Total laparoscopic pancreaticoduodenectomy: a retrospective study of 27 cases treated in a single institution
Introduction
Gagne and Pomp performed the first reported laparoscopic pancreaticoduodenectomy (LPD) in a 30-year-old woman with chronic pancreatitis in 1994 (1). Because this procedure is extremely difficult and there is a high incidence of postoperative complications, some researchers have raised doubts about its efficacy (2-4). Acceptance has probably been slowed by both the inherent technical limitations of laparoscopy and the need for advanced endoscopic skills which, until recently, surgeons have not acquired during their training (5,6).
Four techniques are currently employed for LPD: pure laparoscopy, hand-assisted laparoscopy, laparoscopic-assisted surgery, and robotic-assisted laparoscopy (7-14). With the increasing use of LPD, multicenter randomized controlled studies have shown that LPD is safe, and that intraoperative bleeding, R0 resection rate, number of lymph nodes resected, and incidence of postoperative complications are not worse than with open pancreaticoduodenectomy (PD). Numerous published studies have now reported that LPD is safe, feasible, and adequate (13,15-19).
Thus far, because of the limited experience with total laparoscopic pancreaticoduodenectomy (TLPD) and the added complexity of vascular resection and reconstruction, major venous involvement has been considered a relative contraindication to choosing a laparoscopic approach for PD. Here, we review our initial experience with LPD as performed by a single, high-volume pancreatic surgeon with extensive laparoscopic surgical experience in a tertiary care setting, our focus being on our experience of major venous resection and reconstruction with TLPD.
Methods
From May 2014 to June 2016, 27 patients were underwent LPD in our department. They comprised 18 men and 9 women (male:female =2:1) with a mean age of 63.2 years (range, 50–78 years). Preoperative investigations included routine blood, urine, and stool tests, neoplastic markers, chest radiographs, upper abdominal ultrasound, computed tomography (CT) scan, and gastroscopy. The preoperative diagnoses were primary pancreatic neoplasms in 12, duodenal neoplasms in 7, and biliary tract tumors in 8 patients. Contraindications to laparoscopy were severe cardiorespiratory disease, use of anticoagulant drugs; ASA score ≥4; distant metastases; serious electrolyte disorders, and inability to perform endotracheal intubation.
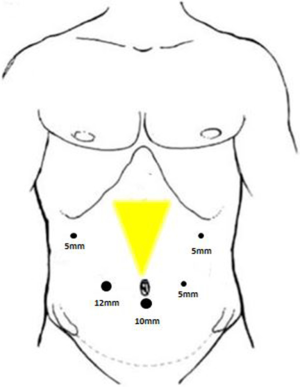
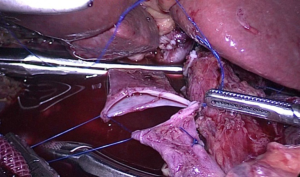
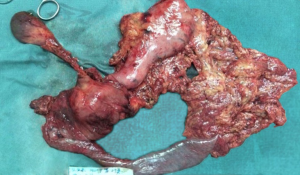
All operations were performed by the same surgeon. The patient was intubated in a supine position with a 20° head-up tilt. After undergoing tracheal intubation and induction of general anesthesia, a CO2 pneumoperitoneum was created via an open Veress-assisted technique. A 30° telescope was used to inspect the peritoneal cavity. Five trocars were used: a 10-mm telescope trocar in the midline above the umbilical incision; two cranially placed 5-mm ports in the left and right anterior axillary lines, and two cranially placed ports 5 mm to the left and 10 mm to the right of the rectus muscles at the level of the umbilicus (Figure 1). First, the duodenum was mobilized by the Kocher maneuver and the inferior vena cava and superior mesenteric vein (SMV) exposed. If tumor was found to be involving these vessels, a decision was usually made convert to open PD. Next, the bile duct and common hepatic artery were dissected; cholecystectomy performed; and sections of the bile duct, stomach, and pancreatic head, and pancreatic uncinate dissected, after which the Whipple specimen was removed through a minimal enlargement of the supraumbilical site using a retrieval bag and a child anastomosis created. Standard lymphadenectomy was always carried out. In the first five cases, “sleeve” anastomoses were created for pancreaticojejunostomy; whereas in the last 22 cases, pancreatic duct to intestinal mucosa anastomoses were created. Portal vein (PV) resection and reconstruction was performed in two cases (Figure 2). We believe that it is very important to perform an “artery first” or “uncinate first” approach before resection and reconstruction of the PV. First, a Kocher incision was made, then the duodenum and pancreatic head freed to expose the anterior inferior vena cava and abdominal aorta to the root of the superior mesenteric artery. After the invaded length of SMV had been identified, resection of the pancreatic uncinate process was completed. Finally, resection of involved vein and entire specimen was completed (Figure 3). Systemic intravenous unfractionated heparin (3,000 to 5,000 units) was administered before clamping the portal system. Vascular anastomosis was achieved by end to end anastomosis using the two-point method (Figure 4).
