Apollon overexpression promotes cell motility of gliomas, and predicts patients’ poor prognosis
Introduction
As the most frequent and malignant primary tumor type in the central nervous system of the body, human gliomas are characterized by aggressive progression, high recurrence and mortality rate (1,2). Based on the World Health Organization (WHO) criteria, there are four grades of human gliomas, including grade I (pilocytic astrocytoma), grade II (diffuse astrocytoma), grade III (anaplastic astrocytoma), and grade IV (glioblastoma, GBM) (3). As the most common subtype of gliomas, GBM represents a kind of highly infiltrative, necrosis-prone, mitotically active and cytologically malignant brain tumor (4). In recent years, there have been great advances in standard treatments for glioma patients, such as neurosurgery, chemotherapy and radiotherapy. However, their therapeutic efficacies and patients’ clinical outcome remain unsatisfactory (5). Therefore, it is necessary to understand the molecular mechanisms of human gliomas, to identify new diagnostic and prognostic markers for precise evaluation of patients' survival, and to develop more efficient therapeutic strategies.
The inhibitors of apoptosis (IAP) family, consisting of eight members with one to three baculovirus inhibitor of apoptosis repeat (BIR) domain, function as endogenous inhibitors of cell apoptosis via binding with caspases and play a crucial role in the regulation of cell death (6). As a member of the human IAP family, apollon, also named as Birc6 or Bruce, is a 528 kDa protein in mammals and consists of a single N-terminal BIR domain and a C-terminal ubiquitin-conjugating domain (7,8). Consistent with other IAP members, apollon can inhibit the activities of caspases-3, 6, 7 and 9 via directly binding to these proteins using its BIR domain (9). Accumulating studies revealed the aberrant expression of apollon protein in various human cancer types, such as acute myeloid leukemia, neuroblastoma, breast cancer, non-small-cell lung cancer, colorectal cancer, cervical cancer, ovarian cancer and prostate cancer, and highlighted its involvement into these malignancies (10-17).
Notably, a previous study of Chen et al. (18) reported that a glioma cell line SNB-78 expressed an increased level of apollon and had distinct resistance against several anti-cancer drugs. However, the role of apollon in human gliomas remains unclear. To address this problem, we aimed to investigate the clinical impact of apollon dysregulation in patients with gliomas, and its functions in malignant phenotypes of glioma cells.
Methods
Ethics statement
The present study was approved by the Research Ethics Committee of Nanjing Medical University, China (ID of ethics approval: NMU2016168). Written informed consent was obtained from all of the patients. All specimens were handled and made anonymous according to the ethical and legal standards.
Patients and tissue samples
A total 96 glioma tissues and 20 nonneoplastic brain tissues, the same as our previous studies (19,20), were collected into the present study. The clinicopathologic features of all the patients were summarized in Supplementary Table S1. The detailed information of this cohort is provided in Supplementary.
Cell culture and transfection
Two human glioma cell lines U251MG and U118MG were purchased from the cell library of the Chinese Academy of Sciences (Shanghai, China). U251MG and U118MG cells were cultured in DMEM (GibCo BRL, Grand Island, NY, USA) supplemented with 10% fetal bovine serum 2 mM L-glutamine, 100 U/mL penicillin-streptomycin mixture (GibCo BRL) at 37 °C, and 5% CO2.
When U251MG and U118MG cells reached 80% confluence, the complete medium was replaced with fresh medium before the transfection. To knockdown the expression of apollon, the apollon-specific siRNA (si-apollon) and control siRNA (si-con) were manufactured by Genechem (Shanghai, China). The sequences of apollon-specific siRNA and control siRNA were as following: si-apollon, 5'-AGA AAU UGA CCU UGA GUU A-3'; si-con, 5'-CAA UCU GUA GAU AUA GUG A-3'. U251MG and U118MG cells were transfected with apollon-specific siRNA or control siRNA by Lipofectamine 2000 according to the instruction for users. Transfected cells were collected for western blot and transwell assays after the transfection for 24–48 h.
Immunohistochemistry analysis
Immunohistochemistry analysis was performed to examine the subcellular localization and expression level of apollon protein in 96 glioma tissues and 20 non-neoplastic brain tissues according to the protocols described by our previous studies (19,20). The mouse monoclonal antibody to apollon protein was purchased from Santa Cruz Biotechnology, Inc. (#sc-390616, Santa Cruz, CA, USA).
