
Programmed death 1 induces cell chemoresistance to 5-fluorouracil in gastric cancer cell lines
Introduction
Gastric cancer is reported to be one of the leading causes of cancer-related death worldwide. Chemotherapy, one of the main methods for the treatment of gastrointestinal carcinoma, plays an important role in comprehensive treatment, because most cases are advanced and surgery is often difficult to obtain the curative effect (1). 5-fluorouracil (5-FU) is one of the most commonly used chemotherapy drugs in treatment of gastrointestinal carcinoma. The drug resistance of tumor cells is the main obstacle to chemotherapy and the clarification of the mechanism will greatly improve the prognosis of patients with tumor chemotherapy (2). Currently, the mechanism of formation of drug resistance are identified to be mainly associated with excessive expression of certain ABC transporters, termed multi-drug resistance (MDR) proteins; increased activities of glutathione transferase glutathione-S-transferase (GST) activity and DNA repair ability and reduced activities of topoisomerase II and apoptosis etc. (3-5).
Immunotherapy is a novel treatment option for cancers because of its less side effects and more effectiveness on cancer cells. Immune surveillance seems to represent an effective tumor suppressor mechanism. However, some cancer cells survive and become variants, being poorly immunogenic and able to enter a steady-state phase (6). Activation of tumor specific CD8+ T cells can effectively inhibit tumor growth. The activation of T cells requires not only antigen-presenting cells to present MHC-antigen peptide to antigen specific T cells as the first signal, also a series of coordinated stimulus molecules as the second signal to make the T cells to produce immune response (7). Lack of stimulating molecules to provide a second signal will result in T-cell tolerance, that is, the failure of T cells to respond (8). Programmed death 1 (PD-1) was originally discovered in the apoptosis of T cell hybridoma and named after its association with cell apoptosis. Several reports show that PD1 on T cells inhibits T cell receptor signaling and immune responses, inducing cancerous immune escape in the host (9). Two ligands (PDL1 and PDL2) for PD1 have been discovered with different distributions. PDL 1 (B7-H1) which belongs to the B7 family plays an important role in the negative regulation of immune response when interacting with PD1. PDL1 has a widely tissue distribution including antigen presenting cells, activated T and B cells, macrophages, placental trophoblastic, myocardial endothelial and thymic epithelial cells with higher expression in some cancer cell lines (10,11). Tumor microenvironment can induce the expression of PDL1 on the tumor cells, favoring the occurrence and growth of the tumor by inducing anti-tumor T cell apoptosis. PDL2 (B7-DC) is restricted to certain types of cells or tissues, such as macrophages and dendritic cells. At present, PD1 ligand is proved to be associated with the poor prognosis of patients with gastric cancer, renal cell carcinoma, and other cancer patients (12,13). Antibodies interfering with binding of PDL1 to PD1 enhanced intratumor CD8+ T-cell and induced regression of established tumors (14,15). A number of clinical trials have revealed that inhibition of PD1/PDL1 pathway received ideal responses to advanced melanoma, non-small cell lung carcinoma, and renal cell carcinoma (16,17), and accumulating evidence demonstrates the potential of PD1 inhibitors in cancer treatment.
The high rate of relapse and failure of chemotherapy is believed to a large degree to result from the emergence of drug resistant cells during treatment. Numerous clinical data revealed that the MDR phenotype in tumors is associated with the expression of certain ABC transporters. P-glycoprotein (P-gp, MDR1, ABCB1) was the first discovered ABC transporter and is likely to be responsible for the most widely observed mechanism in clinical MDR (18). Soon after the cloning and characterization of MDR1, it became evident that other efflux pumps also play significant roles in transport—associated drug resistance. Two other ABC transporters have definitively demonstrated participation in the MDR of tumors: the MDR protein 1 [MRP1, ATP binding cassette subfamily C member 1 (ABCC1)], and the mitoxantrone resistance protein (MXR/BCRP, ABCG2) (19,20). ABCC1 may be independent prognostic markers for gastric cancer treatment (19). Abnormal expression of p53, Bcl-2 or c-myc, which can suppress the apoptosis induced by chemotherapy drugs, at the same time, specific activate triphosphate (ATP) dependent transporters to pump out drugs, may predict a loss of the efficacy of the chemotherapy drugs 5-FU in patients with gastric cancer (21). There have been several reports of the involvement of PD1 in the resistance of different cancer cells to chemotherapeutic reagents. For example, BRAF-targeted therapy results in short lived objective responses in the majority of patients of malignant melanoma because of gradually increased expression of the immunomodulatory molecule PDL1, which may contribute to the resistance (16). Besides, elevated cellular PD1/PD-L1 expression confers acquired resistance to cisplatin in small cell lung cancer cells (22). However, the role of PD1/PDL1 in gastric chemoresistance has not been thoroughly reported. Thus, the present study examined the significance of PD1 expression in regulating the chemoresistant ability of gastric cancer against 5-FU, which may provide new therapeutic strategies to combat gastric cancer.