Results
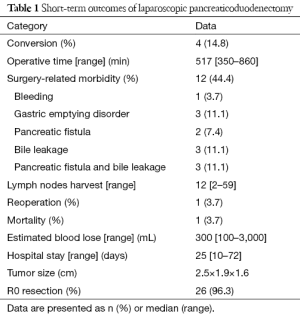
Twenty-seven LPD were carried out. Conversion was required in 4 patients (14.8%), 2 because the PV was involved by tumor; 1 because the tumor was large (4.5 cm × 4.5 cm × 4.0 cm) and surrounded by extensive adhesions, and the fourth because the SMV was involved by tumor. There was no intraoperative mortality. The superior mesenteric artery was injured in 1 patient (3.7%); this was managed by intracorporeal suturing with 4-0 polypropylene (bleeding of 3,000 mL). The mean operation time was 517 (range, 350–860) minutes; mean blood loss 500 (range, 300–3,000) mL; and mean hospital stay was 25 (range, 11–72) days.
PV resection and reconstruction was performed in 2 cases (7.4%) because of involvement by cancer. This procedure was completed without postoperative complications such as anastomotic stenosis, vein thrombosis, anastomotic leakage, or gastric emptying disorder. Total PV clamping time was 32.5 (range, 30–35) minutes.
One patient (3.7%) died on the 14th postoperative day of multiple organ dysfunction syndrome. The overall postoperative morbidity was 44.4% (12 cases). One patient (3.7%) had bleeding requiring open reoperation; intraoperatively, minor crevasse bleeding from the left gastric artery was identified. There were 3 cases (11.1%) of gastric emptying disorder; 2 (7.4%) of simple pancreatic fistula, and 3 (11.1%) of simple biliary leakage. Both pancreatic fistula and biliary leakage occurred in 3 (11.1%) patients (Table 1).
Full table
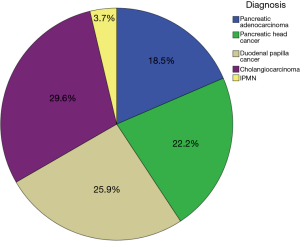
Postoperative pathologic examination of the operative specimen confirmed pancreatic adenocarcinoma in 18.5% of patients (n=5), pancreatic head cancer in 22.2% (n=6), duodenal papillary cancer in 25.9% (n=7), cholangiocarcinoma in 29.6% (n=8), and intraductal papillary mucinous neoplasm (IPMN) in 1 (3.7%) (Figure 5). The mean number of resected lymph nodes was 12 (range, 2–59). R0 resection was achieved in 26 patients (96.3%).
The mean duration of follow-up was 17 (range, 1–44) months. Eight patients are still alive, five being disease-free and the remaining three with recurrence of duodenal adenocarcinoma, pancreatic head cancer, and pancreatic adenocarcinoma, respectively. The remaining patients all died of disease after a mean of 15 (range, 7–18) months.
Discussion
Surgery is the only curative treatment for pancreatic tumors (20). LPD was first described by Gagne and Pomp in 1994 (1). Since then the procedure has been attempted in a relatively small number of patients worldwide and has a high conversion rate of more than 30% (11). Its safety and feasibility were therefore doubted by researchers until the recent emergence of growing evidence that LPD is a feasible alternative to an open approach in appropriately selected patients. Recent small studies have demonstrated that LPD can be performed with less blood loss and resection of more numerous lymph nodes than open surgery (21,22). We believe that surgeon’s attitudes towards LPD have gradually changed because of increasing completion of learning curves for LPD.
The traditional surgical learning curve has three main portions: a slow and potentially arduous beginning, followed by a steep acceleration phase characterized by rapid learning, and finally a plateau involving slower but continued improvements. Since laparoscopic cholecystectomy was first performed in 1996 in our institution, we have increasingly performed laparoscopic operations such as hepatectomies, gastrectomies, splenectomies, hernia repairs and pancreaticoduodenectomies.