Two independent experienced pathologists, blinded to the clinicopathological characteristics and clinical outcomes of glioma patients, were employed to evaluate the results of immunostaining. The immunoreactive score (IRS) of each specimen was calculated in a semiquantitative manner based on the percentage of positively stained cells and the intensity of immunostaining. The percentage of positively stained cells is graded as ‘0’, positively stained cells, <25%; ‘1’, 25% to 75%; and ‘2’, >75%. The intensity of immunostaining is graded as ‘0’, weak yellow; ‘1’, yellow, and ‘2’, dark yellow or brown. Then, the score of the percentage of positively stained cells was multiplied with that of the intensity of immunostaining to yield the IRS score of each sample. Overall, the IRS is graded as: negative (score 0 to 1), low to moderate positive (score 2 to 3), and strong positive (score >3). The number of cells with apollon positive staining showing immunoreactivity in cell cytoplasm in ten representative microscopic fields was counted, and the percentage of positive cells was calculated. The IRS of the two pathologists was compared, and any discrepant scores were trained through reexamining the stainings by both pathologists to achieve a consensus score.
Western blot analysis
Expression levels of apollon protein in transfected cells were detected by western blot analysis according to the protocol as described by previous studies (21,22). In brief, the protein samples were extracted from the cell lysates, and the content of the protein samples was determined by Bradford assay. GAPDH was used as an internal control. The primary antibodies were used as following: anti-apollon antibody (mouse monoclonal antibody, #sc-390616, Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA) and anti-GAPDH antibody (mouse monoclonal antibody, #sc-365062, Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA). The band density was measured with a computer-assisted image-analysis system (Adobe Systems, SanJose, CA, USA) and normalized against the expression level of GAPDH protein.
Cell counting kit-8 (CCK-8) assays in vitro
Followed by the transfection of apollon-specific siRNA, cell proliferation of U251MG and U118MG cells were examined using a CCK-8 (Beyotime, Nantong, China) according to the users’ instructions. Then, the absorbance at 450 nm was measured on an ELX-800 spectrometer reader (Bio-Tek Instruments, Winooski, USA). All experiments were repeated three times.
Cell migration and invasion assays in vitro
Migrated and invaded efficacies of U251MG and U118MG cells were determined using transwell chambers with and without MatrigelTM Basement Membrane Matrix (8 µm pore size, Corning, NY, USA), respectively. In brief, the transfected cells (1×105/well) were resuspended into 100 µL serum-free DMEM medium and then added into the top chamber of the inserts. After that, DMEM medium containing 10% fetal bovine serum was added into the bottom chambers. After 24 h of incubation, the non-migrated/invaded cells in the top chamber were removed, and the migrated/invaded cells in the bottom chamber was fixed and stained with crystal violet. The number of the migrated/invaded cells in five fields was counted under ×200 magnification, and the means for each chamber were determined. All experiments were repeated three times.
Statistical analysis
All data analyses were statistically performed using the software of SPSS version 11.0 for Windows (SPSS Inc., IL, USA). Data were expressed as mean ± SD. The difference in IRS of apollon protein between glioma and normal groups was evaluated by the analysis of variance (ANOVA). The associations between apollon protein expression and various clinicopathological characteristics of glioma patients were analyzed by Fisher’s exact test or the χ2 test. The prognostic value of apollon expression was statistically assessed by the Kaplan-Meier curve, the log-rank and the Cox proportional hazards model for multivariate survival analysis. The statistical significance of the means in different cell groups was calculated using Student’s t-test. Differences were considered statistically significant when P was less than 0.05.
Results
Overexpression of apollon protein in human glioma and nonneoplastic brain tissues
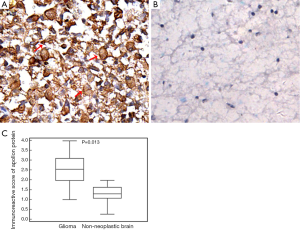
Immunostainings of apollon protein were mainly shown in tumor cell cytoplasm of glioma tissues (Figure 1A), but weakly or negatively observed in nonneoplastic brain tissues (Figure 1B). Statistically, the IRS of apollon protein in glioma tissues was significantly higher than that in the corresponding nonneoplastic brain tissues (tumor vs. control: 2.53±0.73 vs. 1.27±0.44, P=0.013, Figure 1C).
To evaluate the clinical impact of apollon protein in human gliomas, all 96 patients were divided into two groups using the median value of apollon’s IRS (2.53): high apollon expression group (n=50, 52.08%, mean ± SD =3.13±0.41) and low apollon expression group (n=46, 47.92%, mean ± SD =1.88±0.35).