Methods
Cell lines
Human gastric cancer cell line SGC7901 (American Type Culture Collection, Manassas, VA, USA) were cultured in RPMI-1640 medium (Gibco, CA, USA), which was supplemented with 10% heat-inactivated fetal bovine serum (FBS), 100 IU penicillin/mL and 100 µg/mL streptomycin (Invitrogen Life Technologies, China). SGC7901 cells built in 1979 are derived from a 56-year-old female patient with gastric cancer lymph node metastases. The 5-FU-resistant variant SGC7901/5-FU was obtained from the parent cell line (SGC7901) by step by step exposure to increasing concentrations of 5-FU (5, 10, 20 and 40 µg/mL) (Sigma, MO, USA). Briefly, the cells were co-cultured with fixed concentration of 5-FU for 12 hours, then the medium was replaced by fresh medium. When living cells reached 80%, the monolayer cultures were passaged and cultured to logarithmic phase, then treated with increased concentration of 5-FU.
MTT assay
SGC7901 and SGC7901/5-FU cells were respectively seeded into a 96-well plate at a density of 4×103/well and incubated at 37 °C in 95% air and 5% CO2. 5-FU was added so that the final concentrations were 0, 1, 2, 4 and 8 µg/mL. Following 48 h of incubation, the MTT assay was used to evaluate the cell viability. In total, 20 µL of MTT solution (5 mg/mL; Sigma, USA) was added to 100 µf culture media and cells were incubated for a further 4 h at 37 °C. After removing the culture medium, 200 µL DMSO was added to each well and the plates were shaken for 10 min. Then the absorbance (OD) value was measured at A490 nm. Three replicates of each sample were analyzed in each assay. The growth inhibition rate was calculated as: growth inhibition (%) = (1 − OD490/OD490 of 0 µnhi/L) ×100.
Transfection with expression vector and PD1 siRNA
Human codon-optimized human PD1 cDNA was synthesized by Genscript and subcloned into EcoR I and BamH I of pcDNA3.1(+) with T4 DNA Ligase (Fast Digest, MBI Fermentas, USA). Cells were seeded into six-well plates at 5×105 cells per well. The 90% confluent monolayer cultures were cultured in RPMI-1640 medium without serum for 24 h, then tranfected with lipofectamine LTX and PLUS reagents (Life Technologies, USA). Briefly, the cells were washed twice by invitrogen optiMEM medium, and added 1.5 mL optiMEM in every well. The 5 µg plasmid was added to and mixed with 250 µL optiMEM at room temperature for 10 min, then 5 µL plus reagent was added and mixed. In another tube, 15 µL LTX reagent was added to and mixed with 250 µL optiMEM medium at room temperature for 30 min. Then the two tubes were mixed and the complex was added to cells drop by drop, gently mixed, then incubated at 37 °C and 5% CO2 for 6 h. Media was replaced with fresh medium 6 h later. The cells were selected 24 h later with G418 (Amresco, USA) at 1,000 mg/mL and persistently cultured with G418 at 200 mg/mL.
siRNA oligonucleotides for PD1 and Negative control siRNA were purchased from OriGene (Rockville, MD, USA). Cells were seeded into six-well plates at 5×105 cells per well. In total, 90% confluent monolayer cultures were incubated with final concentration of 10 nM PD1 siRNA or Negative control to PD1 using 6 µL SiTran1.0 (OriGene, Rockville, MD, USA) for 24 h. Downregulation of PD1 was assayed by IFA and real-time PCR (RT-PCR) after 48 hours.