Moore et al. first reported resection and reconstruction of the SMV during PD (23). Since then, increasing numbers of reports have described various techniques for reconstructing the SMV and/or PV. It is widely accepted that PV resection increases the resectability rate in patients with cancers of the pancreatic head (24). Some researchers have suggested that, with appropriate patient selection and surgeon experience, venous resection and reconstruction can be performed safely during PD (25) and may even increase survival (26). The Mayo Clinic reported the first series of TLPD with major venous resection and reconstruction (19). Several published reports have shown that TLPD is feasible and has several potential advantages over open PD (27). The Mayo Clinic reported that TLPD with major vascular resection is not only feasible and safe, but also achieves similar morbidity, mortality, and oncologic outcomes to open PD with major vascular resection. Thus, vascular involvement is not an absolute contraindication to TLPD in centers with experienced surgeons (28). After incorporating advances in laparoscopic experience and techniques and developing a learning curve by operating on animals, we performed vascular resection and reconstruction in two patients. The median clamping time was 32.5 (range, 30–35) minutes, which is within safe limits according to van Riel et al.’s research (29). We therefore concluded that laparoscopic venous resection and reconstruction is feasible.
We reviewed data from other institutions in which major venous resection and reconstruction had been performed during TLPD and have compared the results in Table 2 [data from Croome et al. (28), Palanisamy et al. (30), Awad (31), and Dokmak et al. (32)].

Full table
Conclusions
Our experience suggests that TLPD is feasible and safe and that major venous resection and reconstruction can be performed in carefully selected patients. However, there is still uncertainty regarding the reproducibility of our outcomes. We have performed only one type of venous resection and reconstruction; obviously evidence needs to be accumulated for different types of venous resection (such as SMV alone or combined PV and SMV resection) and reconstruction [such as segmental resection (SR), tangential resection (TR), primary suture closure, and patch venorrhaphy]. Large multicenter randomized trials are needed to provide more reliable information about the usefulness of LPD versus open surgery (with or without major venous resection and reconstruction).
Acknowledgments
The authors would like to acknowledge Prof. Yi Lei Mao’s help with manuscript preparation.
Funding: This work was supported by the Scientific Innovation Team Project of Ningbo (2013B82010).
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2016.12.15). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Ningbo Medical Center of Lihuili Hospital ethical committee. Written informed consent was obtained from the patients.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Gagner M, Pomp A. Laparoscopic pylorus-preserving pancreatoduodenectomy. Surg Endosc 1994;8:408-10. [Crossref] [PubMed]
- Cuschieri A. Laparoscopic Pancreatic Resections. Semin Laparosc Surg 1996;3:15-20. [PubMed]
- Park A, Schwartz R, Tandan V, et al. Laparoscopic pancreatic surgery. Am J Surg 1999;177:158-63. [Crossref] [PubMed]
- Underwood RA, Soper NJ. Current status of laparoscopic surgery of the pancreas. J Hepatobiliary Pancreat Surg 1999;6:154-64. [Crossref] [PubMed]
- Subhas G, Mittal VK. Training minimal invasive approaches in hepatopancreatobilliary fellowship: the current status. HPB (Oxford) 2011;13:149-52. [Crossref] [PubMed]
- Vijan SS, Ahmed KA, Harmsen WS, et al. Laparoscopic vs open distal pancreatectomy: a single-institution comparative study. Arch Surg 2010;145:616-21. [Crossref] [PubMed]
- Ammori BJ, Ayiomamitis GD. Laparoscopic pancreaticoduodenectomy and distal pancreatectomy: a UK experience and a systematic review of the literature. Surg Endosc 2011;25:2084-99. [Crossref] [PubMed]
- Lai EC, Tang CN. Current status of robot-assisted laparoscopic pancreaticoduodenectomy and distal pancreatectomy: a comprehensive review. Asian J Endosc Surg 2013;6:158-64. [Crossref] [PubMed]
- Gagner M, Pomp A. Laparoscopic pancreatic resection: Is it worthwhile? J Gastrointest Surg 1997;1:20-5; discussion 25-6. [Crossref] [PubMed]