Overexpression of apollon protein associates with advanced tumor grade of human gliomas
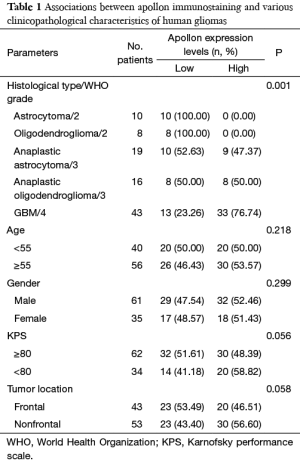
The associations of apollon protein expression with various clinicopathological characteristics were statistically evaluated and summarized in Table 1. Glioma patients with high apollon expression more frequently had higher WHO grade than those with low apollon (P=0.001). However, no statistically significant associations of apollon expression with age at diagnosis, gender of patients and Karnofsky performance scale (KPS) score was found (both P>0.05, Table 1).
Full table
Overexpression of apollon protein predicts poor prognosis of human gliomas
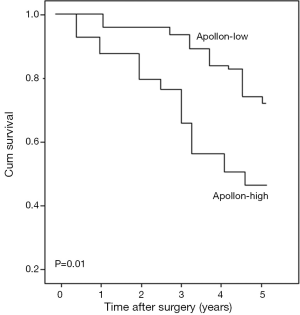
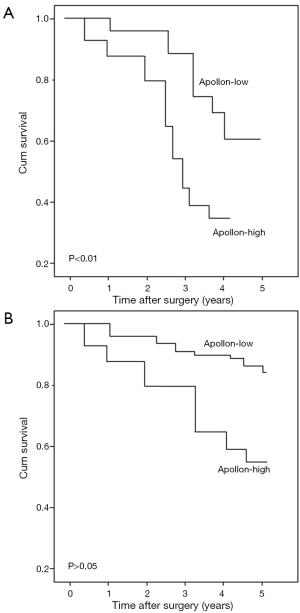
All 96 patients with gliomas were performed follow-up and the median survival of this cohort was 19 months. Glioma patients with high apollon expression had significantly shorter mean survival times than patients with low apollon expression (high vs. low: 16 vs. 28 months). Kaplan–Meier survival curves showed in Figure 2 revealed that overexpression of apollon was significantly associated with poor overall survival (P=0.01). More interestingly, the subgroup analysis also demonstrated that the high-grade glioma patients with high apollon expression had shorter overall survival than those with low apollon expression (P<0.01, Figure 3A). However, there was no difference with statistical significance between high and low apollon expression groups for low-grade glioma patients (Figure 3B).
After the construction of a multivariate Cox proportional hazard model, the results found that apollon expression (P=0.03), WHO grade (P=0.009) and KPS score (P=0.02) seemed to be independent prognostic indicators (Table 2).

Full table
Loss of apollon expression suppresses cell proliferation, migration and invasion of glioma cells
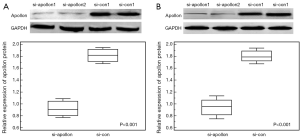
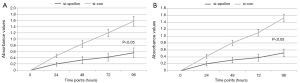
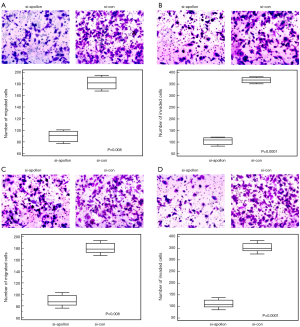
As shown in Figure 4 based on the results of western blot analysis, the expression levels of apollon protein in si-apollon transfected U251MG and U118MG cells were markedly decreased, compared to those in cells transfected with control siRNA (P<0.001). CCK-8 and transwell assays respectively showed that the decreased expression of apollon protein could efficiently suppress cell proliferation, migration and invasion abilities of U251MG and U118MG cells compared to control group (all P<0.05, for CCK-8 assay, Figure 5; for transwell assay, Figure 6).