Immunofluorescence (IFA)
Cells were fixed by 95% ethanol and then incubated with 3% H2O2, and blocked with a normal goat serum for 20 min. Following washing with phosphate buffered saline (PBS) ×3 (5 minutes each), cells were incubated with the mouse monoclonal PD1 antibody (1:250 dilution; Abcam, USA) for 30 min at room temperature. After washing with PBS ×3 (5 minutes each), cells were then incubated with fluorescein isothiocyanate-labeled goat anti-mouse antibody immunoglobulin G (IgG, 1:200 dilution; Sigma, USA) for 30 min at 37 °C, washed, and incubated with 0.5 µg/mL DAPI for 10 min, washed. Then the slides were mounted with 10 mM p-phenylenediamine in glycerol:PBS (9:1), pH 8.5, and analyzed by confocal microscopy (Olympus, Tokyo, Japan).
Annexin V/propidium iodide (PI) staining assay
After various treatments, cells were labelled with 4 µL of FITC-Annexin V and 8 µL of PI for 5 min in darkness and at room temperature, and the specimens were then detected using one flow cytometer (BD Bioscience, USA) for apoptosis assay. Normal living cells and early apoptotic cells could resist the staining by PI, but necrotic cells could not.
RT-PCR
RT-PCR was performed in order to detect the relative levels of the transcripts. RNA of cell samples was isolated using Trizol (Takara, Dalian, China) according to the manufacturer’s instructions. The transcripts were generated from 2 µg RNA extracted from the cells through reverse transcription using RT-PCR Kit (TaKaRa, Dalian, China). PCR was performed under the following conditions: 94iption using generated from Cles of 94 °C or 1 min and 60 °C for 1 min. The med under the following conditions: 94iption using generated from cles of 94 cell samples were isolated 5'-TTTCCCTTCCGCTCACCTCC-3' and 5'-CAGACCCGCCACGATGTTGAC-3' (PD1, 114 bp); 5'-CGTGGACATCCGCAAAGACC-3' and 5'-ATCAACGCAATGTGGGAAAGAACTG-3' (ABCC1, 304 bp); and 5'-TGTGGCCTTCTTTGAGTTCGGT-3' and 5'-CCTATTGCCTCCGAGCCTACG-3' (Bcl-2, 148 bp); 5'-CGCTGAGTTCCTGCGTACCTATG-3' and 5'-GGTGTTGTTGTCGTGGCGTCTTG-3' (β-actin, 211 bp). A melting curve of the reaction system was drawn immediately after the reaction to analyze the specificity of the PCR products. Quantitative analysis of target gene expression data was based on 2−△△Ct method. △△Ct = (average Ct of target gene-average Ct of β-actin in experimental groups) − (average Ct of target gene-average Ct of β-actin in control group) (23).
Statistical analysis
The differences in the mean values between the two groups were statistically analyzed using Student’s t-test. All P values were based on two-tailed statistical analysis. By conventional criteria, if the P value is less than 0.05, the difference between the two samples is considered to be statistically significant. All statistical analysis was performed with the SPSS 12.0 software program.
Results
Correlation between PD1 expression and sensitivity of 5-FU of gastric cancer cell lines
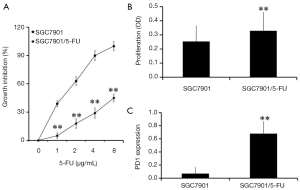
To detect the sensitivity of the SGC7901/5-FU cell line and its parental SGC7901 cell line to 5-FU, an MTT assay was performed. The results demonstrated that compared with the SGC7901 cell line (IC50 =2.2 µg/mL), the SGC7901/5-FU cells (IC50 =8.16 µg/mL) exhibited obvious resistance to 5-FU (Figure 1A). The proliferation of the two cell lines were also measured with MTT method. The result showed that the proliferation of SGC7901/5-FU cells were higher than those of SGC7901 cells (P<0.01, Figure 1B). In order to determine the role of PD1 in the 5-FU-resistant gastric cancer cell line SGC7901/5-FU, RT-qPCR analysis was conducted. The results indicated that PD1 expression was up-regulated in SGC7901/5-FU cells (Figure 1C), suggesting that PD1 may contribute to 5-FU drug resistance in gastric cancer cells, which was consistent with the report of Ishibashi for 5-FU in myeloma cells (24).
