Hodi FS, O'Day SJ, McDermott DF, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med 2010;363:711-23. [Crossref] [PubMed]
- Wolchok JD, Kluger H, Callahan MK, et al. Nivolumab plus ipilimumab in advanced melanoma. N Engl J Med 2013;369:122-33. [Crossref] [PubMed]
- Topalian SL, Hodi FS, Brahmer JR, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med 2012;366:2443-54. [Crossref] [PubMed]
- Le DT, Uram JN, Wang H, Bartlett BR, et al. PD-1 Blockade in Tumors with Mismatch-Repair Deficiency. N Engl J Med 2015;372:2509-20. [Crossref] [PubMed]
- Naidoo J, Page DB, Li BT, et al. Toxicities of the anti-PD-1 and anti-PD-L1 immune checkpoint antibodies. Ann Oncol 2015;26:2375-91. [PubMed]
- Saoulli K, Lee SY, Cannons JL, et al. CD28-independent, TRAF2-dependent costimulation of resting T cells by 4-1BB ligand. J Exp Med 1998;187:1849-62. [Crossref] [PubMed]
- Im SJ, Hashimoto M, Gerner MY, et al. Defining CD8+ T cells that provide the proliferative burst after PD-1 therapy. Nature 2016;537:417-21. [Crossref] [PubMed]
- Blackburn SD, Shin H, Haining WN, et al. Coregulation of CD8+ T cell exhaustion by multiple inhibitory receptors during chronic viral infection. Nat Immunol 2009;10:29-37. [Crossref] [PubMed]