Discussion
Human gliomas represent an aggressive malignancy featured by limited efficient therapeutic strategies and poor prognosis. Therefore, it is of great clinical significance to identify novel prognostic markers and effective therapeutic targets for the treatment of this malignancy. In the present study, we initially examined the sub-cellular localization and expression pattern of apollon protein in human glioma tissues. As a result, the immunostainings of apollon protein were mainly shown in tumor cell cytoplasm of glioma tissues, but weakly or negatively observed in nonneoplastic brain tissues. Statistically, its IRS in glioma tissues was dramatically increased when compared to that in the corresponding nonneoplastic brain tissues. In addition, patients with higher IRS often had advanced WHO grade and shorter overall survival time. Further multivariate analysis identified apollon expression as an independent prognostic factor of glioma patients. Functionally, in vitro experiments revealed that loss of apollon could efficiently suppress proliferation, migration and invasion of glioma cells. These findings highlight the crucial roles of apollon expression in tumor progression, patients’ prognosis and tumor cell motility of human gliomas.
As the largest member of the human IAP family, apollon contains a single N-terminal BIR domain and a C-terminal ubiquitin-conjugating domain, which exerts chimeric E2/E3 ubiquitin ligase activity and antiapoptotic activity (6). Growing evidence shows that the aberrant expression of apollon may play critical roles in tumorigenesis and tumor progression of various human cancer types. For example, Schläfli et al. (10) found significantly lower apollon levels in particular AML subtypes as compared to granulocytes from healthy donors, and described that the knockdown of apollon in acute promyelocytic leukemia cells could distinctly impair neutrophil differentiation, but not cell viability. In contrast, Low et al. (11) reported the elevated apollon protein expression in prostate cancer cell lines and clinical specimens as distinct from their benign counterparts; The authors also determined that the reduction of apollon expression in PCa cell lines could lead to a marked reduction in cell proliferation which was associated with an increase in apoptosis and a decrease in autophagosome formation; Hu et al. (12) suggested that apollon overexpression might be a predictor of poor prognosis in colorectal cancer; Dong et al. (13) revealed that the increased expression of apollon protein in non-small-cell lung cancer tissues might be closely correlated with cancer recurrence and chemoresistance of patients. These reports imply that apollon may function as either an oncogenic factor or a tumor suppressor depending on different cancer types.
Consistent with the previous studies on neuroblastoma (14) and glioma cell line (18), our data here also found the overexpression of apollon protein in clinical glioma tissue specimens and determine its associations with advanced tumor grades and unfavorable patients’ prognosis. Notably, our data, obtained by knock-downing apollon expression, described a role for apollon in modulating the biological properties of glioma cells, including proliferation, migration and invasion in vitro. Loss of this protein led to the suppression of cell motility dramatically.
Conclusions
In conclusion, our findings in the present study imply that the dysregulation of apollon may be implicated into tumorigenesis and various pathological changes of human gliomas. Importantly, this protein might be a potential prognostic marker and novel therapeutic target for patients with this tumor. However, an increasing study has identified several markers for determining the prognosis of glioma patients, such as IDH, MGMT, ATRX, P53, etc. Therefore, the correlation between these established glioma markers and apollon are required to be evaluated, in order to confirm the role of apollon as an independent prognostic marker.
Patients and tissue samples
A total of ninety-six glioma samples were obtained from 96 Chinese patients with gliomas of different grades. Patient characteristics, including the KPS score, were collected before the initial surgery. After surgical resection of their tumors, patients with a high-grade glioma received a course of external beam radiation therapy (standard doses: 40 Gy to the tumor with 3-µm margins, 20 Gy boost to the whole brain) and nitrosourea-based chemotherapy during the course of the disease. Surgically resected tissues were frozen immediately and stored at –80 °C until processing. Tumors were classified histopathologically according to the WHO classification. No patients recruited in this study had chemotherapy or radiotherapy before the surgery. The clinicopathologic features of all the patients were summarized in Supplementary
For the analysis of survival and follow-up, the date of surgery was used to represent the beginning of the follow-up period. All patients who died from diseases other than glioma or from unexpected events were excluded from the case collection. Follow-ups were terminated until May 28, 2009. The median follow-up was 42 months (range, 1–96 months). Treatment modalities after relapse were given according to a uniform guideline as described.

Full table
Acknowledgments
Funding: This work was supported by research grants from the Science and Technology Development Foundation of Huai’an to Dr. lianshu Ding (China, No. HAS2015018).