Downregulation of PD1 decreased resistance of SGC7901/5-FU to 5-FU
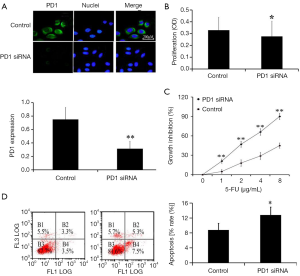
To investigate the association between PD1 and 5-FU resistance in SGC7901/5-FU cells, PD1 siRNA or negative control was transfected into SGC7901/5-FU cells. We examined the expression of PD1 in cells by IFA and RT-PCR, to detect the efficacy of transfection (Figure 2A). After confirming the successful downregulation of PD1 in cells, MTT assay was used to evaluate the cell viability and proliferation in 96-well plate. After 24 hour of transfection, 5-FU was added and incubated with cells for another 48 h. The proliferation of PD1 siRNA cells decreased obviously, when compared with negative siRNA cells (P<0.05, Figure 2B). Moreover, we observed that SGC7901/5-FU promoted apoptosis and decreased resistance to 5-FU, when compared with negative siRNA cells, suggesting that down-regulation of PD1 decreased the chemoresistant ability of SGC7901/5-FU (Figure 2C,D).
Upregulation of PD1 increased resistance of SGC7901 to 5-FU
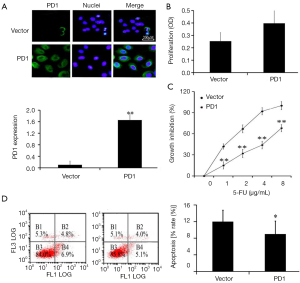
To further determine the effects of PD1 expression on chemoresistance of gastric cancer cells, PD1-expression vector or the control vector was transfected into SGC7901 cells. RT-PCR analysis confirmed that the PD1 vector was able to increase PD1 expression in SGC7901 cells compared with that in the control group (P<0.01, Figure 3A). The proliferation of PD1-expression cells increased obviously, when compared with empty vector control (P<0.05, Figure 3B). The SGC7901 cells which were transfected with the PD1 vector exhibited a significantly higher survival rate than the cells in the empty vector control group following 5-FU exposure (Figure 3C). Moreover, the apoptosis of PD1-expression cells decreased obviously, suggesting that the expression of PD1 may promote resistance of the SGC7901 cells to 5-FU.
PD1 positively regulates ABCC1 and Bcl-2
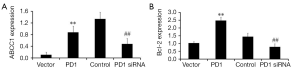
To determine whether ABCC1 or Bcl-2 serve a role in PD1-induced 5-FU resistance, the PD1 gene was decreased or over-expressed. Downregulation of PD1 significantly decreased the expression of ABCC1 or Bcl-2 in the SGC7901/5-FU cells compared with that in the control group (Figure 4A,B). The SGC7901 cells transfected with PD1-expression vector significantly increased the expression of ABCC1 or Bcl-2 compared with that in the negative control group (P<0.01, Figure 4A,B). These results suggested that PD1 may improve the 5-FU resistance by linked to ABC transporter and antiapoptotic mechanism.
Discussion
Over the past few decades, multi-drug chemotherapy has become a standard therapy for the treatment of malignant tumors and has been confirmed to improve survival rate of blood malignancies and solid tumor patients. However, drug resistance is one of the biggest obstacles to clinical practices. 5-FU is the most commonly used chemotherapeutic drug in patients with gastric cancer, and one of the main causes of chemotherapeutic treatment failure in advanced gastric cancer (25). Therefore, the development of novel strategies enhancing the efficacy of 5-FU is essential for effective therapy. It is widely accepted that PD1 is promising to treat many various cancers (26). However, the role of PD1 in drug-resistance of gastric cancer remains to be fully elucidated.