- Lei Z, Zhifei W, Jun X, et al. Pancreaticojejunostomy sleeve reconstruction after pancreaticoduodenectomy in laparoscopic and open surgery. JSLS 2013;17:68-73. [Crossref] [PubMed]
- Pugliese R, Scandroglio I, Sansonna F, et al. Laparoscopic pancreaticoduodenectomy: a retrospective review of 19 cases. Surg Laparosc Endosc Percutan Tech 2008;18:13-8. [Crossref] [PubMed]
- Dulucq JL, Wintringer P, Mahajna A. Laparoscopic pancreaticoduodenectomy for benign and malignant diseases. Surg Endosc 2006;20:1045-50. [Crossref] [PubMed]
- Kim SC, Song KB, Jung YS, et al. Short-term clinical outcomes for 100 consecutive cases of laparoscopic pylorus-preserving pancreatoduodenectomy: improvement with surgical experience. Surg Endosc 2013;27:95-103. [Crossref] [PubMed]
- Nakamura Y, Matsumoto S, Matsushita A, et al. Pancreaticojejunostomy with closure of the pancreatic stump by endoscopic linear stapler in laparoscopic pancreaticoduodenectomy: a reliable technique and benefits for pancreatic resection. Asian J Endosc Surg 2012;5:191-4. [Crossref] [PubMed]
- Kendrick ML, Cusati D. Total laparoscopic pancreaticoduodenectomy: feasibility and outcome in an early experience. Arch Surg 2010;145:19-23. [Crossref] [PubMed]
- Palanivelu C, Rajan PS, Rangarajan M, et al. Evolution in techniques of laparoscopic pancreaticoduodenectomy: a decade long experience from a tertiary center. J Hepatobiliary Pancreat Surg 2009;16:731-40. [Crossref] [PubMed]
- Peng SY, Wang JW, Lau WY, et al. Conventional versus binding pancreaticojejunostomy after pancreaticoduodenectomy: a prospective randomized trial. Ann Surg 2007;245:692-8. [Crossref] [PubMed]
- Cho A, Yamamoto H, Nagata M, et al. Laparoscopic major hepato-biliary-pancreatic surgery: formidable challenge to standardization. J Hepatobiliary Pancreat Surg 2009;16:705-10. [Crossref] [PubMed]
- Kendrick ML, Sclabas GM. Major venous resection during total laparoscopic pancreaticoduodenectomy. HPB (Oxford) 2011;13:454-8. [Crossref] [PubMed]
- Afaneh C, Gerszberg D, Slattery E, et al. Pancreatic cancer surgery and nutrition management: a review of the current literature. Hepatobiliary Surg Nutr 2015;4:59-71. [PubMed]
- Zhou NX, Chen JZ, Liu Q, et al. Outcomes of pancreatoduodenectomy with robotic surgery versus open surgery. Int J Med Robot 2011;7:131-7. [Crossref] [PubMed]
- Zureikat AH, Breaux JA, Steel JL, et al. Can laparoscopic pancreaticoduodenectomy be safely implemented? J Gastrointest Surg 2011;15:1151-7. [Crossref] [PubMed]
- Moore GE, Sako Y, Thomas LB. Radical pancreatoduodenectomy with resection and reanastomosis of the superior mesenteric vein. Surgery 1951;30:550-3. [PubMed]
- Müller SA, Hartel M, Mehrabi A, et al. Vascular resection in pancreatic cancer surgery: survival determinants. J Gastrointest Surg 2009;13:784-92. [Crossref] [PubMed]
- Tseng JF, Raut CP, Lee JE, et al. Pancreaticoduodenectomy with vascular resection: margin status and survival duration. J Gastrointest Surg 2004;8:935-49; discussion 949-50. [Crossref] [PubMed]
- Storkholm JH, Hansen CP. Mesenterico-portal vein resection in patients with pancreatico-duodenal cancer is safe and may increase survival. Dan Med J 2014;61:A4757. [PubMed]
- Croome KP, Farnell MB, Que FG, et al. Total laparoscopic pancreaticoduodenectomy for pancreatic ductal adenocarcinoma: oncologic advantages over open approaches? Ann Surg 2014;260:633-8; discussion 638-40. [Crossref] [PubMed]
- Croome KP, Farnell MB, Que FG, et al. Pancreaticoduodenectomy with major vascular resection: a comparison of laparoscopic versus open approaches. J Gastrointest Surg 2015;19:189-94; discussion 194. [Crossref] [PubMed]
- van Riel WG, van Golen RF, Reiniers MJ, et al. How much ischemia can the liver tolerate during resection? Hepatobiliary Surg Nutr 2016;5:58-71. [PubMed]
- Palanisamy S, Deuri B, Naidu SB, et al. Major venous resection and reconstruction using a minimally invasive approach during laparoscopic pancreaticoduodenectomy: One step forward. Asian J Endosc Surg 2015;8:468-72. [Crossref] [PubMed]
- Awad ZT. Totally laparoscopic pancreaticoduodenectomy for pancreatic head cancer with involvement of the superior mesenteric vein-portal vein confluence. Ann Surg Oncol 2014;21:3439. [Crossref] [PubMed]
- Dokmak S, Chérif R, Duquesne I, et al. Laparoscopic Pancreaticoduodenectomy with Reconstruction of the Portal Vein with the Parietal Peritoneum. Ann Surg Oncol 2016;23:2664. [Crossref] [PubMed]