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2016.12.17). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Research Ethics Committee of Nanjing Medical University, (NMU2016168) and written informed consent was obtained from all patients.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Wen PY, Kesari S. Malignant gliomas in adults. N Engl J Med 2008;395:492-507. [Crossref] [PubMed]
- Liu N, Tu Y. Systematic Review of MicroRNAs and its Therapeutic Potential in Glioma. Cancer Transl Med 2015;1:50-66. [Crossref]
- Louis DN, Ohgaki H, Wiestler OD, et al. The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol 2007;114:97-109. [Crossref] [PubMed]
- Li Q, Tu Y. Genetic Characteristics of Glioblastoma: Clinical Implications of Heterogeneity. Cancer Transl Med 2015;1:176-80. [Crossref]
- Ohgaki H, Kleihues P. Epidemiology and etiology of gliomas. Acta Neuropathol 2005;109:93-108. [Crossref] [PubMed]
- Bartke T, Pohl C, Pyrowolakis G, et al. Dual role of BRUCE as an antiapoptotic IAP and a chimeric E2/E3 ubiquitin ligase. Mol Cell 2004;14:801-11. [Crossref] [PubMed]
- Hao Y, Sekine K, Kawabata A, et al. Apollon ubiquitinates SMAC and caspase-9, and has an essential cytoprotection function. Nat Cell Biol 2004;6:849-60. [Crossref] [PubMed]
- Chu L, Gu J, Sun L, et al. Oncolytic adenovirus-mediated shRNA against Apollon inhibits tumor cell growth and enhances antitumor effect of 5-fluorouracil. Gene Ther 2008;15:484-94. [Crossref] [PubMed]
- Lopergolo A, Pennati M, Gandellini P, et al. Apollon gene silencing induces apoptosis in breast cancer cells through p53 stabilisation and caspase-3 activation. Br J Cancer 2009;100:739-46. [Crossref] [PubMed]
- Schläfli AM, Torbett BE, Fey MF, et al. BIRC6 (APOLLON) is down-regulated in acute myeloid leukemia and its knockdown attenuates neutrophil differentiation. Exp Hematol Oncol 2012;1:25. [Crossref] [PubMed]
- Low CG, Luk IS, Lin D, et al. BIRC6 protein, an inhibitor of apoptosis: role in survival of human prostate cancer cells. PLoS One 2013;8:e55837 [Crossref] [PubMed]
- Hu T, Weng S, Tang W, et al. Overexpression of BIRC6 Is a Predictor of Prognosis for Colorectal Cancer. PLoS One 2015;10:e0125281 [Crossref] [PubMed]
- Dong X, Lin D, Low C, et al. Elevated expression of BIRC6 protein in non-small-cell lung cancers is associated with cancer recurrence and chemoresistance. J Thorac Oncol 2013;8:161-70. [Crossref] [PubMed]
- Luk SU, Xue H, Cheng H, et al. The BIRC6 gene as a novel target for therapy of prostate cancer: dual targeting of inhibitors of apoptosis. Oncotarget 2014;5:6896-908. [Crossref] [PubMed]
- Wang L, Chen YJ, Hou J, et al. Expression and clinical significance of BIRC6 in human epithelial ovarian cancer. Tumour Biol 2014;35:4891-6. [Crossref] [PubMed]
- Lamers F, Schild L, Koster J, et al. Identification of BIRC6 as a novel intervention target for neuroblastoma therapy. BMC Cancer 2012;12:285. [Crossref] [PubMed]
- Van Houdt WJ, Emmink BL, Pham TV, et al. Comparative proteomics of colon cancer stem cells and differentiated tumor cells identifies BIRC6 as a potential therapeutic target. Mol Cell Proteomics 2011;10:M111.011353.
- Chen Z, Naito M, Hori S, et al. A human IAP-family gene, apollon, expressed in human brain cancer cells. Biochem Biophys Res Commun 1999;264:847-54. [Crossref] [PubMed]
- Ding L, Sun X, You Y, et al. Expression of focal adhesion kinase and phosphorylated focal adhesion kinase in human gliomas is associated with unfavorable overall survival. Transl Res 2010;156:45-52. [Crossref] [PubMed]
- Liu C, Tu Y, Sun X, et al. Wnt/beta-Catenin pathway in human glioma: expression pattern and clinical/prognostic correlations. Clin Exp Med 2011;11:105-12. [Crossref] [PubMed]
- Fan S, Zhao C, Zhang L, et al. Knockdown of PFTK1 Inhibits the Migration of Glioma Cells. J Mol Neurosci 2015;57:257-64. [Crossref] [PubMed]
- Hu Y, Chen F, Liu F, et al. Overexpression of TIP30 inhibits the growth and invasion of glioma cells. Mol Med Rep 2016;13:605-12. [PubMed]