In the present study, the association between PD1 and drug-resistance of gastric cancer to 5-FU was investigated, and it was confirmed that PD1 promoted cell survival and 5-FU resistance in human gastric cancer. These results indicated that anti-PD1 therapy may be used as a therapeutic approach in 5-FU-resistant gastric cancer. Next, we explored the relationship between PD1 and some of the mechanisms that have been linked to cell chemoresistant abilities, such as ABCC1 and Bcl-2 protein expression. ABCC1 (ABC transporter family)-mediated drug efflux is widely recognized as one of original causes of tumor multidrug resistance (27). Bcl-2 is also one of main oncogenes resulting in resistance to chemotherapeutic agents through antiapoptotic effects (28). We observed that SGC7901/5-FU cells have new cytological characteristics, such as PD1, ABCC1 and Bcl-2 expression, enhanced proliferation. Inhibiting PD1 in SGC7901/5-FU cells leads to decreased expression of Bcl-2 and ABCC1, at the same time leading to decreased proliferation and resistance to 5-FU.
Our results suggest that increased resistance of gastric cancer cells to 5-FU induced by PD1 possibly results from promoted proliferation and inhibitory apoptosis and expression of certain ABC transporters. However, we did not detect other ABC transporters and other resistant mechanism related with 5-FU resistance. Thus, detailed association of PD1 with other resistant mechanism needs further study.
In summary, PD1 signaling in gastric cancer could participate in the chemoresistant ability of cells in vitro, possibly through enhanced proliferation, inhibitory apoptosis and expression of MDR transporters.
Acknowledgments
Funding: This research was supported by the National Natural Science Foundation of China (No. 81472338) and Science and Technology Development Project in Medicine and Health of Shandong province (No. 2016WS0283).
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2016.12.01). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). Institutional ethical approval and informed consent were waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Lordick F, Janjigian YY. Clinical impact of tumour biology in the management of gastroesophageal cancer. Nat Rev Clin Oncol 2016;13:348-60. [Crossref] [PubMed]
- Zhang D, Fan D. New insights into the mechanisms of gastric cancer multidrug resistance and future perspectives. Future Oncol 2010;6:527-37. [Crossref] [PubMed]
- Yu P, Du Y, Cheng X, et al. Expression of multidrug resistance-associated proteins and their relation to postoperative individualized chemotherapy in gastric cancer. World J Surg Oncol 2014;12:307. [Crossref] [PubMed]
- Geng M, Wang L, Chen X, et al. The association between chemosensitivity and Pgp, GST-π and Topo II expression in gastric cancer. Diagn Pathol 2013;8:198. [Crossref] [PubMed]
- Ott K, Rachakonda PS, Panzram B, et al. DNA repair gene and MTHFR gene polymorphisms as prognostic markers in locally advanced adenocarcinoma of the esophagus or stomach treated with cisplatin and 5-fluorouracil-based neoadjuvant chemotherapy. Ann Surg Oncol 2011;18:2688-98. [Crossref] [PubMed]
- Mendes F, Domingues C, Rodrigues-Santos P, et al. The role of immune system exhaustion on cancer cell escape and anti-tumor immune induction after irradiation. Biochim Biophys Acta 2016;1865:168-75.
- Foell J, Hewes B, Mittler RS. T cell costimulatory and inhibitory receptors as therapeutic targets for inducing anti-tumor immunity. Curr Cancer Drug Targets 2007;7:55-70. [Crossref] [PubMed]
- Ochsenbein AF, Sierro S, Odermatt B, et al. Roles of tumour localization, second signals and cross priming in cytotoxic T-cell induction. Nature 2001;411:1058-64. [Crossref] [PubMed]
- Wei F, Zhong S, Ma Z, et al. Strength of PD-1 signaling differentially affects T-cell effector functions. Proc Natl Acad Sci U S A 2013;110:E2480-9. [Crossref] [PubMed]
- Bertucci F, Finetti P, Mamessier E, et al. PDL1 expression is an independent prognostic factor in localized GIST. Oncoimmunology 2015;4:e1002729 [Crossref] [PubMed]
- Sponaas AM, Moharrami NN, Feyzi E, et al. PDL1 Expression on Plasma and Dendritic Cells in Myeloma Bone Marrow Suggests Benefit of Targeted anti PD1-PDL1 Therapy. PLoS One 2015;10:e0139867 [Crossref] [PubMed]
- Tamura T, Ohira M, Tanaka H, et al. Programmed Death-1 Ligand-1 (PDL1) Expression Is Associated with the Prognosis of Patients with Stage II/III Gastric Cancer. Anticancer Res 2015;35:5369-76. [PubMed]
- Xu F, Xu L, Wang Q, et al. Clinicopathological and prognostic value of programmed death ligand-1 (PD-L1) in renal cell carcinoma: a meta-analysis. Int J Clin Exp Med 2015;8:14595-603. [PubMed]
- Ngiow SF, Young A, Jacquelot N, et al. A Threshold Level of Intratumor CD8+ T-cell PD1 Expression Dictates Therapeutic Response to Anti-PD1. Cancer Res 2015;75:3800-11. [Crossref] [PubMed]
- Fu J, Malm IJ, Kadayakkara DK, et al. Preclinical evidence that PD1 blockade cooperates with cancer vaccine TEGVAX to elicit regression of established tumors. Cancer Res 2014;74:4042-52. [Crossref] [PubMed]
- Cooper ZA, Juneja VR, Sage PT, et al. Response to BRAF inhibition in melanoma is enhanced when combined with immune checkpoint blockade. Cancer Immunol Res 2014;2:643-54. [Crossref] [PubMed]
- Guilleminault L, Carmier D, Heuzé-Vourc'h N, et al. Immunotherapy in non-small cell lung cancer: inhibition of PD1/PDL1 pathway. Rev Pneumol Clin 2015;71:44-56. [Crossref] [PubMed]
- Bar-Zeev M, Assaraf YG, Livney YD. β-casein nanovehicles for oral delivery of chemotherapeutic Drug combinations overcoming P-glycoprotein-mediated multidrug resistance in human gastric cancer cells. Oncotarget 2016;7:23322-34. [PubMed]
- Ge J, Chen Z, Huang J, et al. Upregulation of autophagy-related gene-5 (ATG-5) is associated with chemoresistance in human gastric cancer. PLoS One 2014;9:e110293 [Crossref] [PubMed]
- Mosaffa F, Kalalinia F, Lage H, et al. Pro-inflammatory cytokines interleukin-1 beta, interleukin 6, and tumor necrosis factor-alpha alter the expression and function of ABCG2 in cervix and gastric cancer cells. Mol Cell Biochem 2012;363:385-93. [Crossref] [PubMed]
- Geng M, Wang L, Li P. Correlation between chemosensitivity to anticancer drugs and Bcl-2 expression in gastric cancer. Int J Clin Exp Pathol 2013;6:2554-9. [PubMed]
- Yan F, Pang J, Peng Y, et al. Elevated Cellular PD1/PD-L1 Expression Confers Acquired Resistance to Cisplatin in Small Cell Lung Cancer Cells. PLoS One 2016;11:e0162925 [Crossref] [PubMed]
- Wang L, Wu G, Qin X, et al. Expression of Nodal on Bronchial Epithelial Cells Influenced by Lung Microbes Through DNA Methylation Modulates the Differentiation of T-Helper Cells. Cell Physiol Biochem 2015;37:2012-22. [Crossref] [PubMed]
- Ishibashi M, Tamura H, Sunakawa M, et al. Myeloma Drug Resistance Induced by Binding of Myeloma B7-H1 (PD-L1) to PD-1. Cancer Immunol Res 2016;4:779-88. [Crossref] [PubMed]
- Wei Z, Liang L, Junsong L, et al. The impact of insulin on chemotherapeutic sensitivity to 5-fluorouracil in gastric cancer cell lines SGC7901, MKN45 and MKN28. J Exp Clin Cancer Res 2015;34:64. [Crossref] [PubMed]
- Gandini S, Massi D, Mandalà M. PD-L1 expression in cancer patients receiving anti PD-1/PD-L1 antibodies: A systematic review and meta-analysis. Crit Rev Oncol Hematol 2016;100:88-98. [Crossref] [PubMed]
- Kamata S, Kishimoto T, Kobayashi S, et al. Expression and localization of ATP binding cassette (ABC) family of drug transporters in gastric hepatoid adenocarcinomas. Histopathology 2008;52:747-54. [Crossref] [PubMed]
- Yan LH, Wang XT, Yang J, et al. Reversal of multidrug resistance in gastric cancer cells by E2F-1 downregulation in vitro and in vivo. J Cell Biochem 2014;115:34-41. [Crossref] [PubMed]


